Abstract
Clear cell histology is the hallmark of oligodendroglioma (OG) but also characterizes clear cell ependymoma (CCE) and central neurocytoma (CN). Immunohistochemistry for glial and neuronal proteins may support differential diagnosis. We investigated systematically diagnostic value and limits of immunohistochemistry using representative tumor specimens (>1 cm in diameter) of well-defined OGs, CCEs, and CNs (n=10, respectively). Antibodies comprised anti-neuron specific nuclear protein (NEUN), anti-synaptophysin, anti-neuron-specific enolase, anti-microtubule-associated protein 2, anti-phosphorylated neurofilament protein, anti-non-phosphorylated neurofilament protein, anti-glial fibrillary acidic protein (GFAP), anti-S100 protein, anti-vimentin (VIM), and anti-epithelial membrane antigen (EMA). Among the panel of antibodies anti-NEUN, anti-VIM and anti-EMA proved most useful for differential diagnosis. Prominent (>90%) anti-NEUN immunolabeling of tumor cells clearly distinguished CNs from OGs and CCEs. Anti-VIM immunolabeling and a characteristic cytoplasmic dot-like anti-EMA immunoreactivity pattern of tumor cells were detectable only in OGs and CCEs. Furthermore, prominent anti-VIM immunoreactivity and anti-EMA cell membrane staining including ring-like staining pattern is characteristic for CCEs. Additionally, a widespread gliofibrillary and minigemistocytic cytoplasmic anti-GFAP immunostaining pattern is restricted to some OGs. Our data indicate that immunohistochemistry using anti-NEUN, anti-VIM, and anti-EMA on representative tumor specimens allows clear-cut distinction of CNs vs OGs and CCEs. Anti-VIM, anti-EMA, and anti-GFAP support differential diagnosis of OGs vs CCEs. Nevertheless, it is noted that due to focal expression of glial proteins in CNs and, conversely, of neuronal proteins in OGs and CCEs, immunohistochemistry is of limited value on small tumor specimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clear cell histology is the classic hallmark of oligodendroglioma (OG) but also characterizes clear cell ependymoma (CCE) and central neurocytoma (CN). The OGs, CCEs, and CNs show different clinical and biological behavior; thus, prognosis and therapeutic approaches differ significantly [18]. In particular, diagnosis or exclusion of OG has become a major topic of scientific and clinical interest in recent years due to the implications with regard to postoperative therapy decision making [7]; thus, exact histopathological typing of these tumors is mandatory. The OGs, CCEs, and CNs display distinctive histological features allowing clear-cut differential diagnosis on the basis of plain histology in most cases [17]. According to the World Health Organization (WHO) classification of tumors of the central nervous system (CNS), OGs are defined as neoplasms composed of tumor cells with rounded, homogeneous nuclei, and, on paraffin sections, clear perinuclear halos (“honeycomb” appearance). Additional features include microcalcifications and a dense network of delicate branching capillaries. In ependymoma, key histological features comprise perivascular pseudorosettes and ependymal rosettes. The CCEs also display tumor cells with a clear cell appearance. The CNs are composed of isomorphic tumor cells with small round nuclei and fibrillary cell-free areas (i.e., neuropil islands). Despite the distinctive histopathological features of OGs, CCEs, and CNs, differential diagnosis may be difficult in cases in which clear cell morphology is predominant and in small (e.g., stereotactic) biopsies [8, 13, 21]. In such cases, immunohistochemistry with antibodies against glial and neuronal antigens is employed as a diagnostic aid.
A continuously increasing number of anti-glial and anti-neuronal markers is used for diagnostic purposes [5, 17, 28, 32, 36]. In previous studies, immunostaining of glial and neuronal antigens was considered a reliable means for identification of glial or neuronal differentiation of CNS neoplasms [4, 11, 19, 20, 35]. In recent years, however, expression of neuronal antigens, in addition to glial antigens, has been documented in OGs and also in ependymal neoplasms [23, 26, 34]. Conversely, CNs may express glial antigens, in addition to neuronal antigens [15]. In consequence, none of the glial or neuronal antigens appear to be specific and thus diagnostic per se for OG, CCE, or CN; however, the diagnostic value and limits of the various anti-glial and anti-neuronal markers have not been precisely determined to date.
In the present study, we addressed this issue and analyzed systematically anti-glial and anti-neuronal markers that are commonly used in the routine diagnostic setting on well-defined cohorts of OGs, CCEs, and CNs.
Materials and methods
We retrieved archived formalin-fixed and paraffin-embedded tumor specimens of 10 OGs, 10 CCEs, and 10 CNs from the files of the Institute of Neurology, Medical University Vienna. Tumor specimens were selected according to appropriate size (>1 cm in diameter) and diagnostic histomorphology on hematoxylin and eosin (HE)-stained sections. Review and typing of CCEs and CNs was performed on a multi-headed microscope. For tumor typing we used the current WHO 2000 criteria [17]. According to these criteria, each case showed diagnostic tissue features and, in addition, large clear cell areas (see Fig. 1a–f). Diagnostic histopathological features were considered as follows: (a) OG: diffusely infiltrating clear cell primary brain tumor with delicate vascular network; (b) CCE: primary brain tumor containing characteristic perivascular pseudorosettes and/or ependymal tubules; and (c) CN: primary brain tumor containing typical neuropil islands. Electron microscopy investigations were not performed. The OGs were retrieved from a series of 79 oligodendroglial neoplasms, in which chromosome arms 1p and 19q status [9] were determined according to previously published protocols [1, 2, 6]. The DNA deletions on chromosome arms 1p and 19q are characteristic molecular cytogenetic alterations of oligodendroglial neoplasms [29] and all OGs in the present study had a combined 1p and 19q deletion status. Four OGs and 4 CCEs were classified as WHO grade II, and 6 OGs and 6 CCEs as WHO grade III. All CNs were classified as WHO grade II.
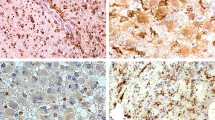
Representative histology of investigated tumor samples (hematoxylin–eosin stain: a–f; magnification: a–c ×100; d–f ×200) and immunohistochemistry results (magnification: g–i ×100; j ×200, k,l ×400). a–c Characteristic histological features of central neurocytoma (CN), oligodendroglioma (OG), and clear cell ependymoma (CCE). The CN showing neuropil islands (a); OG showing honeycomb appearance, fine capillary network and calcifications (b); CCE showing perivascular pseudorosettes (c). d–f Clear cell histology in CN (d), OG (e) and CCE (f). g–l Prominent anti- neuron-specific nuclear protein (NEUN) labeling (>90% of tumor cells) is seen only in CNs (g). OGs (h) and CCEs (i) may show partial anti-NEUN labeling. A globular cytoplasmic anti- synaptophysin staining pattern is characteristic for OGs (j). Dot-like anti-epithelial membrane antigen (EMA) is seen only in OGs (k) and CCEs (l). In addition, CCEs show staining of cell surface structures (l, inset)
Immunohistochemistry was performed in all cases using a panel of anti-neuronal and anti-glial antibodies according to protocols shown in Table 1. As secondary system the ChemMate detection kit (Dako, Denmark) was used. Immunoreactivity was determined semiquantitatively. Semiquantitative evaluation was performed as follows: category ++: immunostaining of ≥90% tumor cells/fibrillary matrix (“prominent immunostaining”); category +: immunostaining of ≤90% tumor cells/fibrillary matrix (“partial immunostaining”); category −: no immunoreactivity. In addition, we assessed characteristic patterns of immunostaining.
The significance of differences of protein expression in the three tumor types were assessed statistically using Kruskal-Wallis test and subsequent Mann-Whitney tests. To control results of these tests, we shifted the cutoff value of ≥ or <90% tumor cells/fibrillary matrix staining to a cutoff value of ≥ or <50% tumor, and repeated analysis. A two-tailed p value of <0.05 was considered as significant.
Results
We considered for evaluation tumor cell nuclei, tumor cell somata, and fibrillary matrix. Evaluating the fibrillary matrix, the difficulty in distinguishing expression of glial fibrillary acidic protein (GFAP) and synaptophysin (SYN) in tumor cell processes vs non-neoplastic cell processes arose.
Results of semiquantiative evaluation are summarized in Table 2. Most notably, total anti-neuron-specific nuclear protein (NEUN) immunolabeling of tumor cell nuclei can only be seen in CNs (see Fig. 1g). Nevertheless, we observed focal anti-NEUN immunostaining also in some OGs and CCEs (see Fig. 1h,i). Anti-vimentin (VIM) immunoreactivity proved to be detectable only in some OGs and CCEs, but not in CNs. Furthermore, prominent anti VIM-immunostaining is seen in 6 of 10 CCEs, but not in OGs and CNs. In OGs, anti-VIM staining is seen only in WHO grade-III tumors, whereas CCEs showed VIM expression both in low- and high-grade lesions.
Anti-epithelial membrane antigen (EMA) immunoreactivity is detectable in tumor cell somata of OGs and CCEs. Anti-EMA immunoreactivity in OGs and CCEs displays a characteristic dot-like pattern (see Fig. 1k,l). In addition, 5 of 10 CCEs show staining of cell membrane structures (see Fig. 1i) including previously described ring-like structures [12]. Prominent anti-GFAP immunostaining of tumor cell somata is visible in 3 of 10 OGs, displaying gliofibrillary and minigemistocytic immunolabeling patterns. We observed widespread anti-GFAP immunoreactive gliofibrillary oligodendrocytes and minigemistocytes in some OGs but not in CNs or CCEs. Interestingly, all OGs containing GFAP-positive gliofibrillary oligodendrocytes or minigemistocytes are WHO grade-III tumors. Anti-GFAP immunostaining of fibrillary matrix was seen in CNs, OGs, and CCEs. In most instances it was difficult or impossible to distinguish anti-GFAP immunoreactivity of tumor cell processes vs cell processes of preexisting non-neoplastic cells. Anti-S100 protein is considered as a glial marker [32]. Only OGs showed prominent immunoreactivity and were never completely negative as were some CNs and some CCEs. Tumor-specific expression of SYN in fibrillary matrix was difficult to distinguish from preexisting non-neoplastic matrix structures. In addition to fibrillary matrix staining we saw partial anti-SYN immunostaining of tumor cell somata in OGs and CCEs. Nevertheless, anti-SYN immunoreactivity patterns showed characteristic differences between CNs, OGs, and CCEs: prominent staining of fibrillary matrix was detectable in all CNs but not in OGs and CCEs. A characteristic globular cytoplasmic anti-SYN staining pattern was seen in OGs (see Fig. 1j).
Antibodies of minor diagnostic value
Immunostaining of glial tumors by the neuronal marker anti-neuron-specific enolase (NSE) is well known in the literature [33]. We observed widespread anti-NSE staining of CNs, OGs, and CCEs. The neuronal marker anti-microtubule associated protein 2 (MAP2) has been reported to show strong cellular immunolabeling of OGs, labeling of fibrillary processes in CNs, and no labeling of CCEs [3]. We observed prominent anti-MAP2 staining of CNs but also of OGs and CCEs. Immunoreactivity was seen in perinuclear cytoplasm and in fibrillary processes. As reported previously, anti-phosphorylated neurofilament protein (pNFP) did not immunolabel any tumor tissue structure, not even in CNs [15, 34]. Antibodies against non-phosphorylated neurofilament protein (npNFP) showed only partial immunostaining of some CNs and OGs.
Statistical analysis
Testing the semiquantitatively assessed data statistically, we found significant differences of expression of EMA, GFAP in fibrillary matrix (GFAPmat), VIM, S-100, npNFP, NEUN, and SYN between the three tumor types (Kruskal-Wallis test). Changing the cut off value from >or <90% tumor cells/fibrillary matrix staining to a cutoff value of >or <50%, statistical results remained unchanged except for GFAP in cell somata (GFAPsom) which became significant (exact p=0.069 vs exact p=0.010). Comparing CNs and OGs (Mann-Whitney test), EMA expression (exact p=0.007), as well as GFAPmat expression (exact p<0.001) was significantly stronger in OGs. The NEUN expression was significantly stronger in CNs than in OGs (exact p<0.001), as well as SYN expression (SYNmat p<0.001, SYNsom p=0.002).
Comparing CNs and CCEs, we observed that EMA expression (exact p<0.001), GFAPmat expression (exact p=0.002), and VIM expression (exact p<0.001) were significantly stronger in CCEs. In contrast, expression of NEUN (exact p<0.001), SYN (exact p<0.001), and npNFP (exact p=0.023) were significantly stronger in CNs.
When we compared OGs and CCEs, we found that expression of S-100 was significantly stronger in OGs (exact p=0.015), whereas VIM expression was significantly stronger in CCEs (exact p=0.001).
Discussion
In the past 25 years, immunohistochemistry has become an indispensable method for analysis of tissue samples in virtually all fields of clinical pathology. In the field of tumor pathology, immunohistochemical visualization of cell-type-specific proteins reveals patterns of differentiation and may significantly contribute to classification of biopsy samples. In primary CNS neoplasms, immunohistochemistry is used for identifying expression of neuronal and glial proteins [20, 28, 32, 35, 36]. The protein expression profile may provide diagnostic information even in brain tumor biopsies, in which plain histology is inconclusive. Small (e.g., stereotactic) biopsy samples displaying clear cell histology are a particular challenge with regard to differential diagnosis. To date, no systematic studies investigating value and limits of immunohistochemistry in differential diagnosis of clear cell CNS neoplasms have been published. Furthermore, selection of the most useful markers from the great number of antibodies recommended for the diagnostic setting is not only important for practical but also for financial reasons. To this end, we investigated the diagnostic value of anti-glial and anti-neuronal markers that are commonly used in the routine diagnostic setting on well-defined cohorts of OGs, CCEs, and CNs. Evaluating the diagnostic impact of the antibodies, we determined semiquantitatively the prominence of immunoreactivity and assessed characteristic patterns of immunolabeling. The panel of antibodies we tested comprised antibodies against antigens preferentially localized in the cell nucleus, perinuclear cytoplasm, or cell processes.
Comparison of semiquantitatively assessed immunohistochemistry data shows that the value of the different anti-neuronal and anti-glial antibodies for differential diagnosis of primary clear cell CNS tumors is variable. Antibodies that proved most useful for differential diagnosis in our hands comprised anti-NEUN, anti-VIM, and anti-EMA.
Anti-NEUN is directed against a neuron-specific protein in the cell nucleus [22]. Prominent anti-NEUN immunolabeling of tumor cells clearly delineated CNs from OGs and CCEs (see Fig. 1g). Nevertheless, we also observed focal anti-NEUN immunostaining in some OGs and CCEs. In our study anti-VIM showed immunoreactivity only in CCEs and OGs, but not in CNs. In OGs, anti-VIM staining was seen only in WHO grade-III tumors, whereas CCEs showed VIM expression both in low- and high-grade lesions. Furthermore, prominent anti-VIM staining was seen only in CCEs; therefore, anti-VIM seems to identify specifically a glial nature of a primary clear cell CNS tumor and seems to be helpful for identification of CCEs.
We investigated also the diagnostic role of anti-epithelial membrane antigen (EMA) immunoreactivity. The EMA is a protein, which is expressed abundantly in epithelial cells [27]. In the CNS, anti-EMA immunostains ependymal cells and is therefore commonly used for identification of ependymal features in brain tumor biopsies. In some of our CCEs, we detected a characteristic cell membrane staining including ring-like staining pattern which has been described previously as highly specific for ependymoma [12]. In addition, a characteristic cytoplasmic punctuate anti-EMA immunostaining pattern could be observed in all cases (see Fig. 1l). The punctuate anti-EMA immunostaining pattern has been described previously and is considered as a characteristic feature of ependymal neoplasms [31]. The subcellular substrate of punctuate anti-EMA labeling has not been definitely clarified. As candidate structures, blepharoblasts or intracytoplasmic microlumina have been discussed [17, 31]. Punctuate anti-EMA staining has been reported recently not only in ependymal neoplasms, but also in astrocytoma, glioblastoma, and infrequently in oligodendroglioma [12]. In our study, we found the punctuate anti-EMA staining pattern in CCEs and in 7 of 10 OGs but in none of the CNs (see Fig. 1k,l); thus, the punctuate anti-EMA immunostaining pattern in primary CNS tumors can be considered to be a specific feature of glial neoplasms. Dot-like anti-EMA immunoreactivity comparable to the punctuate anti-EMA in glial neoplasms has also been described in anaplastic large cell lymphoma and in nodular lymphocyte predominant Hodgkin’s lymphoma. As a subcellular location, the golgi apparatus was proposed [16, 30].
Anti-synaptophysin (SYN) is traditionally considered as the most useful neuronal marker for identification of neurocytomas [8, 10]. In our hands tumor-specific expression of SYN in fibrillary matrix was difficult to distinguish from preexisting non-neoplastic matrix structures. In addition to fibrillary matrix staining we observed partial anti-SYN immunostaining of tumor cell somata in OGs and CCEs. Nevertheless, anti-SYN immunoreactivity patterns showed characteristic differences between CNs, OGs, and CCEs: prominent staining of fibrillary matrix was detectable in all CNs, but not in OGs and CCEs. Furthermore, a characteristic globular cytoplasmic anti-SYN staining pattern was seen only in OGs.
In OGs, cytoplasmic perinuclear ring-like anti-GFAP immunoreactivity is a characteristic feature. These cells have been designated “gliofibrillary oligodendrocytes” [14]. We observed widespread anti-GFAP immunoreactive gliofibrillary oligodendrocytes and minigemistocytes in some OGs but not in CNs or CCEs; thus, widespread GFAP-positive, gliofibrillary oligodendrocytes, and minigemistocytes in clear cell brain tumor biopsies seem to be firm indicators of OG and are particularly helpful for distinction of OG vs CCE.
Summary and conclusion
This study showed that the diagnostic value of anti-glial and anti-neuronal antibodies for differential diagnosis of clear cell primary brain tumors is highly variable. Choice of antibodies used in the routine diagnostic setting should therefore be based on careful and systematic analysis of their diagnostic value and limits. In this study, anti-NEUN, anti-VIM, and anti-EMA proved most useful. Our data indicate that anti-NEUN, anti-VIM, and anti-EMA on representative tumor specimens allows clear-cut distinction of CNs vs OGs and CCEs. Anti-VIM, anti-EMA, and anti-GFAP support differential diagnosis of OGs vs CCEs. Nevertheless, the value of immunohistochemistry is of limited value on small tumor specimens due to focal expression of glial proteins in CNs and, conversely, of neuronal proteins in OGs and CCEs. In such situations, neuroradiological images and clinical data may significantly support differential diagnosis [24]. Furthermore, molecular cytogenetic analysis may amend histopathology and immunohistochemistry. In particular, losses of chromosome arms 1p and 19q have been proven to be of diagnostic value [25, 26, 29].
References
Ambros PF, Ambros IM (2001) Pathology and biology guidelines for resectable and unresectable neuroblastic tumors and bone marrow examination guidelines. Med Pediatr Oncol 37:492–504
Birner P, Piribauer M, Fischer I, Gatterbauer B, Marosi C, Ambros PF, Ambros IM, Bredel M, Oberhuber G, Rossler K, Budka H, Harris AL, Hainfellner JA (2003) Vascular patterns in glioblastoma influence clinical outcome and associate with variable expression of angiogenic proteins: evidence for distinct angiogenic subtypes. Brain Pathol 13:133–143
Blumcke I, Becker AJ, Normann S, Hans V, Riederer BM, Krajewski S, Wiestler OD, Reifenberger G (2001) Distinct expression pattern of microtubule-associated protein-2 in human oligodendrogliomas and glial precursor cells. J Neuropathol Exp Neurol 60:984–993
Caceres A, Binder LI, Payne MR, Bender P, Rebhun L, Steward O (1984) Differential subcellular localization of tubulin and the microtubule-associated protein MAP2 in brain tissue as revealed by immunocytochemistry with monoclonal hybridoma antibodies. J Neurosci 4:394–410
Clark HB (1984) Immunohistochemistry of nervous system antigens: diagnostic applications in surgical neuropathology. Semin Diagn Pathol 1:309–316
Cooke HJ, Hindley J (1979) Cloning of human satellite III DNA: different components are on different chromosomes. Nucleic Acids Res 6:3177–97
Engelhard H, Stelea A, Cochran E (2002) Oligodendroglioma: pathology and molecular biology. Surg Neurol 58:111–117
Figarella-Branger D, Pellissier JF, Daumas-Duport C, Delisle MB, Pasquier B, Parent M, Gambarelli D, Rougon G, Hassoun J (1992) Central neurocytomas. Critical evaluation of a small-cell neuronal tumor. Am J Surg Pathol 16:97–109
Gelpi E, Ambros IM, Birner P, Luegmayr A, Drlicek M, Fischer I, Kleinert R, Maier H, Huemer M, Gatterbauer B, Anton J, Rossler K, Budka H, Ambros PF, Hainfellner JA (2003) Fluorescent in situ hybridization on isolated tumor cell nuclei: a sensitive method for 1p and 19q deletion analysis in paraffin-embedded oligodendroglial tumor specimens. Mod Pathol 16:708–715
Giangaspero F, Cenacchi G, Losi L, Cerasoli S, Bisceglia M, Burger PC (1997) Extraventricular neoplasms with neurocytoma features. A clinicopathological study of 11 cases. Am J Surg Pathol 21:206–212
Haan EA, Boss BD, Covan WM (1982) Production and characterization of monoclonal antibodies against the “brain-specific” proteins 14–3-2 and S-100. Proc Natl Acad Sci USA 79:7585–7589
Hasselblatt M, Paulus W (2003) Sensitivity and specificity of epithelial membrane antigen staining patterns in ependymomas. Acta Neuropathol (Berl) 106:385–388
Hassoun J, Gambarelli D, Grisoli F, Pellet W, Salamon G, Pellissier JF, Toga M (1982) Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol 56:151–156
Herpers MJ, Budka H (1984) Glial fibrillary acidic protein (GFAP) in oligodendroglial tumors: gliofibrillary oligodendroglioma and transitional oligoastrocytoma as subtypes of oligodendroglioma. Acta Neuropathol 64:265–272
Ishiuchi S, Tamura M (1997) Central neurocytoma: an immunohistochemical, ultrastructural and cell culture study. Acta Neuropathol 94:425–435
Jaffe E, Harris N, Stein H, Vardiman J (2001) World Health Organisation classification of tumours: Pathology and genetics of tumours of haematopoietic and lymphoid tissues, 1st edn. IARC Press, Lyon
Kleihues P, Cavenee W (2000) World Health Organisation classification of tumours: pathology and genetics of tumours of the nervous system, 2nd edn. IARC Press, Lyon
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK (2002) The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61:215–225
Lee V, Wu H, Schlaepfer W (1982) Monoclonal antibodies recognize individual neurofilament triplet proteins. Proc Natl Acad Sci USA 79:6089–6092
McLendon R, Bigner D (1994) Immunohistochemistry of the glial fibrillary acidic protein: basic and applied considerations. Brain Pathol 4:221–228
Min KW, Scheithauer BW (1997) Clear cell ependymoma: a mimic of oligodendroglioma: clinicopathologic and ultrastructural considerations. Am J Surg Pathol 21:820–826
Mullen R, Buck C, Smith A (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211
Parker JR, Armstrong DL, Strother D, Rudman DM, Dauser RC, Laurent JP, Deyd J, Rouah PE (2001) Antineuronal nuclei immunohistochemical staining patterns in childhood ependymomas. J Child Neurol 16:548–552
Perry A (2003) Pathology of low-grade gliomas: an update of emerging concepts. Neuro-oncol 5:168–178
Perry A, Fuller CE, Banerjee R, Brat DJ, Scheithauer BW (2003) Ancillary fish analysis for 1p and 19q status: preliminary observations in 287 gliomas and oligodendroglioma mimics. Front Biosci 8:A1–A9
Perry A, Scheithauer BW, Macaulay RJ, Raffel C, Roth KA, Kros JM (2002) Oligodendrogliomas with neurocytic differentiation. A report of 4 cases with diagnostic and histogenetic implications. J Neuropathol Exp Neurol 61:947–955
Pinkus G, Kurtin P (1985) Epithelial membrane antigen: a diagnostic discriminant in surgical pathology: immunohistochemical profile in epithelial, mesenchymal, and hematopoietic neoplasms using paraffin sections and monoclonal antibodies. Hum Pathol 16:929–940
Royds JA, Ironside JW, Taylor CB, Graham DI, Timperley WR (1986) An immunohistochemical study of glial and neuronal markers in primary neoplasms of the central nervous system. Acta Neuropathol 70:320–326
Smith JS, Alderete B, Minn Y, Borell TJ, Perry A, Mohapatra G, Hosek SM, Kimmel D, O’Fallon J, Yates A, Feuerstein BG, Burger PC, Scheithauer BW, Jenkins RB (1999) Localization of common deletion regions on 1p and 19q in human gliomas and their association with histological subtype. Oncogene 18:4144–4152
ten Berge R, Snijdewint F, Mensdorf-Pouilly S von, Poort-Keesom R, Oudejans J, Meijer J, Willemze R, Hilgers J, Meijer C (2001) MUC1 (EMA) is preferentially expressed by ALK positive anaplastic large cell lymphoma, in the normally glycosylated or only partly hypoglycosylated form. J Clin Pathol 54:933–939
Uematsu Y, Rojas-Corona RR, Llena JF, Hirano A (1989) Distribution of epithelial membrane antigen in normal and neoplastic human ependyma. Acta Neuropathol 78:325–328
Van Eldik LJ, Jensen RA, Ehrenfried BA, Whetsell WO (1986) Immunohistochemical localization of S100 beta in human nervous system tumors by using monoclonal antibodies with specificity for the S100 beta polypeptide. J Histochem Cytochem 34:977–982
Vinores SA, Bonnin JM, Rubinstein LJ, Marangos PJ (1984) Immunohistochemical demonstration of neuron-specific enolase in neoplasms of the CNS and other tissues. Arch Pathol Lab Med 108:536–540
Wharton SB, Chan KK, Hamilton FA, Anderson JR (1998) Expression of neuronal markers in oligodendrogliomas: an immunohistochemical study. Neuropathol Appl Neurobiol 24:302–308
Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE (1986) Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci USA 83:3500–3504
Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blümcke I (1996) NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem 44:1167–1171
Acknowledgements
This work was carried out within the Austrian Neuro-Oncology Network (ANN, www.ann.at), supported by the Children’s Cancer Research Institute (CCRI), St. Anna Children’s Hospital Vienna, and by the Scientific Funds of the Mayor of Vienna (project no. 2026).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koperek, O., Gelpi, E., Birner, P. et al. Value and limits of immunohistochemistry in differential diagnosis of clear cell primary brain tumors. Acta Neuropathol 108, 24–30 (2004). https://doi.org/10.1007/s00401-004-0856-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0856-9