Abstract
We immunohistochemically examined the brain and peripheral sympathetic ganglia from eight patients with multiple system atrophy (MSA), using an antibody specific for phosphorylated α-synuclein (anti-PSer129). Phosphorylated α-synuclein was deposited in five cellular locations: oligodendroglial cytoplasm and nucleus, and neuronal cytoplasm, processes and nucleus. Many neuronal cytoplasmic inclusions (NCIs) were found in the pontine and inferior olivary nuclei and, to a lesser extent, in the substantia nigra, locus ceruleus, and neocortical and hippocampal neurons. NCIs were also found in the sympathetic ganglia in two out of the eight cases. Moreover, anti-PSer129 immunohistochemistry revealed extensive neuropil pathology; swollen neurites were abundant in the pontine nucleus, delicate neurites were observed in the deeper layers of the cerebral cortex and thalamus, and neuropil threads and dot-like structures were distributed in the basal ganglia and brainstem. Diffuse neuronal cytoplasmic staining (pre-NCI) was frequently found in the pontine and inferior olivary nuclei. Thus, the widespread accumulation of phosphorylated α-synuclein in both glial and neuronal cells is a pathological feature in patients suffering from MSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-Synuclein is a major component of neuronal and glial inclusions in Parkinson’s disease (PD), dementia with Lewy bodies (DLB), Hallervorden-Spatz disease (HSD) and multiple system atrophy (MSA), collectively referred to as α-synucleinopathies [2, 30, 31, 32]. Neuronal α-synuclein aggregates are also found in the limbic system in sporadic and familial Alzheimer’s disease as well as in other tauopathies [3, 6, 9, 11, 15, 16, 23, 34, 35]. Recently, Fujiwara et al. [4] revealed that α-synuclein deposited in α-synucleinopathy lesions was phosphorylated at Serine129 and that anti-phosphorylated α-synuclein antibody (anti-PSer129) intensely immunolabeled cortical and brainstem-type Lewy bodies (LBs) and Lewy neurites in PD and DLB, dystrophic neurites in HSD, and glial cytoplasmic inclusions (GCIs) in MSA. Saito et al. [26] reported that LB-related pathology occurred in 40 out of 157 cases (25.5%) of aged population, using two antibodies specific for phosphorylated α-synuclein (anti-PSer129 and psyn#64). However, the extent and distribution of phosphorylated α-synuclein in neuronal component in MSA has not been determined.
In the present study, we performed immunohistochemical analysis of the brain and peripheral sympathetic ganglia of patients with MSA, using anti-PSer129 antibody. Here, we report that α-synuclein accumulated in the neuronal cytoplasm, processes and nucleus in the central and peripheral nervous systems is phosphorylated, and that there exists widespread neuropil pathology in MSA.
Materials and methods
Subjects
Eight patients with MSA (aged 63–75 years, mean 68.5 years) served as the subjects of this study. Disease duration ranged from 2.5 to 10 years (mean 5.8 years). For routine histological examination, the brains, spinal cords and sympathetic ganglia of these subjects were fixed with formalin for 3–4 weeks and then embedded in paraffin. Four-micrometer-thick sections were cut and stained with hematoxylin and eosin and by the Klüver-Barrera method. We also examined brain tissues from four patients with PD (aged 65–82 years, mean 73.3 years), two patients with DLB (74 and 77 years), and four control subjects (aged 53–82 years, mean 68.3 years). In each patient, the diagnosis was confirmed by neuropathological examinations, including α-synuclein immunohistochemistry.
Immunohistochemistry
Four-micrometer-thick sections of the temporal lobe, motor cortex, basal ganglia, midbrain, upper pons, medulla oblongata, cerebellum and sympathetic ganglia (paravertebral and/or celiac ganglia) from patients with MSA, PD, DLB and controls were cut and immunostained using the avidin-biotin-peroxidase complex (ABC) method with a Vectastain ABC kit (Vector, Burlingame, CA). The following antibodies and dilutions were used: polyclonal anti-α-synuclein (NACP-6; 1:5,000) [24] and polyclonal anti-phosphorylated α-synuclein (anti-PSer129; 1:200) [4]. NACP-6 was raised by immunizing rabbits with recombinant α-synuclein. Anti-PSer129 was raised against a synthetic peptide that corresponds to amino acid residues 124–134 of human α-synuclein with a phosphorylated Ser129 residue. The specificity of these antibodies has been described previously [4, 24]. The sections were pretreated with 99% formic acid for 5 min for anti-PSer129. Diaminobenzidine was used as the chromogen. The immunolabeled sections were lightly counterstained with hematoxylin.
Paraffin sections from MSA cases were processed for double immunolabeling. Deparaffinized sections were incubated with 5% normal serum for 1 h at room temperature to block nonspecific binding. Sections were then incubated simultaneously with polyclonal anti-PSer129 (1:20) and monoclonal anti-phosphorylated neurofilament (SMI31; Sternberger Immunochemicals, Baltimore, MD; 1:200) antibodies overnight at 4°C. Sections were then rinsed and allowed to react with the secondary antibody solution made up of fluorescein isothiocyanate-conjugated anti-rabbit IgG (Vector; 1:50) and Texas red-conjugated anti-mouse IgG (Vector; 1:50) for 1 h at room temperature. After rinsing, sections were transferred to a glass slide and mounted under glass coverslips with anti-fading medium. The sections were examined with a fluorescence microscope.
Semiquantitative analysis
As a semiquantitative assessment of pathological structures immunostained with anti-PSer129, the number of GCIs, neuronal cytoplasmic inclusions (NCIs), diffuse neuronal cytoplasmic staining and neuritic changes was evaluated. The number of NCIs and diffuse neuronal cytoplasmic staining was counted in one unilateral section from each brain region and was graded using the following semiquantitative scale: −, no inclusions; +, 1–5 inclusions; ++, 6–20 inclusions; and +++, more than 20 inclusions. The number of GCIs and neuritic changes was evaluated as follows: −, absent; +, several; ++, some; +++, many.
Results
Immunostaining with NACP-6 showed diffuse or punctate synaptic staining in controls as well as intense immunolabeling of LBs and Lewy neurites in PD and DLB. By contrast, anti-PSer129 antibody produced intense staining of LB-related pathology without staining of normal presynaptic structures (Fig. 1A–C). Neuritic plaques in the hippocampus contained anti-PSer129-immunoreactive dystrophic neurites, and immunoreactive glial inclusions were occasionally found in PD and DLB brains (Fig. 1D).
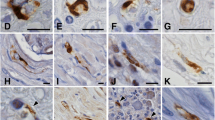
Phosphorylated α-synuclein immunoreactivity in Parkinson’s disease (A, C, D), dementia with Lewy bodies (B) and multiple system atrophy patients (E–R). A Ring-like staining of Lewy bodies in the substantia nigra. B Cortical Lewy bodies in the parahippocampal gyrus. Threads and dot-like structures in the neuropil are also evident. C Numerous Lewy neurites in the sympathetic ganglia. D Glial inclusions in the substantia nigra. E GCIs in the pontine base. F α-Synuclein aggregates in the oligodendroglial cytoplasm and nucleus. G NCIs in the substantia nigra. H NCIs in the dentate granule cells. I A pyramidal neuron in the motor cortex showing accumulation of α-synuclein in the cytoplasm, nucleus and proximal axon. J NCIs in the sympathetic ganglia. K Neuronal nuclear inclusions in the pontine nucleus. L–O Morphological progression of NCIs in the pontine nucleus. L Diffuse neuronal cytoplasmic staining. M Focal α-synuclein aggregates in the cytoplasm. N Irregularly shaped fibrillary aggregates in the cytoplasm. O Typical NCIs. P Swollen neurites in the pontine nucleus. Q Delicate neurites in the centromedian nucleus of the thalamus. R Neuropil threads (arrows) and dot-like structures (arrowheads) in the inferior olivary nucleus (GCIs glial cytoplasmic inclusions, NCIs Neuronal cytoplasmic inclusions). Bars A, F–H, J–O 5 µm; B–E, I, P–R 20 µm
The distribution and extent of phosphorylated α-synuclein-immunoreactive abnormal structures in MSA are summarized in Table 1. Anti-PSer129 antibody strongly immunolabeled numerous GCIs throughout the central nervous system (Fig. 1E). GCI-bearing glial cells occasionally contained rod-like inclusions in their nucleus (glial nuclear inclusions, GNIs) (Fig. 1F). No α-synuclein aggregates were found in Schwann cells in the peripheral sympathetic ganglia.
NCIs were also intensely immunolabeled with anti-PSer129; many NCIs were found in the pontine and inferior olivary nuclei and, to a lesser extent, in the substantia nigra (Fig. 1G), locus ceruleus, dentate granule cells (Fig. 1H), and neocortical and hippocampal pyramidal neurons (Fig. 1I). Several NCIs were also found in the sympathetic ganglia in two out of the eight cases (Fig. 1J). These NCI-containing neurons in the brain and peripheral ganglia often contained intranuclear fibrillary inclusions positive for phosphorylated α-synuclein (neuronal nuclear inclusions, NNIs) (Fig. 1G, I, K).
In addition to typical NCIs, anti-PSer129 immunohistochemistry revealed diffuse neuronal cytoplasmic staining and focal fibrillary aggregates in the cytoplasm (Fig. 1L–O). Diffuse neuronal cytoplasmic staining was found in some brain regions, particularly in the pontine and inferior olivary nuclei (Fig. 1L). Such staining was not noted in sections stained with NACP-6. Focal α-synuclein aggregates were frequently found within the diffuse neuronal cytoplasmic staining (Fig. 1M, N).
Anti-PSer129 immunohistochemistry further revealed extensive neuropil pathology in MSA. We have classified the phosphorylated α-synuclein-positive neuritic changes into four structures morphologically; swollen neurites (Fig. 1P), delicate neurites (Fig. 1Q), neuropil threads (Fig. 1R) and dot-like structures (Fig. 1R). Swollen neurites were abundant in the pontine base and a few in the substantia nigra. Delicate neurites were observed in the deeper layers of the motor cortex and thalamus. Neuropil threads and dot-like structures were preferentially localized in the neostriatum, locus ceruleus and inferior olivary nucleus. No immunoreactive axons were observed in the cerebral white matter in MSA.
Contiguous sections stained with NACP-6 and anti-PSer129 revealed that almost all the GCIs, GNIs, NCIs and NNIs were phosphorylated (Fig. 2). Anti-PSer129-immunoreactive neuritic structures outnumbered NACP-6-immunoreactive structures in the neuropil.
Contiguous sections of pontine (A, B, G, H) and inferior olivary nuclei (C–F) stained with NACP-6 (A, C, E, G) and anti-PSer129 (B, D, F, H). Almost all the GCIs (A), NCIs (C), NNIs (E) and neuritic changes (C, G) immunopositive for NACP-6 are also labeled with anti-PSer129. The neuronal cytoplasm, dot-like structures and NCIs are more intensely stained with anti-PSer129 (D, F) (NNIs neuronal nuclear inclusions). Bars A–D, G, H 20 µm; E, F 10 µm
Double-labeling immunofluorescence revealed that the swollen and delicate neurites, neuropil threads and dot-like structures were occasionally colocalized with epitope of phosphorylated neurofilament, a marker of axons (Fig. 3).
Double-labeling immunofluorescence demonstrating cellular co-localization of the phosphorylated α-synuclein and SMI31 epitopes in swollen neurites in the pontine nucleus (A–C, center) and dot-like structures in the inferior olivary nucleus (D–F, lower right). Phosphorylated α-synuclein appears green (A, D) and SMI31 appears red (B, E). The overlap of phosphorylated α-synuclein and SMI31 appears yellow (C, F) (g glial cytoplasmic inclusion, n neuronal cytoplasmic inclusion). Bar 10 µm
Discussion
In the present study, we demonstrated that α-synuclein accumulated in the following five cellular locations is phosphorylated: oligodendroglial cytoplasm and nucleus, neuronal cytoplasm and nucleus, and neurites. Under normal conditions, the immunoreactivity of α-synuclein has been localized in neurons and presynaptic nerve terminals [10, 17] and α-synuclein has not been identified in neuronal perikarya or glial cells in formalin-fixed tissue sections. However, recent in vitro studies have shown that α-synuclein mRNA and protein are expressed in cultured rat oligodendrocytes and human astrocytes, and that α-synuclein immunoreactivity is observed both in the cytoplasm and nucleus [25, 29]. Mori et al. [19] demonstrated that α-synuclein immunoreactivity was localized in the neuronal perikarya and axons as well as in the cytoplasm of oligodendrocytes and astrocytes in vibratome section of brain tissue taken from normal human subjects. Based on the above findings, it is likely that a significant amount of α-synuclein is present in the neuronal and glial cytoplasm and nucleus in the normal human brain, and that the cytoplasmic and intranuclear α-synuclein both in the neurons and oligodendroglial cells are up-regulated and phosphorylated in MSA. Recently, several investigators demonstrated that extensive phosphorylation of α-synuclein in neurons and glial cells is a highly pathological event, leading to the formation of insoluble aggregates [12, 20, 21, 28]. Hasegawa et al. [7] revealed that the phosphorylated α-synuclein in LB disorders, HSD and MSA is ubiquitinated. However, ubiquitination of α-synuclein is not required for inclusion body formation in α-synucleinopathies [27]. It is unknown whether the ubiquitination of α-synuclein may have some role in the degradation of this protein.
In the present study, phosphorylated α-synuclein immunohistochemistry revealed a novel type of α-synuclein accumulation in MSA; diffuse neuronal cytoplasmic staining was widely distributed in the brain and was more numerous in the predilection sites for NCIs (i.e., pontine and inferior olivary nuclei). Moreover, focal α-synuclein aggregates were frequently found within the diffuse neuronal cytoplasmic staining. In PD and DLB, diffuse cytoplasmic α-synuclein staining is also found in the brainstem pigmented nuclei [26, 31]. The process of LB formation in these areas appears to progress from diffuse neuronal cytoplasmic staining to focal cytoplasmic aggregates (corresponding to pale bodies), and then to typical LBs. Therefore, diffuse neuronal cytoplasmic staining found in MSA is considered to represent an early stage of NCI formation. The process of NCI formation appears to progress from diffuse neuronal cytoplasmic staining to focal cytoplasmic aggregates, and then to typical NCIs.
Recently, Saito et al. [26] revealed three types of phosphorylated α-synuclein lesions in the neuropil in LB disorders: neuropil thread-like structures (Lewy threads); dot-like structures similar to argyrophilic grains (Lewy dots); and swollen axons in the white matter (Lewy axons). We demonstrated swollen neurites, delicate neurites, neuropil threads and dot-like structures in MSA brain. The dot-like structures, neuropil threads, and swollen neurites detected with phosphorylated α-synuclein immunohistochemistry are morphologically analogous to the Lewy dots, Lewy threads and Lewy axons/neurites observed in LB disorders. The neuropil pathology visualized with anti-PSer129 antibody was more widespread than reported previously. These neuritic α-synuclein aggregates might cause impairment of axonal transport and synaptic transmission in MSA.
Autonomic symptoms are one of the cardinal clinical features in MSA [1, 33]. Previous histopathological studies have shown that neuronal loss in the dorsal vagal nucleus, intermediolateral nucleus and Onuf’s nucleus [5, 13, 14, 22] is related to various autonomic symptoms including the orthostatic hypotension and bowel and urinary dysfunctions. In addition, peripheral sympathetic ganglia may be involved in patients with MSA clinically diagnosed as having pure autonomic failure [8]. In the present study, obvious neuronal loss was not noted in the sympathetic ganglia in MSA cases by conventional histopathological examination. Although small in number, NCIs were found in the sympathetic ganglia in two out of eight cases. To our knowledge, this is the first demonstration of abnormal α-synuclein aggregation outside the central nervous system in MSA. Although α-synuclein immunoreactivity has been reported to be present in normal Schwann cells [18], no α-synuclein aggregates were found in these cells in the sympathetic ganglia in MSA. These findings suggest that glial cells in the peripheral nervous system are not involved in the disease process of MSA.
In conclusion, widespread accumulation of phosphorylated α-synuclein in both glial and neuronal cells is a pathological feature in patients with MSA. Anti-phosphorylated α-synuclein immunohistochemistry is a useful tool to evaluate the cytoplasmic and neuropil pathology in α-synucleinopathies.
References
Bannister R, Oppenheimer DR (1972) Degenerative diseases of the nervous system associated with autonomic failure. Brain 95:457–474
Duda JE, Lee VM, Trojanowski JQ (2000) Neuropathology of synuclein aggregates. J Neurosci Res 61:121–127
Forman MS, Schmidt ML, Kasturi S, Perl DP, Lee VM-Y, Trojanowski JQ (2002) Tau and α-synuclein pathology in amygdala of parkinsonism-dementia complex patients of Guam. Am J Pathol 160:1725–1731
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg S, Shen J, Takio K, Iwatsubo T (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164
Gray F, Vincent D, Hauw JJ (1988) Quantitative study of lateral horn cells in 15 cases of multiple system atrophy. Acta Neuropathol (Berl) 75:513–518
Hamilton RL (2000) Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol 10:378–384
Hasegawa M, Fujiwara H, Nonaka T, Wakabayashi K, Takahashi H, Lee VM-Y, Trojanowski JQ, Mann D, Iwatsubo T (2002) Phosphorylated α-synuclein is ubiquitinated in α-synucleinopathy lesions. J Biol Chem 277:49071–49076
Hishikawa N, Hashizume Y, Yoshida M, Hirayama M, Sobue G (2002) Neuropathology of pure autonomic failure. Neurol Med (Tokyo) 57:35–43
Hishikawa N, Hashizume Y, Ujihara N, Okada Y, Yoshida M, Sobue G (2003) α-Synuclein-positive structures in association with diffuse neurofibrillary tangles with calcification. Neuropathol Appl Neurobiol 29:280–287
Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, Rohan de Silva HA, Kittel A, Saitoh T (1995) The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14:467–475
Jellinger KA (2003) α-Synuclein pathology in Parkinson’s and Alzheimer’s disease brain: incidence and topographic distribution—a pilot study. Acta Neuropathol 106:191–201
Kahle PJ, Neumann M, Ozmen L, Müller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C (2002) Hyperphosphorylation and insolubility of α-synuclein in transgenic mouse oligodendrocytes. EMBO Rep 3:583–588
Kennedy PGE, Duchen LW (1985) A quantitative study of intermediolateral column cells in motor neuron disease and the Shy-Drager syndrome. J Neurol Neurosurg Psychiatry 48:1103–1106
Konno H, Yamamoto T, Iwasaki Y, Iizuka H (1986) Shy-Drager syndrome and amyotrophic lateral sclerosis. Cytoarchitectonic and morphometric studies of sacral autonomic neurons. J Neurol Sci 73:193–204
Lippa CF, Fujiwara H, Mann DMA, Giasson B, Baba M, Schmidt ML, Nee LE, O’Connell B, Pollen DA, St. George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VM-Y, Iwatsubo T, Trojanowski JQ (1998) Lewy bodies contain altered α-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 153:1365–1370
Lippa CF, Schmidt M, Lee VM, Trojanowski JQ (1999) Antibodies to α-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol 45:353–357
Marouteaux L, Campanelli JT, Scheller RH (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8:2804–2815
Mori F, Inenaga C, Yoshimoto M, Umezu H, Tanaka R, Takahashi H, Wakabayashi K (2002) α-Synuclein immunoreactivity in normal and neoplastic Schwann cells. Acta Neuropathol 103:145–151
Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K (2002) Demonstration of α-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and fromic acid pretreatment. Exp Neurol 176:98–104
Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Müller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C (2002) Misfolded proteinase K-resistant hyperphosphorylated α-synuclein in aged transgenic mice with locomotor deterioration and in human α-synucleinopathies. J Clin Invest 110:1429–1439
Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, Meijer L, Kahle PJ, Haass C (2000) Constitutive phosphorylation of the Parkinson’s disease associated α-synuclein. J Biol Chem 275:390–397
Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94:79–100
Parkkinen L, Soininen H, Alafuzoff I (2003) Regional distribution of α-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol 62:363–367
Piao Y-S, Mori F, Hayashi S, Tanji K, Yoshimoto M, Kakita A, Wakabayashi K, Takahashi H (2003) α-Synuclein pathology affecting Bergmann glia of the cerebellum in patients with α-synucleinopathies. Acta Neuropathol 105:403–409
Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM-Y (2000) α-Synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res 62:9–14
Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, Arai T, Nagura H, Yamanouchi H, Hasegawa M, Iwatsubo T, Murayama S (2003) Accumulation of phosphorylated α-synuclein in aging human brain. J Neuropathol Exp Neurol 62:644–654
Sampathu DM, Giasson BI, Pawlyk AC, Trojanowski JQ, Lee VM-Y (2003) Ubiquitination of α-synuclein is not required for formation of pathological inclusions in α-synucleinopathies. Am J Pathol 163:91–100
Takahashi M, Kanuka H, Fujiwara H, Koyama A, Hasegawa M, Miura M, Iwatsubo T (2003) Phosphorylation of α-synuclein characteristic of synucleinopathy lesions is recapitulated in α-synuclein transgenic Dorosophilia. Neurosci Lett 336:155–158
Tanji K, Imaizumi T, Yoshida H, Mori F, Yoshimoto M, Satoh K, Wakabayashi K (2001) Expression of α-synuclein in a human glioma cell line and its up-regulation by interleukin-1β. Neuroreport 12:1909–1912
Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM (1998) Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol 44:415–422
Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H (1998) Accumulation of α-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol 96:445–452
Wakabayashi K, Yoshimoto M, Fukushima T, Koide R, Horikawa Y, Morita T, Takahashi H (1999) Widespread occurrence of α-synuclein/NACP-immunoreactive neuronal inclusions in juvenile and adult-onset Hallervorden-Spatz disease with Lewy bodies. Neuropathol Appl Neurobiol 25:363–368
Wenning GK, Shlomo YB, Magalhaes M, Daniel SE, Quinn NP (1994) Clinical features and natural history of multiple system atrophy: an analysis of 100 cases. Brain 117:835–845
Yamazaki M, Arai Y, Baba M, Iwatsubo T, Mori O, Katayama Y, Oyanagi K (2000) α-Synuclein inclusions in amygdala in the brains of patients with the parkinsonism-dementia complex of Guam. J Neuropathol Exp Neurol 59:585–591
Yokota O, Terada S, Ishizu H, Tsuchiya K, Kitamura Y, Ikeda K, Ueda K, Kuroda S (2002) NACP/α-synuclein immunoreactivity in diffuse neurofibrillary tangles with calcification (DNCT). Acta Neuropathol 104:333–341
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and grants from The Naito Foundation of Japan, The Karoji Memorial Foundation for Medical Research, and The Uehara Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishie, M., Mori, F., Fujiwara, H. et al. Accumulation of phosphorylated α-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol 107, 292–298 (2004). https://doi.org/10.1007/s00401-003-0811-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0811-1