Abstract
We performed a systematic immunohistochemical study on 378 brain tumors using 37 antibodies and tissue microarray (TMA) technology. The aim of this study was to find new diagnostic biomarkers using antibodies established in our laboratory. Our TMA consisted of a grid of 1.5-mm cores that were extracted from individual donor blocks. Staining for each antibody was scored using a three-point system. We used hierarchical clustering analysis to interpret these data, which resulted in separation of all the brain tumors into seven groups. Although there were some exceptions, cases with the same histological diagnosis were generally grouped together. We then carried out statistical analyses to find the most useful antibodies for grouping of brain tumors. Ten antibodies [glial fibrillary acidic protein (GFAP), Olig2, vimentin, epithelial membrane antigen (EMA), cytokeratin (AE1/AE3), alpha-internexin, nestin, pinealocytes PP5, aquaporin-4 (AQP4) M13d and AQP4M13e] discriminated between astrocytomas and oligodendroglial tumors. Six antibodies [EMA, AE1/AE3, TUJ1, nestin, neurofilament protein-MH (NF-MH) and perivascular cells GP-1] showed significant differences between high-grade and low-grade gliomas. Our data have revealed new antibodies with potential diagnostic utility (Olig2, PP5, GP-1) and demonstrate that TMA technology is highly useful for evaluating newly established antibodies in brain-tumor research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue microarrays (TMAs) are powerful, large-scale tools for tumor analysis [12], which have markedly increased the number of available specimens for tumor research. Since staining conditions for all the tissue cores on each TMA slide are identical, technical variation among cases is avoided. Furthermore, the use of reagents is minimized, which leads to cost-effectiveness. TMA technology is still being developed and improved. While at present TMAs are mainly used for immunohistochemistry, they have also been utilized for DNA fluorescence in situ hybridization on paraffin blocks (TMA-FISH) [2, 5, 10], for frozen tissue [4, 14], and cell lines [7].
Brain tumors include many histological subtypes. In the current World Health Organization (WHO) classification, brain tumors are classified into as many as 126 types [11]. Since therapeutic strategies and prognosis are highly dependent on the histological type of tumors, accurate pathological diagnosis is indispensable. Therefore, pathologists need to have adequate knowledge of the morphological and immunohistochemical features of brain tumors, but considerable pathological variety and rareness (eight to ten cases occur per 100,000 Japanese per year) of brain tumors seem to have prevented large-scale immunohistochemical analyses. Some publications applied TMAs to brain-tumor profiling [5, 16, 20, 22, 23]; however, there is still a need for analysis of larger numbers of cases and antibodies. For research, development and application of new diagnostic biomarkers are necessary. Our laboratory has established many polyclonal and monoclonal antibodies against nervous-system antigens [1, 17–19, 26–31]. We are already using some of these antibodies for routine pathological diagnosis. However, the expression patterns of our biomarkers among many types of brain tumors remain unknown.
In this study, we applied TMA technology for exhaustive immunoprofiling of brain tumors in an attempt to find new biomarkers with diagnostic utility.
Materials and methods
Case materials
Paraffin blocks of primary brain tumors were acquired from the pathology archives of Gunma University Hospital and Kantoh Neurosurgical Hospital. The cases dated from 1991 to 2005. Tumors with marked heat degeneration at surgery or marked calcification were not used. The total of 378 cases listed in Table 1 were selected to build a TMA.
TMA construction
Tissue microarray production started with a pathological review of all selected cases. A representative area of each tumor was marked both on a hematoxylin–eosin (H&E) section and an original donor block. One tissue core of 1.5-mm diameter was extracted from the marked area of each donor block using an arraying machine (MTA-1, Beecher Instruments, Sun Prairie, WI, USA). The core was fit into a vertical hole that was bored in a recipient paraffin block in advance. This sampling procedure for each case was repeated many times to produce TMA blocks. Finally, recipient blocks were incubated at 60°C for 5 min, pressed on a hot plate for 3 min, and cooled in ice water. This procedure made the paraffin melt and congeal, which enabled tissue cores to integrate into the recipient block and thereby prevented tissue core loss at sectioning. Sections of 3-μm thickness were cut from each array block. Unused sections were stored at minus 80°C with paraffin coating.
Immunohistochemistry
Immunohistochemical studies were carried out by a streptavidin–biotin–immunoperoxidase technique (Histofine SAB-PO Kit; Nichirei, Tokyo, Japan). The 37 antibodies listed in Table 2 were used in this study. Various antibodies we had established in our laboratory were chosen, including antibodies against pinealocytes (PP1–PP7), pineal interstitial cells (PI1, PI2, and PX1), perivascular cells of the central nervous system (GP-1, GP-2), Schwann cells (Schwann/2E), protoplasmic astrocytes (PRAS-1, PRAS-4), oligodendrocytes (Olig2), and neurofilament protein (NF1D).
Sections of 3-μm thickness were deparaffinized, rehydrated, treated with 0.3% H2O2 in methanol for 30 min to inhibit endogenous peroxidase activity, and rinsed in phosphate buffered saline (PBS). For antigen retrieval before staining with some antibodies, pretreatment was performed as follows: (1) autoclaving in PBS or 0.1 mol/l citrate buffer pH 6.0 for 10 min at 121°C; (2) pronase treatment (0.05% protease, type XXVII, Sigma, St. Louis, MO, USA) for 5 min at room temperature. Sections were then covered with 10% normal goat or rabbit serum for 30 min. Sections were overlaid with optimally diluted antibodies and incubated overnight in a moist chamber at 4°C. Sections were washed three times with PBS and incubated with biotinylated anti-mouse or anti-rabbit IgG for 30 min. After three washes in PBS, sections were incubated with peroxidase-conjugated streptavidin for 30 min. Finally, peroxidase activity was visualized by incubation with 0.02% 3,3′-diaminobenzidine-4HCl (Wako Pure Chemicals, Osaka, Japan) in 0.05 mol/l Tris–HCl buffer (pH 7.6) containing 0.005% H2O2 for 5 min. Sections were washed, counterstained lightly with hematoxylin, dehydrated, and mounted. Incubation, except that with primary antibodies, was carried out at room temperature.
Scoring of immunostaining data
Each tumor core was considered to be suitable for evaluation if the tumor occupied more than 10% of the core area. The scoring system was as follows: score 0 (less than 5% of tumor cells stained), score 1 (5–25% of tumor cells stained), and score 2 (more than 25% of tumor cells stained).
Data analysis and statistics
Staining data were recorded directly into Microsoft Excel worksheets and reformatted into a suitable form for hierarchical clustering analysis. This reformat procedure was performed using the TMA-Deconvoluter program, freely available at the Stanford TMA software Web site (http://www.genome-www.stanford.edu/TMA/) [15]. The hierarchical clustering analysis was performed with the Cluster program, and the results were visualized using the TreeView program. Both programs were freely available at the Eisen Lab Web site (http://www.rana.lbl.gov/EisenSoftware.htm). Clustered data were displayed with antibodies on the horizontal axis and cases on the vertical axis. Cases or antibodies were placed next to each other if they had the most similar expression profiles. Length of branches was inversely proportional to profiling similarity. Cases with 50% or less interpretable scores were excluded from the hierarchical clustering analysis.
Staining results were compared between two tumor types by Fisher’s exact test using StatView for Windows version 5.0 software (SAS Institute Inc.). A significant difference was declared if the P value was less than 0.05. If multiple significance tests are conducted in parallel, inflation of the overall type-1 error may occur. Because the significance level is assumed to be 5% in this study, 5% of results are expected to be false positives. Usually, no strict correction of significance levels of the single tests is carried out in statistical analyses of TMA data. Among all conducted tests, a certain number of false positive results is accepted.
Results
Hierarchical clustering analysis
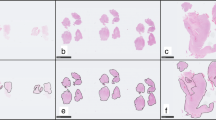
Nineteen cases were excluded from the hierarchical clustering analysis because their data were insufficient (i.e., interpretable scores were 50% or less). The reasons for insufficient data were mainly inadequate tumor volume or tissue core loss at sectioning. The clustering of the remaining 359 cases produced seven groups, group A to group G (Fig. 1a). All the groups included more than one histological type. The number of cases and the major histological type in each group were, respectively, as follows: group A (eight cases, germinomas); group B (95 cases, gliomas); group C (19 cases, malignant lymphomas and hemangioblastomas); group D (131 cases, meningiomas); group E (37 cases, schwannomas); group F (50 cases, pituitary adenomas); group G (19 cases, medulloblastomas, neuronal tumors and pineal parenchymal tumors). Occasionally, cases with the same histological diagnosis were placed into different groups. For example, two of 126 meningiomas were placed into group C or E, not into group D. The other results of clustering were as follows: ependymomas were placed into group B; hemangiopericytomas were placed into group F; craniopharyngiomas were placed into group F.
Hierarchical clustering analysis of brain tumors. a A full-length view of the diagram with cases on the vertical axis and antibodies on the horizontal axis. b A close-up view of the diagram of antibody clustering. Two biomarkers with a close relationship are located next to each other. c A close-up view of the diagram of clustering analysis of a part of group B. Red, brown, and green cubes indicate score 2, score 1, and score 0, respectively. Grey cubes indicate the absence of staining data. Gliomas of different cell of origin or grade are placed together
In the same way, clustering analysis of the 37 antibodies used for immunohistochemistry was carried out (Fig. 1b). The length of branches above antibody names in the figure is inversely proportional to the profiling similarity. Two biomarkers with a close relationship were placed next to each other [for example, neurofilament (NF) 1D and NF70/200k, antibodies against neurofilament protein]. However, two adjacent antibodies did not always have a close relationship (for example, Glut5 and vimentin). A close-up view of the clustering analysis results for a part of group B is shown in Fig. 1c. Gliomas of different histological types are placed therein. However, gliomas were not completely divided in accord with their cell of origin or grade. To find biomarkers that further differentiate gliomas, we adopted another method: statistical analysis.
Statistical analysis
All gliomas were examined in terms of cell of origin and grade. Ten antibodies [glial fibrillary acidic protein (GFAP), Olig2, vimentin, epithelial membrane antigen (EMA), cytokeratin (AE1/AE3), alpha-internexin, nestin, PP5, aquaporin-4 (AQP4) M13d and AQP4M13e] significantly distinguished between astrocytomas and oligodendrogliomas. The expression of these biomarkers in astrocytic tumors and oligodendroglial tumors is shown in Table 3a, Fig. 2a, and Table 3b, Fig. 2b, respectively (p values are indicated in Table 4).
Comparison of marker expression patterns between astrocytic tumors (a) and oligodendroglial tumors (b). Three-step bars show percentages of tumors positive for each antibody. Red, brown, and green bars indicate score 2, 1, and 0, respectively. All the antibodies here showed significant immunostaining (P<0.05)
Six antibodies [EMA, AE1/AE3, neuronal class III beta tubulin clone TUJ1, nestin, neurofilament protein-MH (NF-MH) and GP-1] differentiated between high-grade (WHO grade III or IV) and low-grade (WHO grade I or II) gliomas. The expression of these biomarkers in high- and low-grade gliomas is shown in Table 5a, Fig. 3a and Table 5b, Fig. 3b, respectively (P values are indicated in Table 6).
Discussion
We applied TMA for exhaustive profiling of a wide variety of brain tumors. As a result, we observed distinct immunostaining features with several antibodies. Specifically, some antibodies obtained previously in our laboratory were found to be useful for subtyping or grading of gliomas, including Olig2, PP5, and GP-1. In addition, immunostaining results we obtained using commonly used antibodies were in accord with previous findings (for example, schwannomas were positive for S-100). We also found that some antibodies yielded unexpected clues with potential diagnostic usefulness. Additional experiments will be needed to draw definitive conclusions regarding these issues.
For discrimination between astrocytic and oligodendroglial tumors, ten biomarkers were useful (Tables 3, 4; Fig. 2). The distinction between these two types of tumors is important because oligodendroglial tumors often show chemosensitivity to procarbazine, lomustine (CCNU), and vincristine (PCV) therapy and a more favorable clinical behavior. A previous study showed that the expression of several oligodendroglial lineage genes was frequently increased in gliomas; nevertheless, no significant expression difference between astrocytic tumors and oligodendroglial tumors was detected [21]. In our study, we found Olig2 to be useful for distinguishing between these tumors. Olig2 recognizes a transcription factor that regulates oligodendroglial development. Olig2 labels the nuclei of oligodendrocytes and oligodendroglial tumors, and besides, astrocytomas are also positive for Olig2 to a lesser extent [31]. Our data are in accord with that finding. Another useful antibody we had obtained was PP5, an antibody against human pineal antigens [19, 27].
Other biomarkers that discriminated between astrocytoma and oligodendroglial tumors included vimentin and AQP4. Antivimentin immunoreactivity has been shown in gliomas and ependymomas but not in neuronal tumors [13]. Our data obtained in the current study were in accord with those findings. Additionally, vimentin was expressed more frequently in astrocytic tumors than in oligodendroglial tumors. AQP4 is a water channel protein that is highly expressed in astrocytes. AQP4 is considered to be involved in regulating water flux between vascular and glial compartments and responsible for development of vasogenic edema in the brain. It has been shown that AQP4 and potassium channel protein Kir4.1 are redistributed in glioblastomas [24], and low- and high-grade gliomas [25], respectively. However, our study showed significant differences of the expression profiles of AQP4 between astrocytic tumors and oligodendroglial tumors rather than between high- and low-grade gliomas.
Glioma grade markedly affects patients’ prognosis. This work showed that six biomarkers were significantly able to discriminate between high- and low-grade gliomas (Tables 5, 6; Fig. 3). Interestingly, most of these biomarkers were epithelial markers (EMA, AE1/AE3) or neuronal markers (TUJ1, nestin and NFP-MH). Gliomas tend to have EMA immunoreactivity more frequently as their grade becomes higher [6]. In addition, according to a previous study, TUJ1 immunoreactivity is significantly stronger in high-grade astrocytomas than in low-grade astrocytomas [9]. Our data were in accord with those findings. The antigens reacting with GP-1 were expressed more frequently in high-grade gliomas than in low-grade gliomas. GP-1 is an antibody that recognizes lysosomal proteins in the perivascular cells of the central nervous system [30].
In this study, we addressed two technical issues related to TMA technology, namely, TMA construction and data analysis. One limitation of TMAs is that a given tissue core is not always representative of the whole tumor. To overcome this problem, in most recent studies, multiple 0.6-mm cores were prepared at tissue extraction. We attached importance to consistent, reproducible histological observation rather than to examining a number of cores mounted on one recipient block. To ensure the adequate representation of diverse brain tumor tissues, we extracted one 1.5-mm core instead of two or three 0.6-mm cores. Although the tissue of a 1.5-mm core is 6.25 times as large as that of a 0.6-mm core [(1.5/0.6)2=6.25], the tissue loss in each donor block was trivial. We paid careful attention to the conditions for storage of unused TMA sections. A previous study demonstrated that each core on a section lost its antigenicity within 1 month if a section was stored unwrapped in room air [3]. That study indicated that frozen storage of slides with paraffin coating held antigenicity loss to a minimum. Using this method, we obtained satisfactory immunostaining results even 10 months after sectioning.
The other issue was the strategy for data analysis. Because the amount of data generated by TMAs is huge, novel analytic methods are required. In fact, the number of our data points was 13,986 (378 cases with 37 antibodies). To analyze this enormous amount of data, we performed two-step analysis. First, we qualitatively classified all cases with a hierarchical clustering analysis. Then, we quantitatively and statistically evaluated tumor marker expression. This procedure allowed both a simple overview and detailed investigation of all the cases.
Finally, we demonstrated that TMA technology was a powerful method for brain-tumor profiling. On the other hand, TMAs generate such a large amount of data that it is necessary to verify and analyze the data carefully. TMAs can be used for identifying prognostic parameters. For example, loss of heterozygosity on chromosome arms 1p and 19q is predictive of chemosensitivity and longer survival in oligodendroglial tumors [8]. This genetic alteration can be found by TMA with FISH. Our findings demonstrated that TMA technology is highly useful for assessing the usefulness of newly established antibodies for brain-tumor research.
References
Arai H, Hirato J, Nakazato Y (1998) A novel marker of Schwann cells and myelin of the peripheral nervous system. Pathol Int 48:206–214
Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, Willi N, Mihatsch MJ, Sauter G, Kallioniemi OP (1999) Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res 59:803–806
DiVito KA, Charette LA, Rimm DL, Camp RL (2004) Long-term preservation of antigenicity on tissue microarrays. Lab Invest 84:1071–1078
Fejzo MS, Slamon DJ (2001) Frozen tumor tissue microarray technology for analysis of tumor RNA, DNA, and proteins. Am J Pathol 159:1645–1650
Fuller CE, Wang H, Zhang W, Fuller GN, Perry A (2002) High-throughput molecular profiling of high-grade astrocytomas: the utility of fluorescence in situ hybridization on tissue microarrays (TMA-FISH). J Neuropathol Exp Neurol 61:1078–1084
Hasselblatt M, Paulus W (2003) Sensitivity and specificity of epithelial membrane antigen staining patterns in ependymomas. Acta Neuropathol (Berl) 106:385–388
Hoos A, Cordon-Cardo C (2001) Tissue microarray profiling of cancer specimens and cell lines: opportunities and limitations. Lab Invest 81:1331–1338
Jeuken JW, von Deimling A, Wesseling P (2004) Molecular pathogenesis of oligodendroglial tumors. J Neurooncol 70:161–181
Katsetos CD, Del Valle L, Geddes JF, Assimakopoulou M, Legido A, Boyd JC, Balin B, Parikh NA, Maraziotis T, de Chadarevian JP, Varakis JN, Matsas R, Spano A, Frankfurter A, Herman MM, Khalili K (2001) Aberrant localization of the neuronal class III beta-tubulin in astrocytomas. Arch Pathol Lab Med 125:613–624
Kay E, O’Grady A, Morgan JM, Wozniak S, Jasani B (2004) Use of tissue microarray for interlaboratory validation of HER2immunocytochemical and FISH testing. J Clin Pathol 57:1140–1144
Kleihues P, Cavenee WK. (2000) World Health Organization classification of tumours, pathology and genetics of tumours of the nervous system. IARC, Lyon
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847
Koperek O, Gelpi E, Birner P, Haberler C, Budka H, Hainfellner JA (2004) Value and limits of immunohistochemistry in differential diagnosis of clear cell primary brain tumors. Acta Neuropathol (Berl) 108:24–30
Kylaniemi M, Koskinen M, Karhunen P, Rantala I, Peltola J, Haapasalo H (2004) A novel frozen brain tissue array technique: immunohistochemical detection of neuronal paraneoplastic autoantibodies. Neuropathol Appl Neurobiol 30:39–45
Liu CL, Prapong W, Natkunam Y, Alizadeh A, Montgomery K, Gilks CB, van de Rijn M (2002) Software tools for high-throughput analysis and archiving of immunohistochemistry staining data obtained with tissue microarrays. Am J Pathol 161:1557–1565
Lusis EA, Chicoine MR, Perry A (2005) High throughput screening of meningioma biomarkers using a tissue microarray. J Neurooncol 73:219–223
Nakazato Y, Ishizeki J, Takahashi K, Yamaguchi H, Kamei T, Mori T (1982) Localization of S-100 protein and glial fibrillary acidic protein-related antigen in pleomorphic adenoma of the salivary glands. Lab Invest 46:621–626
Nakazato Y, Sasaki A, Hirato J, Ishida Y (1987) Monoclonal antibodies which recognize phosphorylated and nonphosphorylated epitopes of neurofilament protein. Biomed Res 8:369–376
Nakazato Y, Hirato J, Sasaki A, Yokoo H, Arai H, Yamane Y, Jyunki S (2002) Differential labeling of the pinealocytes and pineal interstitial cells by a series of monoclonal antibodies to human pineal body. Neuropathology 22:26–33
Neben K, Korshunov A, Benner A, Wrobel G, Hahn M, Kokocinski F, Golanov A, Joos S, Lichter P (2004) Microarray-based screening for molecular markers in medulloblastoma revealed STK15 as independent predictor for survival. Cancer Res 64:3103–3111
Riemenschneider MJ, Koy TH, Reifenberger G (2004) Expression of oligodendrocyte lineage genes in oligodendroglial and astrocytic gliomas. Acta Neuropathol (Berl) 107:277–282
Sallinen SL, Sallinen PK, Haapasalo HK, Helin HJ, Helen PT, Schraml P, Kallioniemi OP, Kononen J (2000) Identification of differentially expressed genes in human gliomas by DNA microarray and tissue chip techniques. Cancer Res 60:6617–6622
Tynninen O, Carpen O, Jaaskelainen J, Paavonen T, Paetau A (2004) Ezrin expression in tissue microarray of primary and recurrent gliomas. Neuropathol Appl Neurobiol 30:472–477
Warth A, Kroger S, Wolburg H (2004) Redistribution of aquaporin-4 in human glioblastoma correlates with loss ofagrin immunoreactivity from brain capillary basal laminae. Acta Neuropathol (Berl) 107:311–318
Warth A, Mittelbronn M, Wolburg H (2005) Redistribution of the water channel protein aquaporin-4 and the K+ channel protein Kir4.1 differs in low- and high-grade human brain tumors. Acta Neuropathol (Berl) 109:418–426
Yamaguchi H (1980) Studies on the immunohistochemical localization of S-100 and glial fibrillary acidic proteins in the rat nervous system and in human brain tumors (in Japanese). No To Shinkei 32:1055–1064
Yamane Y, Mena H, Nakazato Y (2002) Immunohistochemical characterization of pineal parenchymal tumors using novel monoclonal antibodies to the pineal body. Neuropathology 22:66–76
Yokoo H, Nakazato Y (1996) A monoclonal antibody that recognizes a carbohydrate epitope of human protoplasmic astrocytes. Acta Neuropathol (Berl) 91:23–30
Yokoo H, Sasaki A, Hirato J, Nakazato Y (1996) A monoclonal antibody that specifically recognizes a novel mitochondrial protein of human astrocytes. J Neuropathol Exp Neurol 55:716–721
Yokoo H, Sasaki A, Hirato J, Nakazato Y (1998) Immunohistochemical characterization of two novel monoclonal antibodies that recognize human perivascular cells of the central nervous system and macrophage subsets. Pathol Int 48:678–688
Yokoo H, Nobusawa S, Takebayashi H, Ikenaka K, Isoda K, Kamiya M, Sasaki A, Hirato J, Nakazato Y (2004) Anti-human Olig2 antibody as a useful immunohistochemical marker of normal oligodendrocytes and gliomas. Am J Pathol 164:1717–1725
Acknowledgments
We thank Professor K. Takata (Department of Anatomy and Cell Biology, Gunma University Graduate School of Medicine) for providing antibodies (AQP4M13d and AQP4M13e). We also thank Dr. M. Kamiya for helpful advice. The technical assistance of Ms. A. Kumagai and Ms. A. Kodama is gratefully acknowledged. This work was supported in part by a Grant-in-Aid for Scientific Research (B) (no. 15300113) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (to YN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikota, H., Kinjo, S., Yokoo, H. et al. Systematic immunohistochemical profiling of 378 brain tumors with 37 antibodies using tissue microarray technology. Acta Neuropathol 111, 475–482 (2006). https://doi.org/10.1007/s00401-006-0060-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0060-1