Abstract
Aims
We report our experience concerning lead performance and re-surgery rate of the Medtronic EnRhythm MRI SureScan pacemaker system (MRI-PM) in comparison to standard pacemaker (PM) systems and leads used at our institution.
Methods
All patients (except patients with transvenous left ventricular leads) with successful PM implantation performed at our institution from 1 March 2009 to 31 October 2009 were included in this analysis and followed until mid January 2010. Lead measurements (assessed at implantation, prehospital discharge interrogation (1st follow-up) and at the first scheduled out-patient follow-up (2nd follow-up) were compared between atrial leads 4592–53 cm and 5086MRI–52 cm (lead group 1), and between ventricular leads 4092–58 cm and 5086MRI–52 cm/-58 cm (lead group 2), respectively. Causes for re-operations were assessed and compared between patients with standard dual chamber PM (DC-PM) and the MRI-PM.
Results
A total of 140 patients (VVI-PM: 36 patients; DDD-PM: 102 patients; biventricular PM: 1 patient) were successfully implanted with a PM within the implantation period. Two patients with transvenous left ventricular leads were excluded from further analysis. In an atrial position, lead 4592 was implanted in 51 patients and lead 5086MRI–52 cm was implanted in 40 patients, respectively. Ventricular leads were lead 4092–58 cm (64 patients) and lead 5086MRI (41 patients), respectively. Patients were followed for 26 ± 11 weeks. Comparison of lead measurements of lead group 1 showed significant differences for pacing impedance and pacing threshold at implantation, and for sensing at the 2nd follow-up. Comparison of lead measurements within lead group 2 showed significant differences for pacing impedance at implantation, for pacing threshold at the 1st follow-up, and for sensing, pacing threshold, and impedance at the 2nd follow-up. All assessed mean values were favorable for all leads at any follow-up. The number of re-operations was high in both dual chamber PM groups, but did not differ significantly between the two groups (DC-PM: 5 patients, 8.5%; MRI-PM: 5 patients, 13.2%).
Conclusion
Our study demonstrates favorable lead measurements of lead model 5086MRI in comparison to lead 4592 and 4092 in a short-term follow-up. The number of re-operations was higher in the MRI-PM group, but not statistically different in comparison with the standard dual chamber PM group.
Zusammnefassung
Hintergrund
Wir berichten unsere initiale Erfahrung hinsichtlich Elektrodenverhalten und Reoperationsrate des Medtronic EnRhythm MRI Sure Scan Schrittmachersystems im Vergleich zu den an unserer Abteilung benützten Standardschrittmachersystemen und -elektroden.
Methoden
Alle Patienten mit erfolgreicher Schrittmacherimplantation (außer jenen mit transvenösen linksventrikulären Elektroden) an unserer Abteilung zwischen 01.03.2009 und 31.10.2009 wurden in diese retrospektive Studie eingeschlossen und bis Mitte Januar 2010 nachbeobachtet. Elektrodenmesswerte (erhoben bei Implantation, vor Entlassung [1. Kontrolle] und bei der 4- bis 6-Wochen-Kontrolle [2. Kontrolle]) wurden verglichen zwischen den atrial implantierten Elektroden 4592–53 und 5086MRI–52 (Elektrodengruppe 1) und den ventrikulär implantierten Elektroden 4092–58 und 5086MRI–52/58 (Elektrodengruppe 2). Gründe für Reoperationen wurden ebenfalls erhoben und zwischen den Patienten mit Standard-DDD-Schrittmachern (DDD-SM) sowie den Patienten mit dem EnRhythm MRI SureScan Schrittmachersystem (MRI-SM) verglichen.
Ergebnisse
Im Implantationszeitraum wurde 140 Patienten erfolgreich ein Schrittmachersystem (VVI-SM: 36; DDD-SM: 102; biv. SM: 1) implantiert. Zwei Patienten mit transvenösen linksventrikulären Elektroden wurden von der weiteren Analyse ausgeschlossen. Rechtsatrial wurde das Elektrodenmodell 4592–53 bei 51 Patienten implantiert, das Modell 5086MRI–52 bei 40 Patienten. Elektrodenmodell 4092–58 wurde bei 64 Patienten rechtsventrikulär implantiert, Elektrodenmodell 5086MRI bei 41 Patienten. Die Nachbeobachtungszeit betrug 26 ± 11 Wochen. Beim Vergleich der Elektrodenmesswerte von Elektrodengruppe 1 zeigten sich signifikante Unterschiede für die Stimulationsimpedanz und Reizschwelle bei Implantation sowie für die Wahrnehmungsamplitude bei der 2. Kontrolle. Der Vergleich der Elektrodenmesswerte der Elektrodengruppe 2 zeigte signifikante Unterschiede für die Stimulationsimpedanz bei Implantation, für die Reizschwelle bei der 1. Kontrolle sowie für alle drei Parameter (Wahrnehmungsamplitude, Reizschwelle, Stimulationsimpedanz) bei der 2. Kontrolle. Insgesamt waren alle Elektrodenmesswerte bei jeder Kontrolle für alle Elektroden akzeptabel. Die Anzahl der Reoperationen war in beiden DDD-Schrittmachergruppen hoch, jedoch bestand kein signifikanter Unterschied (DDD-SM: 5 Patienten, 8,5%; MRI-SM: 5 Patienten, 13,2%).
Schlußfolgerung
Unsere Studie zeigt in dem kurzen Beobachtungszeitraum akzeptable Elektrodenmesswerte für die 5086MRI-Elektroden im Vergleich mit den Elektrodenmodellen 4595 und 4092. Die Anzahl der Reoperationen war in der MRI-SM-Gruppe zwar höher, der Unterschied war jedoch statistisch nicht signifikant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Magnet resonance imaging (MRI) has become an important diagnostic tool for diagnosis and therapy control of certain diseases [1]. Patients with cardiac implantable electronic devices (CIED) such as permanent pacemakers and implantable defibrillators are usually excluded from participation of these developments since the majority of implantable devices have a contraindication for MRI [2, 3]. Some larger series with pacemaker patients who underwent MRI suggest an acceptable risk–benefit ratio by taking MRI and pacemaker-related precautions. [4, 5] Recently, Roguin et al. [2] summarized the potential adverse effects of magnet resonance on pacemakers in their position paper on “Magnet resonance imaging in individuals with cardiovascular implantable devices.” Potential adverse effects include direct interference between the magnetic field and the hardware (e.g. mechanical forces on ferromagnetic components, activation of reed switch with consecutive asynchronous pacing, pacemaker reprogramming or reset, heating of leads with consecutive heating and possible damage of surrounding cardiac tissue), as well as due to magnet resonance-induced artifact oversensing or undersensing (e.g., rapid atrial pacing, radio frequency induced rapid ventricular pacing, inhibition of pacing) [2]. Application of MRI in patients with CIEDs not compatible to MRI had remained controversial. The decision to perform MRI in individuals with CIEDs must be made on a case-by-case basis and—if performed—requires certain precautions [2, 4, 5].
At the end of 2008, the discussion concerning MRI in individuals with pacemakers changed, since a newly designed pacemaker system (EnRhythm MRI™ SureScan™ and CapsureFix MRT 5086MRI leads, Medtronic Corp., Minneapolis, MN, USA) eligible for the use with MRI became available in Europe. We report our initial experience concerning lead performance and re-surgery rates in the clinical routine use of this MRI safe dual chamber pacemaker system in comparison to standard pacemakers and leads used at our institution.
Methods
All patients with successful pacemaker implantation at our institution from 1 March 2009 to 31 October 2009 were included in this study. Permanent pacemakers were implanted according with current guidelines [6, 7]. The selection of type and model of a specific pacemaker system was on the decision of the admitting or implanting physician.
Since the purpose of our study was to assess the performance of the magnetic resonance safe lead model CapsureFix MRT 5086MRI in clinical routine, lead specific measurements (assessed at implantation, before hospital discharge, and at the first scheduled out-patient follow-up) were compared between lead model 5086MRI–52 cm and lead model 4592–53 cm (atrial leads; lead group 1), and between lead model 5086MRI–52/58 cm and lead model 4092–58 cm (ventricular leads; lead group 2), respectively. Of note, leads 4592–53 cm and 4092–58 cm are the most commonly used leads at our institution.
The surgical revision rate was compared between the EnRhythm MRI™ SureScan™ dual chamber pacemaker system and all other within the implantation period with different passive fixation leads implanted dual chamber pacemaker systems (“standard dual chamber pacemakers”).
EnRhythm MRI SureScan system
The EnRhythm MRI SureScan system is a dual chamber pacemaker system designed for safe use with magnetic resonance imaging at 1.5 Tesla. This pacemaker system is a modified version of the EnRhythm pacemaker. The system has modified hardware to reduce the level of energy transmitted from the leads to the device. In addition, the device has a programmable SureScan™ feature, with which MRI-related oversensing shall be eliminated when it is activated during MRI scan. Data collection and monitoring functions of the device can be temporarily suspended if the SureScan™ feature is on, whereas asynchronous pacing is provided if needed [8]. The pacemaker carries a radiopaque label, identifying it to be MRI conditional.
The newly designed lead used with the EnRhythm MRI SureScan system is model CapsureFix MRT SureScan 5086MRI, which is a modified version of CapSureFix® Novus model 5076 lead. Lead model 5086MRI is a steroid eluting, bipolar, silicone insulated lead with an active fixation mechanism (helix electrode extension is indicated by a radiopaque indicator). The lead body diameter is 2.3 mm. Available lengths are 45, 52, and 58 cm, respectively [9, 10]. Likewise, lead 5086MRI is also identifiable by a radiopaque label as being MRI conditional.
The major differences to model 5076 is that lead 5086MRI is eligible for use with MRI, based on modified internal wiring composition to decrease the risk of overheating during an MRI scan, and, therefore, to reduce potentially dangerous heating at the leads tip [11]. The diameter of the inner conductor coil of lead 5086MRI is larger than of lead 5076 (0.036” vs. 0.026”) to maintain good torque.
In vitro tests have demonstrated considerably reduced heating of lead 5086MRI in comparison to a standard lead (SureFix® model 5072, Medtronic Inc.) when exposing the leads to MRI at 1.5 Tesla [12]. Meanwhile, safety and effectiveness of the EnRhythm MRI SureScan pacemaker system were proven in an in vivo magnetic resonance environment [13].
Lead 4592–53 cm and 4092–58 cm
The most commonly used leads with permanent pacemakers at our institution are lead models CapSure SP Novus 4592–53 cm for atrial implantation and lead model CapSure SP Novus 4092–58 cm for ventricular implantation. Both leads are steroid eluting, bipolar, passive fixation leads with 5.3 Fr diameter. Lead 4592–53 cm is J-shaped at its distal end, whereas lead 4092 is straight. Neither lead is MRI compatible.
Pacemaker implantation
Implantations were performed in the catheter room by four experienced cardiologists and two colleagues in training. All devices have been placed right or left pectorally. After incision of the skin parallel to the clavicle, access to the subclavian vein was achieved in all cases using Seldinger’s technique. Medtronic 5086MRI leads were introduced via 8 Fr peel-away sheaths, whereas leads 4592–53 cm and 4092–58 cm were introduced using 7 Fr peel-away sheaths.
The leads were then introduced to the right ventricle or the right atrium under fluoroscopic guidance. Preferably, atrial leads were placed at the right atrial appendage. Lead 4092–58 cm usually was placed near the right ventricular apex, while ventricularly used 5086MRI leads were implanted at the right ventricular apex or at the ventricular septum. After confirmation of acceptable values for sensing (mV), pacing threshold (V/0.5 ms), pacing impedance (Ω/5.0 V), and slew rate (V/s) using an external measuring device (AnalyzerTM, Medtronic Inc.), leads were fastened to the surrounding tissue using suture sleeves. Thereafter the leads were connected to the generator, which was then inserted into the preformed pocket (subfascially or subpectorally). Usually, the final generator and lead locations were documented by a short fluoroscopic movie. Finally, the wound was closed layer by layer. The overall X-ray time was documented at the end of the session. In addition, time of day that the surgery was performed was assessed (6 h time periods, beginning at 07:30 a.m.), and if the operating cardiologist was on duty on a normal working day, on a day with night shift or on a day the night shift has ended in the morning. For the purpose of this retrospective study, fluoroscopic movies documenting locations of 5086MRI leads were screened retrospectively for correct helix electrode extension as indicated by the specific radiopaque indicator (Fig. 1).
Radiopaque indicator (A indicator ring; B drive mechanism) of screw condition (top helix extended (gap between A and B); bottom Helix retracted) [10]
Pacemaker follow-up
The first pacemaker interrogation is usually performed 1 day after implantation (1st follow-up) at our institution. The next (out-patient) interrogation (2nd follow-up) is usually scheduled at 4–6 weeks after implantation, then every 12 months. Atrial and ventricular lead measurements (sensing (mV), pacing threshold (V/0.4 ms), and pacing impedance (Ω/5.0 V)) are assessed at every follow-up, and the device’s programming is adjusted to the patient’s needs.
For the purpose of this study, a telephone follow-up was performed by mid January 2010 to assess the status of the patient as well as to exclude external re-surgeries in this patient cohort.
Statistical analysis
Data were collected prospectively and analyzed retrospectively. For the purpose of this study, assessed lead measurements were compared between the leads of group 1 (atrial leads: 4592–53 cm, 5086MRI–52 cm) and between leads of group 2 (ventricular leads: 4092–58 cm, 5086MRI–52/58 cm). Values are expressed as mean ± standard deviation. Continuous variables were compared using the unpaired Student’s t test. Categorical variables were compared using the χ2 test, and the Fisher’s exact test, where appropriate. For comparison of the survival free of surgical revision of any cause, Kaplan–Meier survival curves using the log rank test were calculated for the EnRhythm MRI SureScan systems and standard dual chamber pacemaker systems using different passive fixation leads (patients with a mixture of passive and active fixation leads were excluded from survival analysis). A p < 0.05 was considered significant. All analyses were performed using SPSS for Windows statistical software package (version 17; SPSS, Chicago, IL, USA).
Results
Within the implantation period, 140 patients were successfully implanted with permanent pacemakers at our department. Two patients who had transvenous left ventricular pacing leads (1 patient with a CRT-P, 1 patient with VVI PM) were excluded from further analysis because of differences in implantation technique and potentially higher complication rates with transvenous coronary sinus leads [14]. The demographics of patients included in this analysis are shown in Tab. 1. A total of 36 patients were implanted with VVI PM, 102 patients with dual chamber PM, of whom 39 were implanted with the EnRhythm MRI SureScan system. Indication for pacemaker implantation was in the majority of patients (43%) higher degree AV block (Tab. 1). Thirty-nine patients were implanted with the EnRhythm MRI SureScan system, the remaining with conventional pacemaker systems. Patients implanted with the EnRhythm MRI SureScan system were significantly younger than patients implanted with standard dual chamber pacemakers (72 ± 14 years vs. 79 ± 8 years; p = 0.009). Actually, age was the leading reason for choosing the MRI safe pacemaker system in specific patients of this patient cohort.
In an atrial position, lead model 4592–53 cm was implanted in 51 patients, lead model 5086MRI–52 cm in 40 patients, respectively. In a right ventricular position lead model 4092–58 cm was implanted in 64 patients, lead model 5086MRI–52 cm in 1 patient, and lead model 5086MRI–58 cm in 39 patients (Tab. 1). Statistical analysis of lead measurements assessed at implantation, the 1st follow-up and the 2nd follow-up was restricted only to the leads within lead group 1 and lead group 2.
One patient with an unsuccessful ventricular implantation attempt of a 5086MRI–58 cm lead received a conventional dual chamber pacemaker in combination with a 5086MRI–52 cm lead in an atrial position and a conventional passive fixation lead in a ventricular position. This patient was excluded from statistical analyses comparing standard dual chamber PM with EnRhythm MRI PM. Another patient with a single chamber pacemaker indication was implanted with a 5086MRI–58 cm lead in the ventricular position in combination with a standard VVI pacemaker system for future use of a potentially upcoming MRI suitable VVI pacemaker system manufactured by Medtronic. Eighteen pacemaker implantations were performed by the two colleagues in training.
Patients were followed for a mean of 26 ± 11 weeks. Prehospital discharge interrogation (1st follow-up) was performed 1.3 ± 0.9 days after implantation, the 2nd follow-up 6.6 ± 3.7 weeks after pacemaker implantation.
In 1 patient, the first out-patient pacemaker follow-up was postponed to a later date due to a prolonged hospital stay, and another patient has not observed the scheduled follow-up visit due to his reduced general condition.
Eleven patients (8%; 4 women; age at implantation: 83 ± 6 years; implanted pacemaker system: VVI (n = 6), standard DDD (n = 4), MRI eligible DDD (n = 1)) died within the observational period (mean 8 ± 3 weeks after PM implantation). Causes of death were end-stage heart failure in 3 patients, severe pulmonary disease in 1, end-stage renal failure in 1, major stroke in 1, and multiorgan failure in 1 patient. One patient suffered from sudden cardiac death. The circumstances of death remain unknown in 3 patients.
Lead measurements
Mean values for sensing amplitudes, pacing thresholds and pacing impedances assessed at implantation, prehospital discharge interrogation and at the 2nd follow-up were within clinically accepted ranges and were favorable at any time for all analyzed lead models. Since active fixation 5086MRI leads were compared with passive fixation leads (4592/4092), differences of measured values were expected, but were clinically irrelevant at the end.
Atrial leads
A summary of the assessed measurements of lead 4592 and 5086MRI are given in Tab. 2.
Comparison of lead measurements of lead group 1 showed significant differences for pacing impedance (4592/5086MRI: 486 vs. 659; p < 0.001) and pacing threshold (4592/5086MRI: 0.7 vs. 0.9; p = 0.048) at implantation, and for sensing (4592/5086MRI: 3.3 vs. 2.6; p = 0.34) at the 2nd follow-up. X-ray time within group 1 was not significantly different when comparing the two leads.
With focus on the individual lead, one-way ANOVA showed a significant difference (p = 0.046) for pacing impedance for lead model 4592 from implantation to the 2nd follow-up, based on a significant increase (p < 0.001) of pacing impedance from the 1st follow-up to the 2nd follow-up. Lead 5086MRI showed a significant difference for pacing threshold (p = 0.001) and impedance (p < 0.001) between the follow-ups. The t-test revealed a significant decrease for sensing (p = 0.008), pacing threshold (p < 0.001), and pacing impedance (p < 0.001) from implantation to the 2nd follow-up, and a significant increase of sensing (p = 0.009) and pacing threshold (p < 0.001) from the 1st follow-up to the 2nd follow-up.
Review of fluoroscopic documents of lead positions (not available in 2 patients) showed that in two cases the radiopaque indicator of the atrial 5086MRI lead was not in the position of a properly extended helix (no gap between A and B (Fig. 1, Fig. 5)).
Ventricular leads
A summary of the assessed measurements of lead 4092 and 5086MRI is shown in Tab. 3.
Comparison of lead measurements within lead group 2 showed significant differences for pacing impedance (4092/5086MRI: 844/972; p = 0.041) at implantation, for pacing threshold at the 1st follow-up (4092/5086MRI: 0.5 vs. 0.6; p = 0.016), and for sensing (4092/5086MRI:14.1 vs. 9.9; p ≤ 0.001), pacing threshold (4092/5086MRI: 0.6 vs. 1.00; p = 0.008), and impedance (4092/5086MRI: 714 vs. 560; p < 0.001) at the 2nd follow-up.
X-ray time was statistically not different when comparing ventricular lead models 4092 and 5086MRI, but showed a tendency towards longer X-ray time for lead model 5086MRI.
The individual statistical analysis with one-way ANOVA showed for lead model 4092 significant differences for sensing (p = 0.023), pacing threshold (p = 0.001), and pacing impedance (p = 0.002) between the follow-ups. These differences are based on a significant decrease of pacing threshold (p < 0.001) and of pacing impedance (p < 0.001) between implantation and 1st follow-up, and of a significant increase of sensing (p = 0.02) and pacing threshold (p = 0.002) from the 1st follow-up to the 2nd follow-up.
For ventricular lead 5086MRI, one-way ANOVA revealed significant differences for pacing threshold (p = 0.014) and pacing impedances (p < 0.001) between the follow-ups. There was a significant decrease (p = 0.003) of pacing threshold and of pacing impedance (p < 0.001) from implantation to 1st follow-up. From 1st follow-up to the 2nd follow-up, pacing threshold increased significantly (p = 0.005), pacing impedance decreased significantly (p = 0.001).
Review of fluoroscopic documents of lead positions (not available in 2 patients) showed that in 5 cases the radiopaque indicator of the ventricular 5086MRI lead was not in the position of a properly extended helix (no gap between A and B (Fig. 1)). An example of a properly extended atrial 5086MRI screw and an improperly extended ventricular 5086MRI screw is shown in Fig. 5.
Irrespective the implant procedures performed by the two colleagues in training and implantations preceded by coronary angiography X-ray time differed significantly between both pacemaker systems (standard dual chamber PM: 4.5 ± 3.8 min; EnRhythm MRI: 6.9 ± 5.6 min; p = 0.031).
Re-surgeries
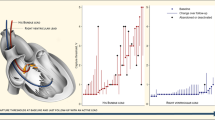
Surgical revisions were necessary in 5 patients of each dual chamber pacemaker group. Worsening of ventricular threshold due to micro or macro dislocation of the ventricular lead led to surgical revision in 5% of all patients (Tab. 4). One patient with a VVI pacemaker suffered from lead dislodgment caused by Twiddler’s syndrome, whereas in 6 patients with dual chamber pacemakers the ventricular lead had to be re-positioned. Four of these patients had been implanted with the EnRhythm MRI SureScan system. Atrial lead dislodgment occurred in 1 patient of each dual chamber pacemaker group. In one case, there was a connection problem with the pacemaker’s port for the IS-1 pin of the ventricular lead. Another patient who suffered from systemic infection with multiple vegetations on the leads had to be explanted. The overall revision rate was 8%. The log rank test revealed no statistically significant difference in survival free of surgical revision between standard dual chamber pacemakers (n = 58) and the EnRhythm MRI SureScan system (n = 39) as well as for lead groups 1 and 2 (Fig. 2, Fig. 3, Fig. 4). The time of day that the surgery was performed and the type of duty the operating cardiologist had had no statistically significant impact on survival free of re-surgery. In univariate analysis, only the indication for pacemaker implantation and the operating cardiologist was a predictor for re-surgery. In fact, one of the two colleagues in training for pacemaker implantations was responsible for five re-surgeries (33% of PM implantations performed by this colleague).
Discussion
The new lead model 5086MRI showed stable lead measurements in short-term follow-up. The differences in designs of active and passive fixation leads are suggested to be responsible for the significant differences in lead measurements observed within this patient cohort [15, 16]. Of note, the differences in measured mean values for sensing amplitudes, pacing thresholds, and pacing impedances were clinically not relevant and were within clinically accepted ranges and were favorable at any time for all three analyzed lead models.
From a statistical point of view, the rate of surgical revision of the new EnRhythm MRI SureScan system is statistically not different in comparison to standard pacemaker systems used with passive fixation atrial and ventricular leads at our institution. In fact, 13.2% of implanted EnRhythm MR SureScan systems underwent surgical revision because of lead dislodgements (n = 5, 6.7% of all leads implanted with EnRhythm MRI SureScan PM), whereas “only” 5.1% (n = 3, 2.5% of all leads implanted with standard dual chamber PM) did.
Since standard leads at our institution are usually passive fixation leads for atrial and ventricular pacing, some handling problems with lead 5086MRI reported by all implanting physicians—as well as the re-surgery rate—may be seen as a consequence of lacking experience with implantation of active fixation leads. The total percentage of re-operations considerably exceeds previously reported rates of re-surgeries after pacemaker implantation [14, 15, 16, 17, 18, 19, 20].
Retrospective review of the fluoroscopic movies of the final lead positions brought to light that in 7 of the 5086MRI leads (6 patients, 15% of patients implanted with EnRhythm MRI PM; atrial lead: 2 patients, 5%; ventricular lead: 5 patients, 12.5%) the helix electrode was not properly extended (in all cases no gap between A and B (Fig. 1, Fig. 5)). Interestingly, none of the affected leads had to be revised within the observational period of this study (nor thereafter).
Five of the 11 re-surgeries (2 in patients with the EnRhythm MRI SureScan systems) had to be indicated after first implantation performed by one of the colleagues in training for pacemaker implantation. Inexperienced operators were a significant predictor for lead complications in a recently published large population-based cohort study [14]. Disregarding the pacemaker implantations performed by this colleague, the over-all re-surgery rate would have been 4%, and 8% for the EnRhythm MRI SureScan system, respectively.
Eight of the 11 patients with pacemaker related re-surgeries were initially implanted within the first half of the implantation period, reflecting a learning curve for pacemaker implantation in general as well as for experienced implanters using leads they are unfamiliar with.
The findings of this study may be seen as an internal quality assessment and may raise some questions. First of all, why this complication rate happened without obvious consequences. It is probably the result of a lacking alert system, based on a real time quality assessment. The second question is whether the training for pacemaker implantations is properly structured. Third, why a new active fixation lead (with handling differences even to other active fixation leads) is introduced without appropriate training at an implantation center usually using passive fixation leads.
Limitations
The major limitation of this study is the small patient number; therefore, statistics have only limited power. Another limitation may be or may be not the high revision rate produced by one implanting cardiologist. The extraordinary high revision rate may lead to distortion of reality. But, actually, it was the feeling of something is going wrong that triggered this analysis—including its uncomfortable findings.
Conclusion
Our results demonstrate favorable lead measurements of atrial and ventricular implanted lead models 5086MRI until the first follow-up after hospital discharge.
The rate of re-surgeries after implantation of the Medtronic EnRhythm MRI SureScan system is high, but is statistically not different compared to standard dual chamber pacemakers at our institution.
So far, implanting cardiologists and surgeons usually using passive fixation leads should be aware of differences in handling the CapsureFix MRI 5086MRI leads, especially when implanted in a ventricular location. It is strongly recommended to consider lead implantation recommendations for lead model 5086MRI given by the manufacturer [9, 10].
References
American College of Radiology (2011) ACR Practice Guidelines, http://www. acr.org. Accessed 20 Nov 2011
Roguin A, Schwitter J, Vahlhaus C et al (2008) Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace 10:336–346
Kalin R, Stanton MS (2005) Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 28:326–328
Sommer T, Naehle CP, Yang A et al (2006) Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 Tesla in the presence of cardiac pacemakers in non-pacemaker dependent patients. A prospective study with 115 examinations. Circulation 114:1285–1292
Nazarian S, Roguin A, Zviman MM et al (2006) Clinicial utility of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 Tesla. Circulation 114:1277–1284
Vardas PE, Auricchio A, Blanc JJ et al (2007) Guidelines for cardiac pacing and cardiac resynchronization therapy. The task force for cardiac pacing and cardiac resynchronization therapy of the European society of cardiology. Developed in collaboration with the European heart rhythm association. Eur Heart J 28:2256–2295
Epstein AE, DiMarco JP, Ellenbogen KA et al (2008) American college of cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American college of cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 guideline update for implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 51(21):e1–e62
Surescan™ (2008) Technical manual. Medtronic Inc (http://www.medtronic.com/manuals). Accessed 20 Nov 2011
ENRHYTHM MRI™ SURESCAN EMDR01 (2008) Reference manual. Medtronic Inc (http://www.medtronic.com/manuals). Accessed 20 Nov 2011
CAPSUREFIX MRI™ SURESCAN™ 5086MRI (2009) Manual. Medtronic Inc (http://www.medtronic.com/manuals). Accessed 20 Nov 2011
Medtronic News Release 2008. http://www.medtronic.com. Accessed 20 Nov 2011
Nitschke T, Heuer A, Scheffold T, Heuer H (2009) In-Vitro-Vergleich der Temperaturentwicklung einer MRT-tauglichen Schrittmachersonde im Vergleich mit einer konventionellen Schrittmachersonde im 1,5 Tesla Magnetresonanztomographen. Clin Res Cardiol 98(Suppl 2)
Wilkoff BL, Bello D, Taborsky M et al (2011) Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm 8:65–73
Kirkfeldt RE, Johansen JB, Nohr EA et al (2011) Risk factors for lead complications in cardiac pacing: a population-based cohort study of 28,860 Danish Patients. Heart Rhythm 8:1622–1628
Luria DM, Feinberg MS, Gurevitz OT et al (2007) Randomized comparison of J-shaped atrial leads with and without active fixation mechanism. Pacing Clin Electrophysiol 30:412–417
Ellenbogen KA, Hellkamp AS, Wilkoff BL et al (2003) Complications arising after implantation of DDD pacemakers: the MOST experience. Am J Cardiol 92(6):740–741
Glikson M, Hyberger LK, Hitzke MK et al (1999) Clinical surveillance of a tined, bipolar, J-shaped, steroid-eluting, silicone-insulated atrial pacing lead. Pacing Clin Electrophysiol 22:1079–1081
Fleck T, Khazen C, Wolner E, Grabenwöger M (2006) The incidence of reoperations in pacemaker recipients. Heart Surg Forum 2006
Markewitz A (2009) Annual report 2007 of the German pacemaker registry. Expert group pacemakers and the National Institute for Quality in Health Care (BQS Bundesgeschäftsstelle Qualitätssicherung gGmbH; managing director: Dr. C. Veit), Düsseldorf. Herzschrittmacherther Elektrophysiol 20:191–218
DPR (2011) Danish Pacemaker Register. Department of Cardiology, Odense University Hospital, Denmark (http://www.pacemaker.dk)
Conflict of interest
The corresponding author states the following: Dr. C.G. Wollmann: Biotronik, Boston Scientific, St. Jude Medical (consulting); Dr. H. Mayr: Medtronic (consulting).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wollmann, C., Thudt, K., Vock, P. et al. Clinical routine implantation of a dual chamber pacemaker system designed for safe use with MRI. Herzschr. Elektrophys. 22, 233–242 (2011). https://doi.org/10.1007/s00399-011-0161-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00399-011-0161-y