Abstract
Amyotrophic lateral sclerosis (ALS) is characterized by degeneration of upper and lower motor neurons. In some ALS patients, dementia or aphasia may be present (ALS-D). The dementia is most commonly a frontotemporal dementia (FTD), and many of these cases have ubiquitin-positive, tau-negative inclusions in neurons of the dentate gyrus and superficial layers of the frontal and temporal lobes. Identical inclusions have been found in cases presenting with FTD and have been designated motor neuron disease (MND)-inclusions. Cases of ALS-D without MND-inclusions have been reported to show neocortical gliosis, neuronal loss, and superficial spongiosis, but there have also been scattered case reports of ALS with Alzheimer’s disease (AD). To determine whether AD pathology may play a role in the dementia or aphasia syndromes in ALS, we reviewed 30 cases of sporadic ALS diagnosed at the University of Pittsburgh Medical Center. A clinical history of ALS-D was found in 24.1% of the cases, of which 57% had MND-inclusions. Although the ALS-D cases with MND-inclusions typically had amyloid-beta (Aβ) plaques, there were no neuritic plaques. Three cases of ALS-D had no MND-inclusions, and two of these fulfilled pathological criteria for AD. One ALS-D case showed severe amyloid angiopathy but no neuritic plaques or MND-inclusions. MND-inclusions were not found in any ALS case without dementia; however, four patients without dementia or aphasia showed moderate or frequent numbers of neuritic plaques. In conclusion, we found that approximately 30% of ALS cases with dementia have AD and that some ALS cases without frank dementia have significant AD pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease of motor neurons located in the spinal cord, brainstem, and motor cortex [1]. The clinical hallmarks of the disease are progressive muscle weakness, atrophy, and spasticity [2,3]. Approximately 10% of ALS cases are familial (FALS), with mutations in the SOD1 gene representing about 15% of FALS [4,5]. The neuropathological findings in sporadic and familial forms of ALS are identical, consisting of severe loss of lower motor neurons and variable loss of upper motor neurons. Lower motor neurons often contain cytoplasmic inclusions, including hyaline inclusions, small eosinophilic inclusions known as Bunina bodies, and ubiquitin-positive filamentous inclusions (skeins) [1, 6,7]. These inclusions are not typically found in upper motor neurons. In the motor cortex, neuronal loss and gliosis are most commonly encountered. The ALS/Parkinsonism-dementia complex of Guam (ALS-PDC) is distinct from sporadic and familial ALS because it is associated with the presence of numerous widespread tau-positive neurofibrillary tangles [8,9].
It has become apparent that cognitive difficulties (dementia or aphasia) may accompany, or even precede, the motor symptoms in ALS [10, 11, 12, 13, 14, 15, 16, 17,18]. Over 100 cases of ALS with dementia have been reported in Japan since 1964 [15]. The dementia is clinically of the frontal-lobe type [9, 13,19], and functional imaging studies have supported the frontotemporal distribution of the defects [20, 21,22]. Subtle cognitive abnormalities have also been noted in ALS cases without frank dementia [19, 23,24].
Dementia and aphasia in both sporadic and familial ALS have been associated with ubiquitin-positive, tau-negative cytoplasmic inclusions in neurons of the fascia dentata, entorhinal cortex, and substantia nigra, and in the superficial layers of the frontal and temporal lobes [18, 20, 25, 26, 27, 28,29]. Similar inclusions are found in cases of frontotemporal dementia (FTD) and have been called motor neuron disease (MND)-inclusions [30,31]. Neuronal intranuclear inclusions have recently been proposed to be a distinguishing characteristic in familial FTD-MND cases [32]. However, not all ALS cases with dementia or aphasia (ALS-D) have MND-inclusions, and the pathological basis of the cognitive difficulties is unclear. One possibility is the presence of AD-like pathology that induces cognitive abnormalities in ALS patients. A review of the literature in 1981 reported only one case of ALS-D with neuritic plaques [33]. Since that time, sporadic reports of the concurrence of ALS and AD have appeared in the literature [33, 34, 35, 36,37], though very few neuropathological studies have looked specifically at the association of dementia or aphasia with AD pathology in a large cohort of ALS patients. To determine whether AD pathology might play a role in ALS with dementia or aphasia, we reviewed 30 cases of sporadic ALS diagnosed at the University of Pittsburgh School of Medicine for the presence of amyloid-beta (Aβ) pathology, neuritic plaques, neurofibrillary tangles, Lewy bodies, and MND-inclusions. We report that a clinical history of dementia or aphasia in ALS is often associated with MND-inclusions but that approximately 30% of ALS patients with dementia have AD and that many ALS cases without frank dementia or aphasia have significant AD pathology.
Materials and methods
A retrospective review of autopsy cases at the University of Pittsburgh School of Medicine (UPMC) from 1981–2001 identified 30 cases of ALS. Sixteen of these cases came from the University of Pittsburgh ALS Tissue Bank, which was established in 1997. Clinical history was abstracted from the available medical records, and neuropathologic examination confirmed the clinical diagnosis of motor neuron disease. Duration of disease was estimated from the first report of patient symptoms. The severity of the dementia syndrome could not be estimated in many cases because formal cognitive testing was rarely performed. Cases with aphasia did not include those with only dysarthria.
Available microscopic sections were reviewed, and in many cases additional immunohistochemical studies were performed. Although availability of sections varied, most cases had sections of pre-and post-central gyri, hippocampus, cingulate gyrus, caudate, putamen, globus pallidus, thalamus, amygdala, midbrain, substantia nigra, pons, medulla, and various levels of spinal cord, as well as sections from the frontal, parietal, temporal, and occipital lobes. Paraffin blocks were available on 28/30 cases. All sections were examined with hematoxylin and eosin (H&E) stains, but additional stains included Bielschowsky, congo red, and immunohistochemical stains for Aβ (4G8, Senetek, Maryland Heights, MO, USA), ubiquitin (Dako, Carpinteria, CA, USA), tau (SMI 51, Sternberger and Sternberger), Alz-50 (a kind gift of Peter Davies, Albert Einstein College of Medicine, NY, USA), and alpha-synuclein (Zymed, San Francisco, CA, USA). Severity of neuritic plaque formation was estimated using criteria established by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [38]. Degree of neurofibrillary tangle (NFT) formation was determined using Braak staging [39]. Aβ plaque severity was evaluated using the same CERAD criteria as used for neuritic plaques. MND-inclusions were identified as ubiquitin-positive, tau-negative intraneuronal cytoplasmic inclusions found primarily in the fascia dentata and the upper layers of the neocortex [18,30]. Although the number of MND-inclusions of neocortical and dentate gyrus varied from case to case, no attempt was made to estimate the severity of this pathology in this study. Averages are reported with the standard deviation. Groups were compared using the two-tailed student’s t -test.
Results
The 30 cases of ALS (Table 1) consist of 20 male and 10 female patients. The average age at autopsy was 61.2±10.7 years (range 40–75). Females tended to be slightly older (average age 65.2±5.8 years) than males (average age 59.2±10.9). The diagnosis of ALS was confirmed from clinical history and review of microscopic sections in all cases. One ALS case presented with primary progressive aphasia, but all other cases initially presented with motor neuron-related symptoms.
Dementia and aphasia in ALS
Information regarding cognitive status was available on 29 cases, and 7/29 (24.1%) had been diagnosed with dementia and/or aphasia (ALS-D). The ALS-D cases consisted of five males and two females, roughly the same proportion as in the ALS group as a whole. The average age at autopsy for ALS-D cases was 66.1±7.1 years (range 54–75) and was not significantly different from ALS cases (59.7±11.2 years, range 40–73, p =0.16). Duration of disease was available on 25 cases and was 30.7±17.3 months (range 11–60 months) in the ALS-D group ( n =6) compared with 38.0±26.9 months (range 5–112 months) for the ALS cases ( n =19), with no statistically significant difference, p =0.54. One ALS-D case presented with primary progressive aphasia (Table 1, case 4). Four of the seven ALS-D cases were reported to have both aphasia and dementia. Detailed neuropsychological testing on one of these cases (Table 1, case 2) has been previously reported [40] and showed abnormal executive/frontal functions as well as language deficits, consistent with a recent report that identified deficits in frontal executive functions and diminished word recognition in approximately 50% of ALS patients [41].
Gross
There was no evidence of cortical atrophy in any of the cases. Average brain weights for the ALS-D (1271±193 g) group did not differ significantly from the nondemented ALS cases (1313±166 g, p =0.57). None of the ALS cases had significant vascular or ischemic pathology.
Alzheimer disease-related pathology: Aβ staining
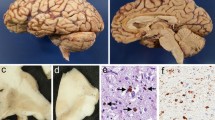
Immunostains for Aβ were available for 28/30 cases, and 14/28 (50%) had Aβ plaques, amyloid angiopathy, or both (Table 1). There were 10 males and four females, roughly in similar proportions to the entire cohort. The average age of ALS cases with Aβ staining (65.2±9.7 years) was significantly older than ALS cases without Aβ (56.7±10.9, p <0.05, student’s t -test, two-tailed). The number of neocortical Aβ plaques was rated as sparse (Fig. 1A), moderate (Fig. 1B) or frequent (Fig. 1C). Of the 14 cases with Aβ staining, six had a history of dementia or aphasia. Three of the ALS-D cases with MND-inclusions had paraffin blocks available for immunostaining, and all three showed moderate or frequent numbers of Aβ plaques (Table 1). The distribution of Aβ plaques in all cases was typical for that seen in aged and AD subjects. The distribution and severity of the Aβ immunostaining was not significantly different in the six ALS-D cases compared with the eight ALS cases lacking dementia.
Neuropathological changes in amyotrophic lateral sclerosis with dementia (ALS-D). Aβ immunostaining ranged from sparse numbers of plaques in four cases ( A, original magnification ×100), to moderate numbers in three cases ( B, original magnification ×100) and frequent Aβ plaques in seven cases ( C, original magnification ×100) (see Table 1). One demented case and one nondemented case showed severe amyloid angiopathy with only rare parenchymal plaques ( D, original magnification ×20). In four cases, motor neuron disease (MND)-inclusions were present and could be detected in H&E-stained sections as faintly basophilic, cytoplasmic inclusions in neurons in the superficial neocortex (layer II) and dentate gyrus ( E, original magnification ×600). These inclusions stained strongly for ubiquitin ( F, original magnification ×400) but were negative for tau and alpha-synuclein
One ALS-D case had severe amyloid angiopathy (Fig. 1D), but only rare parenchymal Aβ plaques and no neuritic plaques (Table 1, case 7). Because this case had a Braak stage of 2 and no MND-inclusions, it is tempting to speculate on the possible role of the amyloid angiopathy in the dementia syndrome of this patient. However, similar severe amyloid angiopathy with minimal parenchymal Aβ was also seen in a nondemented case (Table 1, case 15).
Alzheimer disease-related pathology: Bielschowsky staining
Bielschowsky stains were available for review for all cases. Six of 30 cases (20%) had neuritic plaques, which were found only in cases with Aβ deposits. Two cases without Aβ plaques had a Braak stage of 3 or less, consistent with age (Table 1, cases 26 and 30). Six of the 13 cases with Aβ staining had either moderate or frequent numbers of neocortical neuritic plaques, and 2/6 cases with neuritic plaques were ALS-D. The average age of the six cases with neuritic plaques was 65.3±8.6 years, which was not older than the seven cases with Aβ but without neuritic plaques (64.0±11.2). None of the ALS-D cases with MND-inclusions had neuritic plaques. Of the three ALS-D cases that did not have MND-inclusions, two had a CERAD age-related plaque score of “C,” allowing for the diagnosis of “definite AD” by CERAD criteria. One of these patients was 71 years of age and had a Braak stage of 4, while the other was a 64-year-old with a Braak stage of 2. Because these cases lacked MND-inclusions and had abundant Aβ deposition, as well as widespread neocortical neuritic plaque formation, it seems likely that the AD-related pathology was the substrate of the dementia. Data on disease duration was available for 25 patients (including 5/6 cases with neuritic plaques), and there was a trend for shorter duration of disease in cases with neuritic plaques; the five cases with neuritic plaques had an average duration of 23.0±15.6 months compared with 39.6±25.9 months for the 20 cases without neuritic plaque formation ( p =0.18, student’s t -test, two-tailed).
Lewy bodies
All cases had H&E stained sections of substantia nigra, pons, hippocampus, and cingulate gyrus available for review. No Lewy bodies (LB) were found. Paraffin blocks were available for 28/30 cases, and no LBs were detected by alpha-synuclein immunohistochemistry in the substantia nigra, pons, hippocampus, or cingulate gyrus in any cases.
MND-inclusions
MND-inclusions are ubiquitin-positive, tau-negative spherical cytoplasmic inclusions in neurons of the dentate gyrus and layer II in neocortical areas [18, 26, 27,30]. We identified MND-inclusions in 4/30 cases (Fig. 1E). As noted above, MND-inclusions were positive for ubiquitin (Fig. 1F), but negative for tau and alpha-synuclein immunostaining. Variable amounts of gliosis and neuronal loss were associated with the neocortical inclusions, and some cases showed mild to focally moderate superficial spongiosis. MND-inclusions were found only in cases with a history of dementia or aphasia. Two of these cases had both dementia and aphasia, while one had only dementia and one presented with primary progressive aphasia, which later developed into ALS without evidence of cognitive decline. Interestingly, all four cases were males. Although cases with MND-inclusions had Aβ plaques, none had any neuritic plaques by Bielschowsky stain, and all four cases were at a low Braak stage compatible with age. The presence of MND-inclusions did not markedly affect survival time: 3/25 cases with MND-inclusion had a survival time of 37.7±21.1 months compared with 22 cases without MND-inclusions and an average survival time of 36±25.8 months.
Discussion
To determine whether AD pathology might play a role in the dementia or aphasia syndromes in ALS, we reviewed 30 cases of sporadic ALS diagnosed at the University of Pittsburgh Medical Center. A clinical history of dementia or aphasia was found in 7/29 cases (24.1%). Four of the seven ALS-D cases (57.1%) had MND-inclusions in the dentate gyrus and superficial neocortex. Although these cases typically had Aβ plaques, there were no neuritic plaques. Interestingly, three cases of ALS-D had no MND-inclusions, and two of these cases fulfilled CERAD criteria for definite AD. The last ALS-D case (Table 1, case 7) showed severe amyloid angiopathy but no neuritic plaques or MND-inclusions. Therefore, all of our ALS-D cases exhibited either MND-inclusions, Aβ deposition, or both. Five cases of ALS without dementia or aphasia exhibited significant numbers of Aβ plaques (four with moderate or frequent neuritic plaques), and 50% of all ALS cases had Aβ plaques.
While the incidence of dementia in ALS has historically been reported as less than 10% [42, 43, 44,45], more recent studies have reported that 25–28% of ALS cases have significant cognitive impairment [12,23]. These differences are likely due to the type of testing used in each report. In a recent study of 69 ALS cases, the incidence of dementia rose from 7% to 22% when diagnostic criteria for frontotemporal dementia (FTD) were included in the evaluation [19]. Another study of bulbar ALS patients found that 48% of 23 cases had significant cognitive impairments by neuropsychological testing [46].
Although aphasia is less common than dementia in ALS, numerous reports have noted the association of language deficits and ALS [10, 12, 16, 17, 24, 40,47]. In one study of 16 nondemented ALS cases, all 16 exhibited impaired verbal fluency and picture recall [48]. Word generation and detailed neuropsychological testing may be useful screening tools to identify ALS patients with cognitive abnormalities, and the use of such tests in one recent study has revealed that approximately 50% of ALS patients exhibited frontal deficits and met criteria of possible or probable frontotemporal lobar dementia (FTLD) [41]. Similarly, patients with FTD were assessed for motor neuron deficits, and 50% had either definite or possible ALS [41]. This suggests an overlap between ALS and FTD and indicates that cognitive impairment may be much more common in ALS patients than previously acknowledged.
In this study, MND-inclusions were found in 4/7 ALS-D cases and in none of the 23 ALS cases without dementia. Others have also reported on the association of MND-inclusions and dementia in ALS, but it should be noted that not all ALS cases with MND-inclusions in these studies had evidence of clinical dementia, although a thorough cognitive assessment was not available in every case, as in our series [18, 20, 25, 26, 27, 49,50]. Identical inclusions in the same distribution have been reported in patients presenting with FTD [30, 51, 52, 53, 54, 55,56], and such cases have been termed MND-inclusion dementia. As mentioned above, many patients with FTD can also be diagnosed with ALS or go on to develop symptoms of MND [21,57].
However, there were no MND-inclusions in 3/7 ALS-D cases, and we screened our study subjects for AD pathology. We found Aβ plaques in 14/28 (50%) of our ALS cases. Not unexpectedly, older age was associated with the presence of Aβ staining. Moderate or frequent numbers of neuritic plaques were found in six ALS cases, two of which had a clinical history of dementia and no MND-inclusions. Therefore, two of our ALS-D cases without MND-inclusions exhibited sufficient pathology to warrant a diagnosis of definite AD using CERAD criteria. AD pathology in ALS-D patients has previously been noted to be relatively rare. In 1981 a review of 27 previously reported ALS-D cases found only one with AD pathology [33]. Since that time, several reviews of ALS-D cases have reported only mild AD changes in a few cases and none with sufficient AD pathology for a diagnosis of AD [52, 58, 59, 60,61]. However, individual cases of AD in demented ALS patients have been reported [34, 35, 36,37], and two of these case reports also noted the presence of Lewy bodies [34,37]. Using alpha-synuclein immunohistochemistry, none of our 28 cases with available paraffin blocks had Lewy bodies in the substantia nigra or amygdala; however, since this study was started we have encountered a case of ALS with “incidental” Lewy bodies. As noted by others [29,32], we also found that the MND-inclusions are negative for alpha-synuclein.
AD changes in nondemented ALS cases have also been reported as relatively rare [62, 63, 64,65], although one study found similar amounts of AD pathology in a group of 20 nondemented ALS cases compared with 17 nondemented controls matched for age [66]. We found Aβ plaques in 8/23 (35%) of ALS cases lacking dementia or aphasia (Table 1). In addition, 50% of these cases (4/8) exhibited neuritic plaques, and one had severe amyloid angiopathy. It is interesting to note that a 49-year-old ALS patient with frequent Aβ plaques and moderate levels of neuritic plaques did not have a clinical history of dementia (Table 1, case 8). The lack of formal neuropsychological and neurobehavioral testing in our group limits our ability to determine whether any of these nondemented ALS cases exhibited cognitive abnormalities near their times of death. The lack of cognitive testing also limits our ability to determine whether the ALS-D cases with MND-inclusions had patterns of cognitive difficulties different from those of the ALS-D cases with AD. We do note that the presence of neuritic plaques decreased patient survival time (decreased disease duration), whereas the presence of MND-inclusions did not alter disease duration. Formal testing may have identified subtle cognitive difficulties in other ALS patients as well. Because up to 50% of ALS patients may have some cognitive impairment [41], it may be prudent for ALS research centers to include cognitive testing as part of a routine evaluation of ALS patients. These cognitive tests should include both word generation tests to identify deficits related to FTLD, and Alzheimer disease/hippocampal memory tests to identify deficits related to AD pathology.
Interestingly, two of our cases had severe amyloid angiopathy with minimal parenchymal amyloid. One of these cases was demented and had no MND-inclusions, and it is tempting to speculate on the role of the amyloid angiopathy in the dementia; however, the other case had no clinical history of dementia and had comparable severity of amyloid angiopathy. Isolated amyloid angiopathy is found in some elderly individuals, and “dyshoric” amyloid angiopathy was recently reported in a 79-year-old female ALS case without dementia [65]. Other vascular pathology (e.g., infarcts, lacunes, hippocampal sclerosis, and leukoencephalopathy) was not present to any significant degree in any of our cases.
In summary, we found that 50% of ALS cases had Aβ deposits and that nearly half of these also had neocortical neuritic plaques. The high prevalence of AD pathology in our cohort compared with what has been reported in the literature is most likely due to the fact that previous studies of the pathology of ALS-D have focused primarily on the presence or absence of MND-inclusions, superficial spongiosus, and cortical gliosis, but often did not investigate or report on the prevalence or severity of AD pathology in their cohorts. Even fewer studies looking at nondemented ALS cases have included evaluation of AD pathology, and when included it has often been dismissed as “age-related” changes. While our study indicates that the ALS cases with AD pathology (both beta-A4 deposits and neuritic plaques) are significantly older than those ALS cases without AD pathology, the average age of our cohort overall is similar to that of sporadic ALS cases in the literature. Further, we found that while 4/7 ALS-D cases had MND-inclusions, 2/7 (28.6%) ALS-D cases had AD without MND-inclusions. Finally, 1/7 cases of ALS-D had no MND-inclusions and no neuritic plaques, but did have severe amyloid angiopathy and only sparse numbers of Aβ plaques, with a Braak stage of 2. Moreover, a significant number of ALS cases without overt clinical dementia or aphasia had moderate or frequent numbers of neuritic plaques, and it is possible that mild cognitive impairment could have been demonstrated with appropriate testing.
These findings support routine testing for cognitive impairment in ALS patients because they are at increased risk of developing a dementia/aphasia syndrome, either from MND-inclusion formation (frontotemporal dementia) or from AD-related pathology. It is impossible to say from such a small study if there is a link between these two pathologies, but it seems likely that in any large cohort of ALS patients, there will be some who will have pathological changes associated with AD. Indeed, because the two diseases occur in an older population, it would be surprising not to find such an overlap. While the association of AD pathology and ALS may be coincidental, we have found that in some cases cognitive impairment in ALS is related to the presence of AD pathology and suggest that the presence of MND-inclusions or Aβ plaques may represent part of the clinicopathological spectrum of ALS.
References
Bergmann M (1993) Motor neuron disease/amyotrophic lateral sclerosis—lessons from ubiquitin. Pathol Res Pract 189:902–912
Rowland LP (1994) Natural history and clinical features of amyotrophic lateral sclerosis and related motor neuron diseases. In: Calne DB (ed) Neurodegenerative diseases. Saunders, Philadelphia, pp 507–521
Cleveland DW, Rothstein J (2001) From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2:806–819
Siddique T, Hentati A (1995) Familial amyotrophic lateral sclerosis. Clin Neurosci 3:338–347
Brown RH (1997) Amyotrophic lateral sclerosis. Insights from genetics. Arch Neurol 54:1246–1250
Leigh PN, Anderton BH, Dodson A, Gallo JM, Swash M, Power DM (1988) Ubiquitin deposits in anterior horn cells in motor neurone disease. Neurosci Lett 93:197–203
Matsumoto S, Hirano A, Goto S (1990) Ubiquitin-immunoreactive filamentous inclusions in anterior horn cells of Guamanian and non-Guamanian amyotrophic lateral sclerosis. Acta Neuropathol 80:233–238
Shankar SK, Yanagihara R, Garruto RM, Grundke-Iqbal I, Kosik KS, Gajdusek DC (1989) Immunocytochemical characterization of neurofibrillary tangles in amyotrophic lateral sclerosis and parkinsonism-dementia of Guam. Ann Neurol 25:146–151
Ikemoto A, Hirano A, Akiguchi I (2000) Neuropathology of amyotrophic lateral sclerosis with extra-motor system degeneration: characteristics and differences in the molecular pathology between ALS with dementia and Guamanian ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 1:97–104
Caselli RJ, Windebank AJ, Petersen RC, Komori T, Parisi JE, Okazaki H, Kokmen E, Iverson R, Dinapoli RP, Graff-Radford NR, Stein SD (1993) Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol 33:200–207
Lowe J (1994) New pathological findings in amyotrophic lateral sclerosis. J Neurol Sci 124:38–51
Rakowicz WP, Hodges JR (1998) Dementia and aphasia in motor neuron disease: an underrecognised association? J Neurol Neurosurg Psychiatry 65:881–889
Bak TH, Hodges JR (1999) Cognition, language and behaviour in motor neurone disease: evidence of frontotemporal dysfunction. Dement Geriatr Cogn Disord 10:29–32
Gentileschi V, Muggia S, Poloni M, Spinnler H (1999) Fronto-temporal dementia and motor neuron disease: a neuropsychological study. Acta Neurol Scand 100:341–349
Mitsuyama Y (2000) Dementia with motor neuron disease. Neuropathology 20:S79–81
Tsuchiya K, Ozawa E, Fukushima J, Yasui H, Kondo H, Nakano I, Ikeda K (2000) Rapidly progressive aphasia and motor neuron disease: a clinical, radiological, and pathological study of an autopsy case with circumscribed lobar atrophy. Acta Neuropathol 99:81–87
Bak TH, Hodges JR (2001) Motor neurone disease, dementia and aphasia: coincidence, co-occurrence or continuum? J Neurol 248:260–270
Wilson C, Grace G, Munoz DG, He B, Strong M (2001) Cognitive impairment in sporadic ALS: a pathologic continuum underlying a multisystem disorder. Neurology 57:651–657
Barson FP, Kinsella GJ, Ong B, Mathers SE (2000) A neuropsychological investigation of dementia in motor neurone disease (MND). J Neurol Sci 180:107–113
Anderson VE, Cairns NJ, Leigh PN (1995) Involvement of the amygdala, dentate and hippocampus in motor neuron disease. J Neurol Sci 129:75–78
Garraux G, Salmon E, Degueldre C, Lemaire C, Franck G (1999) Medial temporal lobe metabolic impairment in dementia associated with motor neuron disease. J Neurol Sci 168:145–150
Vercelletto M, Ronin M, Huvet M, Magne C, Feve JR (1999) Frontal type dementia preceding amyotrophic lateral sclerosis: a neuropsychological and SPECT study of five clinical cases. Eur J Neurol 6:295–299
Chari G, Shaw PJ, Sahgal A (1996) Nonverbal visual attention, but not recognition memory of learning, processes are impaired in motor neurone disease. Neuropsychologia 34:377–385
Bak TH, O’Donovan DG, Xuereb JH, Boniface S, Hodges JR (2001) Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain 124:103–120
Okamoto K, Murakami N, Kusaka H, Yoshida M, Hashizume Y, Nakazato Y, Matsubara E, Hirai S (1992) Ubiquitin-positive intraneuronal inclusions in the extramotor cortices of presenile dementia patients with motor neuron disease. J Neurol 239:426–430
Wightman G, Anderson VE, Martin J, Swash M, Anderton BH, Neary D, Mann D, Luthert P, Leigh PN (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Wakabayashi K, Piao YS, Hayashi S, Kakita A, Yamada M, Takahashi H (2001) Ubiquitinated neuronal inclusions in the neostriatum in patients with amyotrophic lateral sclerosis with and without dementia—a study of 60 patients 31 to 87 years of age. Clin Neuropathol 20:47–52
Piao YS, Wakabayashi K, Kakita A, Yamada M, Hayashi S, Morita T, Ikuta F, Oyanagi K, Takahashi H (2003) Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 13:10–22
Al-Sarraj S, Maekawa S, Kibble M, Everall I, Leigh N (2002) Ubiquitin-only intraneuronal inclusion in the substantia nigra is a characteristic feature of motor neurone disease with dementia. Neuropathol Appl Neurobiol 28:120–128
Jackson M, Lennox G, Lowe J (1996) Motor neurone disease-inclusion dementia. Neurodegeneration 5:339–350
Nakano I (2000) Frontotemporal dementia with motor neuron disease (amyotrophic lateral sclerosis with dementia). Neuropathology 20:68–75
Mackenzie IRA, Feldman H (2003) Neuronal intranuclear inclusions distinguish familial FTD-MND type from sporadic cases. Acta Neuropathol 105:543–548
Hudson AJ (1981) Amyotrophic lateral sclerosis and its association with dementia, Parkinsonism and other neurological disorders: a review. Brain 104:217–247
Delisle MB, Gorce P, Hirsch E, Hauw JJ, Rascol A, Bouissou H (1987) Motor neuron disease, parkinsonism and dementia. Report of a case with diffuse Lewy body-like intracytoplasmic inclusions. Acta Neuropathol 75:104–108
Frecker MF, Fraser FC, Andermann E, Pryse-Phillips WE (1990) Association between Alzheimer disease and amyotrophic lateral sclerosis? Can J Neurol Sci 17:12–14
Muller M, Vieregge P, Reusche E, Ogomori K (1993) Amyotrophic lateral sclerosis and frontal lobe dementia in Alzheimer’s disease. Case report and review of the literature. Eur Neurol 33:320–324
Hedera P, Lerner AJ, Castellani R, Friedland RP (1995) Concurrence of Alzheimer’s disease, Parkinson’s disease, diffuse Lewy body disease, and amyotrophic lateral sclerosis. J Neurol Sci 128:219–224
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Lopez OL, Becker JT, DeKosky ST (1994) Dementia accompanying motor neuron disease. Dementia 5:42–47
Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B (2003) Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 60:1094–1097
Kondo K, Hemmi I (1984) Clinical statistics in 515 fatal cases of motor neuron disease. Neuroepidemiology 3:129–148
Gubbay SS, Kahana E, Zilber N, Cooper G, Pintov S, Leibowitz Y (1985) Amyotrophic lateral sclerosis. A study of its presentation and prognosis. J Neurol 232:295–300
Leone M, Chandra V, Schoenberg BS (1987) Motor neuron disease in the United States, 1971 and 1973–1978: patterns of mortality and associated conditions at the time of death. Neurology 37:1339–1343
Capitani E, Della Sala S, Marchetti C (1994) Is there a cognitive impairment in MND? A survey with longitudinal data. Schweiz Arch Neurol Psychiatr 145:11–13
Portet F, Cadilhac C, Touchon J, Camu W (2001) Cognitive impairment in motor neuron disease with bulbar onset. Amyotroph Lateral Scler Other Motor Neuron Disord 2:23–29
Doran M, Xuereb J, Hodges JR (1995) Rapidly progressive aphasia with bulbar neurone disease: a clinical and neuropsychological study. Behav Neurol 8:169–180
Kew JJ, Goldstein LH, Leigh PN, Abrahams S, Cosgrave N, Passingham RE, Frackowiak RS, Brooks DJ (1993) The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain 116:1399–1423
Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y (1991) New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129:233–236
Ince PG, Shaw PJ, Slade JY, Jones C, Hudgson P (1996) Familial amyotrophic lateral sclerosis with a mutation in exon 4 of the Cu/Zn superoxide dismutase gene: pathological and immunocytochemical changes. Acta Neuropathol 92:395–403
Cooper PN, Jackson M, Lennox G, Lowe J, Mann DM (1995) Tau, ubiquitin, and alpha B-crystallin immunohistochemistry define the principal causes of degenerative frontotemporal dementia. Arch Neurol 52:1011–1015
Bergmann M, Kuchelmeister K, Schmid KW, Kretzschmar HA, Schroder R (1996) Different variants of frontotemporal dementia: a neuropathological and immunohistochemical study. Acta Neuropathol 92:170–179
Jackson M, Lowe J (1996) The new neuropathology of degenerative frontotemporal dementias. Acta Neuropathol 91:127–134
Talbot PR (1996) Frontal lobe dementia and motor neuron disease. J Neural Transm Suppl 47:125–132
Kertesz A, Kawarai T, Rogaeva E, St. George-Hyslop P, Poorkaj P, Bird TD, Munoz DG (2000) Familial frontotemporal dementia with ubiquitin-positive, tau-negative inclusions. Neurology 54:818–827
Uchihara T, Sato T, Suzuki H, Ikeda K, Akiyama H, Takatori T (2001) Bunina body in frontal lobe dementia without clinical manifestations of motor neuron disease. Acta Neuropathol 101:281–284
Lomen-Hoerth C, Anderson T, Miller B (2002) The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 59:1077–1079
Wikstrom J, Paetau A, Palo J, Sulkava R, Haltia M (1982) Classic amyotrophic lateral sclerosis with dementia. Arch Neurol 39:681–683
Morita K, Kaiya H, Ikeda T, Namba M (1987) Presenile dementia combined with amyotrophy: a review of 34 Japanese cases. Arch Gerontol Geriatr 6:263–277
Neary D, Snowden JS, Mann DM, Northen B, Goulding PJ, Macdermott N (1990) Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry 53:23–32
Yoshida M, Murakami N, Hashizume Y, Takahashi A (1992) A clinicopathological study on 13 cases of motor neuron disease with dementia. Clin Neurol 32:1193–1202
Tan N, Kakulas BA, Masters CL, Gajdusek DC, Garruto RM, Chen KM, Gibbs CJ, Jr. (1986) Observations on the clinical presentations and the neuropathological findings of amyotrophic lateral sclerosis in Australia and Guam. Ann Acad Med Singapore 15:62–66
Mitsuyama Y (1993) Presenile dementia with motor neuron disease. Dementia 4:137–142
Su M, Wakabayashi K, Tanno Y, Inuzuka T, Takahashi H (1996) An autopsy case of amyotrophic lateral sclerosis with concomitant Alzheimer’s and incidental Lewy body diseases. No To Shinkei (Brain & Nerve) 48:931–936
Primavera J, Lu B, Riskind P, Iulian M, de la Monte SM (1999) Brain accumulation of amyloid-beta in non-Alzheimer neurodegeneration. J Alzheimer Dis 1:183–193
Smitt PA, Troost D, Louwerse ES, de Jong JM, van Kessel DT, de Leeuw MA (1993) Temporal lobe pathology in amyotrophic lateral sclerosis. Do amyotrophic lateral sclerosis and Alzheimer’s disease share a common etiological factor? Clin Neuropathol 12:88–91
Acknowledgments
RLH was supported in part by Research Grant AG05133 from the National Institute of Aging and the Alzheimer’s Disease Research Center at the University of Pittsburgh. RB was supported by a research grant from the National ALS Association. The University of Pittsburgh ALS Tissue Bank is supported by the Mario Lemieux Foundation and the Western Pennsylvania Chapter of the ALS Association.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamilton, R.L., Bowser, R. Alzheimer disease pathology in amyotrophic lateral sclerosis. Acta Neuropathol 107, 515–522 (2004). https://doi.org/10.1007/s00401-004-0843-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0843-1