Abstract
The presence of many neurofibrillary tangles (NFTs) in the central nervous system is a hallmark of amyotrophic lateral sclerosis (ALS) and parkinsonism–dementia complex (PDC) in people living in the Kii peninsula of Japan and in the island of Guam. To determine whether or not ALS and PDC are on a spectrum of a single tauopathy, we investigated the topography of NFTs semiquantitatively in two patients with ALS, three with PDC, and two with “PDC plus ALS” (PDC followed by ALS) on the basis of clinical symptoms. NFTs were counted under ×100 magnification of Gallyas-Braak stained preparations and were plotted on brain maps of the hemisphere, brainstem, and the spinal cord. In all cases, the hippocampus, particularly in the CA1 field, the parahippocampal gyrus, amygdaloid nucleus, and the temporal poles were most severely affected. In the neocortex, layers II–III were more severely affected by NFTs than layers V–VI. In the spinal cord, a few NFTs were revealed in the intermediate gray. NFTs were dense in all cases of PDC and “PDC plus ALS” and variable in density in ALS cases, although the topography was similar between them. We conclude that similar topographical distribution of NFTs in ALS and PDC in people living in the Kii peninsula of Japan suggests a single tauopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Kii peninsula of Japan and the island of Guam are focal points of a high prevalence of amyotrophic lateral sclerosis (ALS) and parkinsonism–dementia complex (PDC) in the western Pacific [5, 14]. Clinically, western Pacific ALS in Kii and Guam is indistinguishable from sporadic ALS in other areas of the world, while PDC is unique in these endemic areas and is characterized by insidious progression of mental slowness and extrapyramidal symptoms of bradykinesia, rigidity, and occasional tremor [2]. Both the ALS and PDC features overlap in some patients presenting with “PDC plus ALS” where PDC features usually precede ALS features.
We previously reported that Kii PDC cases are neuropathologically characterized by nerve cell loss and widespread neurofibrillary tangles (NFTs) of severe degree without senile plaques in the cerebral cortex, basal ganglia, or brainstem, and with upper and lower motor neuron degeneration of varying degrees, while Kii ALS cases are characterized by classical ALS pathology and the additional findings of nerve cell loss and NFTs of varying degree in various brain areas [9, 10]. “PDC plus ALS” cases are neuropathologically characterized by superimposition of PDC pathology and ALS pathology. These findings may suggest that ALS, PDC and “PDC plus ALS” in the Kii peninsula share a common neuropathology of a combination of motor neuron disease and widespread neurofibrillary degeneration, and that they are neuropathologically on a spectrum of a single disease entity despite the difference in clinical manifestations [7, 9, 10].
However, there are no quantitative or semiquantitative data on the regional distribution of NFTs in Kii ALS/PDC. We have, therefore, investigated topography and semiquantitative density of NFTs in Kii ALS, PDC, and “PDC plus ALS” cases in order to clarify whether or not Kii ALS and PDC are on a spectrum of a single entity from the viewpoint of NFT pathology.
Materials and methods
Seven autopsied brains and five spinal cords from seven Kii ALS/PDC patients who were born and raised in the Kii peninsula ALS focal point were submitted for the present study. The clinical features are shown in Table 1. Clinical diagnosis was based on the clinical features: “ALS” refers to pure motor neuron disease features throughout the course of the disease and included two patients. “PDC” refers to progressive parkinsonism and dementia with modest, if present, motor neuron signs, and included three patients. “PDC plus ALS” refers to an overlap of PDC and ALS, where PDC features preceded ALS features and included two patients.
The brains and spinal cords were fixed in formalin solution. The brains were sliced coronally, and the spinal cords were sliced axially, and the slices were embedded in paraffin. The paraffin-embedded specimens were cut into 9-μm thick sections and prepared for hematoxylin-eosin (HE), Klüver-Barrera (KB), Bodian, and Gallyas-Braak staining. For the detection of NFTs, the sections were stained with anti-human tau protein (AT8, monoclonal mouse, dilution at 1:2,000, Innogenetics, Zwijndrecht, Belgium) using the dextranpolymer method with an ENVISION kit (Dako, Glostrup, Denmark). Peroxidase-conjugated streptavidin was visualized with 3,3′-diaminobenzidine (DAB; Wako, Osaka, Japan) as the final chromogen and counterstained lightly with Mayer’s hematoxylin.
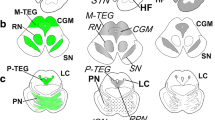
Gallyas-Braak stained sections of the frontal cortex (motor area), frontal pole, temporal pole, amygdaloid nucleus, hippocampus, basal ganglia, Meynert’s nucleus, midbrain, upper and middle pons, medulla oblongata, cerebellum including the dentate nucleus, and spinal cord were used for topographical mapping and counting of NFTs. NFTs were counted and plotted on brain maps under ×100 magnification using an Olympus micrometer (Olympus, Tokyo, Japan).
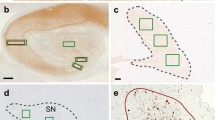
Neurofibrillary tangles were counted in five different areas of 1-mm2 in the temporal cortex, amygdaloid nucleus, substantia nigra, and in the entire area of the unilateral locus ceruleus.
Results
Neuropathological findings
The neuropathological findings of ALS, PDC, and “PDC plus ALS” cases were essentially similar to those previously reported [4, 9] and are summarized in Table 2. The brain maps of NFT topography of representative cases are shown, to the same scale, in Figs. 1 and 2.
Macroscopically, the brains of the ALS cases were unremarkable, whereas those of PDC and “PDC plus ALS” cases showed moderate to marked atrophy of the frontal and temporal lobes as shown in Fig. 1, with a decrease in brain weight (Table 2). The substantia nigra and locus ceruleus were depigmented in not only the PDC and “PDC plus ALS” cases, but also in the ALS cases.
Microscopically, the loss of pigmented nerve cells of the substantia nigra and locus ceruleus was marked in the PDC and “PDC plus ALS” cases, while it was mild to moderate in the ALS cases. Nerve cell loss and NFTs of the cerebral cortex and brainstem were severe in the PDC and “PDC plus ALS” cases, while they were mild in one ALS case and severe in the other. Typical senile plaques and amyloid angiopathy were absent, though a few diffuse plaques were found in the Ammon’s horn of a single PDC case (case 3, Table 2). Neuropil threads were observed in the parahippocampal gyrus to a mild degree in all cases.
Topography and density of NFTs
In all cases, NFTs were distributed in a similar topography with a predilection for the limbic system; NFTs were numerous in the hippocampus, particularly in the CA1 field, parahippocampal gyrus, temporal pole, insular cortex, and amygdaloid nucleus; they were moderate in number in the Meynert nucleus, hypothalamus, periaqueductal gray matter, substantia nigra, locus ceruleus, and brainstem reticular formation; and there were few in the pontine nuclei, basal ganglia, thalamus, dentate nucleus, other cerebral cortices including the primary motor cortex and spinal cord (Figs. 1, 2). The regional densities of NFTs including ghost tangles were high in all PDC and “PDC plus ALS” cases and one ALS case, while it was low in the other ALS case (Fig. 3, Table 3). The density of NFTs did not correlate with age or the duration of the disease in ALS, PDC, or “PDC plus ALS” cases.
Distribution of NFTs in the parahippocampal gyrus (a–c), substantia nigra (d–f), and primary motor cortex (g, h) of Kii ALS and PDC cases. a, d, g; case 1 (ALS with abundant NFTs), b, e; case 4 (PDC), c, f, h; case 6 (“PDC plus ALS” case). NFTs were distributed in dense layers II and III. The topography of NFTs was similar in all cases. Modified Gallyas stain with faint Nissl and Luxol-fast blue stains. a–f scale bar = 200 μm. g and h scale bar = 100 μm
Discussion
Amyotrophic lateral sclerosis and PDC of the Kii peninsula occur in the same geographic area, in members of the same family, and frequently in the same individual simultaneously, which suggests that they are different clinical phenotypes of a single disease entity. Neuropathological findings of NFTs and motor neuron involvement were also common to both ALS and PDC [7, 9, 10].
The present study has demonstrated a similar topographic distribution of NFTs in cases of clinical ALS, PDC, and “PDC plus ALS” phenotypes. In all cases, hippocampus, especially the CA1 field, parahippocampal gyrus, temporal pole, insular cortex, and amygdaloid nucleus were most severely affected. NFTs of the neocortex were denser in the superficial layers II–III than in the layers V–VI, as we reported previously in Kii ALS/PDC [4, 9]. This pattern is different from that of Alzheimer’s disease cases, where NFTs are densest in layers V–VI. Only a few NFTs were observed in the five spinal cord cases that were investigated. They were diffusely distributed, with lamina VII that was described by Rexed [13], which is known as the intermediate gray, showing higher NFT density than other parts of the spinal cord.
In 1979, Anderson et al. [1] reported that the presence of many NFTs was not unique to the brains of Guamanian ALS or PDC patients, but was common to the brains of neurologically normal Guamanian Chamorros. Similar findings were confirmed by several studies [2, 11, 12], and some researchers have suggested that the presence of NFTs in Guamanian brains represents a non-pathological background of Chamorros rather than a pathological hallmark.
Oyanagi et al. [11] reported that the numbers of NFTs were almost similar between normal Guamanian Chamorros and Guamanian ALS patients, and have proposed that “Guam ALS” is not a particular ALS but a classical ALS in Guamanian Chamorros, and that Guamanian PDC is nosologically different from Guamanian ALS.
Recently, Hof et al. [3] reported that the primary motor cortex in Guamanian cases contained high numbers of NFTs, contrasting sharply with the situation in AD and in non-Chamorro cases of ALS. They have suggested that Guamanian ALS/PDC may represent a single disorder with a broad spectrum of neurodegeneration, ranging from relatively pure ALS to PDC.
The present study has shown that the topography of NFTs is similar among ALS, PDC, and “PDC plus ALS” in Kii cases. These findings suggest that ALS and PDC in the Kii peninsula are on a spectrum of a single disease, and abundant NFTs are pathognomonic to Kii ALS/PDC.
In Kii and Guam, the distribution of NFTs of ALS/PDC cases was similar. However, the densities of NFTs were different between Kii and Guam cases. In Guam, pure ALS cases show considerably lower densities of NFTs in the neocortex and hippocampus, excluding the entorhinal cortex, than the PDC cases [2, 3]. Interestingly, in our “ALS” cases, the density of NFTs was abundant in one but only a few in the other. We could not find “pure ALS” cases with a high density of NFTs in Guam or Kii in previous reports. Although more cases are necessary, the variety of the NFT densities might be a peculiarity in the Kii cases.
The duration of the disease was longer in PDC and “PDC plus ALS” cases (6–8 years) than in the ALS cases (3–5 years). Though our data failed to show a correlation between the density of the NFTs and the duration of the disease, the longer survival of PDC and “PDC plus ALS” cases than ALS cases may be one of the causes of denser NFTs and more marked brain atrophy in the former.
Our previous investigation [7] has revealed that the frequency of NFTs in the brains of people without neurodegenerative disease in the ALS focal point of the Kii peninsula was as low as that of the general Japanese population. In addition, a neuropathological study on NFTs in ALS patients from the whole Mie prefectural area including the high incidence focus [6] has shown that the presence of abundant NFTs in the CNS was unique to ALS cases from the high incidence ALS focus area and never occurred in ALS cases outside the focus area. More than 70% of the patients had a positive family history of ALS/PDC, and the rates of incidence and prevalence remain continuously very high despite the dramatic changes in environmental factors, including food and water supply and by westernization of daily life [8]. These findings suggest that genetic factors may play a major role, although further studies on more cases are necessary to clarify the etiopathogenesis of Kii ALS/PDC.
References
Anderson FH, Richardson EP, Okazaki H, Brody JA (1979) Neurofibrillary degeneration on Guam. Frequency in Chamorros and non-Chamorros with no known neurological disease. Brain 102:65–77
Hof PR, Perl DP, Loerzel AJ, Steele JC, Morrison JH (1994) Amyotrophic lateral sclerosis and parkinsonism–dementia from Guam: differences in neurofibrillary tangle distribution and density in the hippocampal formation and neocortex. Brain Res 650:104–116
Hof PR, Perl DP (2002) Neurofibrillary tangles in the primary motor cortex in Guamanian amyotrophic lateral sclerosis/parkinsonism–dementia complex. Neurosci Lett 328:294–298
Itoh N, Ishiguro K, Arai H, Kokubo Y, Sasaki R, Narita Y, Kuzuhara S (2003) Biochemical and ultrastructural study of neurofibrillary tangles in amyotrophic lateral sclerosis/parkinsonism–dementia complex in the Kii peninsula of Japan. J Neuropathol Exp Neurol 62:791–798
Kimura K, Yase Y, Higashi Y, Uno S, Yamamoto K, Iwasaki M, Tsumoto I, Sugiura M, Yoshimura S, Namikawa K, Kumura S, Iwamoto S, Handa Y, Yata M, Yata Y (1961) Epidemiological and geomedical studies on ALS and allied diseases in Kii Peninsula (Japan): preliminary report. Proc Jpn Acad 37:417–420
Kokubo Y, Kuzuhara S, Narita Y (2000) Geographical distribution of amyotrophic lateral sclerosis with neurofibrillary tangles in the Kii peninsula of Japan. J Neurol 415:850–852
Kokubo Y, Kuzuhara S (2004) Neurofibrillary tangles of residents in ALS and parkinsonism–dementia complex focus in Japan. Neurology 63:2399–2401
Kuzuhara S, Kokubo Y, Narita Y, Sasaki R (1998) Continuing high incidence rates and frequent familial occurrence of amyotrophic lateral sclerosis and parkinsonism–dementia complex of the Kii peninsula of Japan. Neurology 50:A173
Kuzuhara S, Kokubo Y, Sasaki R, Narita Y, Yabana T, Hasegawa M, Iwatsubo T (2001) Familial amyotrophic lateral sclerosis and parkinsonism–dementia complex of the Kii Peninsula of Japan: clinical and neuropathological study and tau analysis. Ann Neurol 49:501–511
Kuzuhara S (2004) Clinical genetics and review of the hypotheses on the cause(s) of amyotrophic lateral sclerosis/parkinsonism–dementia complex (ALS/PDC) of the Kii Peninsula. Adv Neurol Sci 48:1–9
Oyanagi K, Wada M (1999) Neuropathology of parkinsonism–dementia complex and amyotrophic lateral sclerosis of Guam: an update. J Neurol 246(Suppl 2):19–27
Perl DP, Hof PR, Purohit DP, Loerzel AJ, Kakulas BA (2003) Hippocampal and entorhinal cortex neurofibrillary tangle formation in Guamanian Chamorros free of overt neurologic dysfunction. J Neuropathol Exp Neurol 62:381–388
Rexed B (1964) A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100:297–379
Yase Y, Matsumoto N, Azuma K, Nakai Y (1972) Amyotrophic lateral sclerosis: association with schizophrenic symptoms and showing Alzheimer’s tangles. Arch Neurol 27:118–128
Acknowledgments
We would like to thank Professor Y. Hashizume and Dr. M.Yoshida of Aichi Medical University for their advice and encouragement. We thank Ms. Hisami Akatsuka and Ms. Chieko Uno for their technical assistance in preparing tissues for histopathology. This study was partly supported by a grant-in-aid of the Research Committee of CNS Degenerative Disease, the Ministry of Health and Welfare, Japan, and by a grant-in-aid for the Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00401-007-0213-x.
Rights and permissions
About this article
Cite this article
Mimuro, M., Kokubo, Y. & Kuzuhara, S. Similar topographical distribution of neurofibrillary tangles in amyotrophic lateral sclerosis and parkinsonism–dementia complex in people living in the Kii peninsula of Japan suggests a single tauopathy. Acta Neuropathol 113, 653–658 (2007). https://doi.org/10.1007/s00401-007-0197-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0197-6