Abstract
The relationship between particle dynamics in a suspension drop during drying and final coffee ring patterns was investigated using suspension systems with spherical and non-spherical ellipsoidal particles. Employing multi-speckle diffusing wave spectroscopy (MSDWS), fast particle Brownian motions in suspension drops containing spherical and ellipsoidal particles were quantitatively compared during the drying process in real time. From the autocorrelation function data and characteristic times for β-relaxation, we confirmed that ellipsoidal particles move more slowly than spherical particles in a suspension drop due to their structural factor. The resulting coffee ring patterns by spherical and ellipsoidal particles are clearly distinguishable from one another and are dependent upon particulate concentration, initial drop volume, and particle shape. Notably, ellipsoidal particles in a suspension drop form coffee rings less readily than spherical particles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding particle motions during solvent evaporation in colloidal suspensions containing particles is important in various academic and industrial fields such as DNA stretching [1,2,3], colloidal self-assembly [4,5,6], electronic materials [7, 8], inkjet printing [9,10,11], and patterning of nanoparticles [12]. The evaporation of a suspension drop with dispersed particles takes place under complex dynamics. Generally, when a particulate suspension drop is dried on a solid substrate, annular deposits form naturally around the drop boundary; these deposits are colloquially called “coffee-rings or coffee-stains” [13, 14]. The coffee ring phenomenon can be explained by a non-uniform evaporation flux that induces fluid flow by capillary action to compensate for the evaporated solvent loss from the drop edge [14, 15]. Simultaneously, the dispersed particles in the liquid move to the edge, due to the fluid flow, and accumulate to form a dried ring pattern [8, 12, 15,16,17,18]. The final drying pattern of a suspension drop is affected by various parameters such as solute-substrate interaction, bulk flow [19, 20], and particle shape [21,22,23]. The patterning mechanism related to the particle shape has practical implications in material technologies that utilize colloidal assemblies [24]. Yunker et al. [21] eloquently analyzed the coffee ring formation of a drop containing colloidal ellipsoids using a confocal microscope by considering both the micro-rheological and physical aspects of complex suspension systems. It was found that the ellipsoidal particles in the drop generally do not migrate toward the edge due to strong long-ranged interparticle attractions and thus loosely packed structures on the air-water interface. Kim et al. [22] scrutinized the suppression of coffee ring effect by ellipsoidal particles in a suspension drop using the ratio of capillary and hydrodynamic forces. Dugyala et al. [23] reported that it is possible to characterize the deposition pattern by tuning the particle aspect ratio and particle-substrate interactions using a liquid model system with monodisperse particles.
The tendency of ellipsoidal particles to not accumulate on the contact line is due to the capillary bonding network structure formed on the suspension surface [21,22,23]. The network layer of agglomerated particles on the suspension surface significantly changes the mechanical response at the air-liquid interface. The ellipsoidal particles exhibit much greater elastic and loss moduli at the surface of the suspension drops for spherical particles in similar surface ranges [25]. Network structures and drying patterns inside a suspension are dependent upon the composition of particles and micro-rheological properties of particulate suspensions during the drying process.
Various methods have been developed and tested to elucidate the properties of colloidal suspensions during drying. Typical methodologies include confocal microscopy [26], echo speckle imaging (ESI) [27], and multi-speckle diffusing wave spectroscopy (MSDWS) based on multiple scattering [28,29,30,31]. Lee et al. [30] employed a line scan camera with a high frame rate, instead of a CCD camera, in performing MSDWS to analyze the drying dynamics of particulate suspension drops and relaxation features of the fast Brownian motion of particles. Oh et al. [31] studied the dynamic motion of bimodal particles in a suspension drop during drying by examining autocorrelation function data obtained by a line scan camera for fast β-relaxation and a CCD camera for slow α-relaxation using MSDWS. α-relaxation for slow particle dynamics is associated with the structural reconstruction of particles, while β-relaxation for fast particle motions is related to the Brownian motion of particles [32,33,34,35,36,37]. The aforementioned studies primarily focused on unimodal or bimodal spherical particles. There have been few reports on the particle dynamics and relaxation of non-spherical (e.g., ellipsoidal) particulate suspensions.
In this study, we clarified the relationship between coffee ring formation and particle dynamics in suspension systems with spherical and ellipsoidal particles during drying using MSDWS, a light scattering technique. The temporal changes of particle motions in a non-ergodic system were successfully quantified using MSDWS. The fast motions of spherical and ellipsoidal particles during drying were thoroughly examined from autocorrelation data obtained by a line scan camera.
Methods
In this study, we employed a drying process in which a suspension drop containing spherical or ellipsoidal particles was evaporated onto a glass slide. We used two kinds of particles: spherical particles with an average diameter of 2 μm and an aspect ratio of 1 and ellipsoidal particles with an aspect ratio of 6 created via the uniaxial elongation of the spherical particles.
Preparation of spherical and ellipsoidal particulate suspensions
Spherical polystyrene (PS) particles, 2 μm in diameter, were synthesized using dispersion polymerization, following procedures described in previous studies [30, 31]. Briefly, 40 g of styrene and 1.6 g of polyvinyl pyrrolidone (MW = 1,300,000 g/mol) were combined inside a 500-mL glass reactor containing 200 g of isopropanol and 10 g of water. Polymerization was carried out in the presence of 0.4 g of 2,2′-azobisisobutyronitrile at 70 °C while the mixture was stirred at 120 rpm for 24 h. The ellipsoidal PS particles with an aspect ratio of 6 were prepared by embedding the spherical PS particles in a prefabricated poly(vinyl alcohol) film, using a modified version of the film stretching method introduced in Ahn et al. [38]. The aspect ratio is defined as the length ratio of the major axis to the minor axis of a particle. The particle shape and aspect ratio were confirmed using scanning electron microscopy (SEM, JSM 5200, Jeol, Japan) (Fig. 1). Two sets of aqueous suspensions with low particle concentrations (1 and 2 wt%) were produced to clearly observe the particle motion within the suspension drop.
Measurement of contact angles for suspension drops
The contact angle at the pinned contact line of the suspension drop was measured in conjunction with the drying time to compare the change in transient bulk flow for two types of suspension systems with spherical and ellipsoidal particles. Changes in the contact angle are related to the pinning feature of contact line and outward radial flow during drying [19, 38]. The contact angles of suspension drops, approximately 1 μL in volume, were measured using a contact angle measurement system (Phoenix 300 Touch, SEO Co. Ltd.) at various drying times.
3D profile patterns of dried suspension drops
Three-dimensional (3D) changes in completely dried patterns of suspension drops were quantified using an optical surface profilometer (NanoSystem NV-3000 equipped with NanoView software, NanoSystem Co. Ltd., Korea). Coffee ring shapes were created using various volume sizes (0.5, 1.0, and 2.0 μL) and concentrations (1 and 2 wt%) of drops with spherical and ellipsoidal particles.
Multi-speckle diffusing wave spectroscopy (MSDWS)
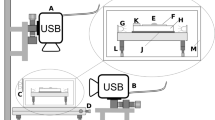
The drying process of the suspension drops was characterized via MSDWS equipped with a laser and CMOS line scan camera (Basler Vision Technologies, spL-4096-39 km, Germany, 38.5 kHz frame rate). The laser source (Jinsung Laser, DPGL-2200, Korea) emitted a 1-mm-diameter, temperature-stabilized green laser with a wavelength of 532 nm. The drying temperature and humidity were maintained at 30 ± 0.3 °C on a hot plate (HP-20D, Witeg, Germany) and 50 ± 2%, respectively. For this test, 1 μL suspension drops with different particle concentrations (1 and 2 wt%) were laid onto the plate and then dried to measure the particle dynamics via MSDWS. The detailed procedure for MSDWS testing is described in previous studies [30, 31].
The correlation of the laser intensity at a certain time-point and a later time-point (i.e., a lag time) can be evaluated from the ensemble average of N speckles in the form of the autocorrelation function, g 2 −1 (Eq. (1)),
where, g 2 −1 denotes the autocorrelation function, I n is the light intensity at the nth pixel, 〈⋯〉 n is the ensemble average over the pixels, t w is the initial measuring time, and τ is the lag time relative to t w . The scale of the autocorrelation function is normalized to have a minimum value of 0 and a maximum value of 1. The drying characteristics of suspension drops with spherical or ellipsoidal particles can be determined by analyzing patterns in the resultant autocorrelation function. One microliter of a suspension was dropped onto slide glass on the hot plate with constant temperature (30 ± 0.3 °C.). Then, laser light illuminated the suspension drop and the CMOS line scan camera captured the light scatter images. The horizontal line obtained from the images contained the brightness (ranging from intensity values of 0 (complete darkness) to 255 (maximum brightness)) of N pixels at a specific lag time. A total of 4096 pixels were used to evaluate the autocorrelation function for fast particle motion. The intensity data were captured at every minute for a lag time of 0.1 s during the drop drying. The fast relaxation (β-relaxation) of each suspension drop was quantified as the characteristic time at which the autocorrelation function decreased to a value of 0.6 [30, 31]. Figure 2 shows a schematic of an MSDWS experiment and the morphological change of a suspension drop during drying.
Results and discussion
Contact angles of particulate suspension drops during drying
The drying patterns of suspension drops with spherical and ellipsoidal particles were first compared by considering changes in their contact angles during drying. Side views of suspension drops, consisting of different particles at different concentrations, throughout the drying process are illustrated in Fig. 3. The contact angle at the nearly pinned contact line gradually decreased along the evaporation time for all suspension cases (Fig. 3a). The position of the contact line did not appreciably shrink from its initial state during drying of suspension drops [19, 21], because the particles moved toward the edge of the drop as explained by the coffee ring mechanism. The contact angles of suspension drops with ellipsoidal particles were relatively lower than those with spherical particles (Fig. 3b). Note that suspension drops with ellipsoidal particles showed slightly different contact angle values at two concentrations, whereas contact angles for cases with spherical particles were very similar.
Structural analysis of dried patterns of suspension drops with spherical and ellipsoidal particles
The final patterns of suspension drops under various conditions, such as particle shape, concentration, and drop size, were visualized using a 3D profiler. Figure 4 displays the overall coffee ring patterns for 1 μL suspension drops with spherical and ellipsoidal particles at different concentrations. (Dried pattern images for suspension drops at different initial volumes can be seen in the supplementary materials.) The top-down and cross-sectional images shown in Fig. 4 were used to quantify the coffee rings’ dimensions such as average diameter, peripheral ring width, and ring height (Fig. 5). Spherical particles formed thicker and higher rings than ellipsoidal particles (Fig. 5b, c). These images clearly substantiate the suppression of coffee ring formation by ellipsoidal particles in a suspension drop.
SEM images (Fig. 6) of evaporated suspension drops show the aggregated particles that form coffee rings. We verified the structural difference between the inside and the edge of dried drops by considering the height data of the 3D profiler and the cross-sectional SEM images. In coffee rings formed by spherical (or ellipsoidal) particles, we observed that the inner region of a drop had fewer layers of particles than the edge region, which had more layers consisting of spherical (or ellipsoidal) particles. Also, ellipsoidal particles generally form loosely packed structure in comparison to the spherical case, making the surface drag and bulk drag noticeably different in terms of Boussinesq number, as notably mentioned in Yunker et al. [21]
Characterization of ellipsoidal and spherical particle motions in the evaporating drop
In this study, fast motions of spherical or ellipsoidal particles in a suspension drop were investigated using MSDWS during drying. The autocorrelation functions of the initial 1 μL suspension drops with spherical and ellipsoidal particles of 1 and 2 wt% were measured at a constant temperature (30 ± 0.3 °C) using MSDWS with a CMOS line scan camera. Figure 7 shows the autocorrelation function (g 2 −1) data for suspension drops with spherical (Fig. 7a, b) and ellipsoidal particles (Fig. 7c, d) at different concentrations within a very short lag regime, as the drying progresses. In the initial drying stage, the autocorrelation function rapidly decreases with increasing lag time, implying that the correlation between particles at the initial drying stage abruptly disappears due to their fast Brownian motion. The autocorrelation function gradually increases with lag time for longer drying times, and finally, the autocorrelation function does not change at the completion of the drying time, around 11 min. Densely packed particles during the evaporation of solvent water result in an increase in the magnitude of the autocorrelation function, addressing a strong correlation between particles. The correlation between ellipsoidal particles is more slowly relaxed than that between spherical particles at the same concentration due to the slower motion of ellipsoidal particles compared to spherical particles.
The β-relaxation time of the autocorrelation function was quantified by determining the characteristic time when the autocorrelation function decreased to a specified value (i.e., 0.6 [30, 31]). Figure 8 displays the relationship between the characteristic time for the β-relaxation of suspension drops with different concentrations and particle shapes and the drying time. The characteristic time gradually increases during drying, indicating that time required to be relaxed to a specified state increases with increasing drying time. An increase in the characteristic time means that the movement of the particles is not fast due to the continuous packing of particles during drying. We confirmed from the comparison of characteristic times that the ellipsoidal particles were slower than the spherical particles under the same conditions.
Characteristic times for β-relaxation of suspension drops with spherical and ellipsoidal particles, using autocorrelation function data of Fig. 7
Spherical particles undergo random motion, which is only affected by the particle size, whereas, ellipsoidal particles have more complex dynamics due to their anisotropic shape and spatial-dependent drag [39]. The shape of ellipsoidal particles results in locally variable drag coefficients and reduced motion, causing motion to primarily occur in the direction parallel to each particle’s major axis rather than in the direction perpendicular to the major axis. Also, self-assembled ellipsoidal particles prevent the transport of particles to the periphery of the air-water interface due to their shape-induced capillary interaction [21, 25]. The locally variable movement of ellipsoidal particles, as can be seen from light scattering experiments, can affect the formation of coffee rings.
In our experiments, relatively low suspension concentrations (1 or 2 wt%) were utilized to allow for more reliable measurements of the particle movement in suspension drops during the course of coffee ring formation. For spherical particles, more particles move from the center of the drop to the side due to capillary action, making larger coffee rings than those seen when using ellipsoidal particles. Autocorrelation functions and characteristic times for suspensions with spherical particles were very similar at low concentration conditions (1 and 2 wt%). Conversely, for ellipsoidal particles, the relaxation time for the 2 wt% suspension was larger than that for the 1 wt% suspension during drying. Different drying patterns of suspensions containing spherical and ellipsoidal particles could be related to the contact angle data shown in Fig. 2. Ellipsoidal particles are more likely to be located on the surface of a drop than spherical particles [40]. It will be hereafter important to link the real-time autocorrelation function data with micro-rheological properties of suspension drops during drying (e.g., Boussinesq number including local elastic modulus and shear stress within a suspension drop [21]), for more quantitative analysis of coffee ring effect.
Characterization of particle motions in larger drop during drying
Using larger volume (2 μL) drops than in the above experiment, particle motions at the center and edge positions of the coffee rings, predicted from MSDWS, were compared for suspension systems with spherical and ellipsoidal particles with a concentration of 1 wt% at 30 °C. Figure 9 shows the characteristic times for larger suspension drops at the center and edge positions.
In the drying of a suspension drop containing spherical particles, characteristic times at the edge were larger than those at the center, presenting that the particle movement at the edge dramatically decreased due to the densely packed particles at the prominent edge. However, in the case of a suspension drop with ellipsoidal particles, which formed a relatively weak coffee ring, the difference in characteristic time data was not appreciable. Thus, MSDWS can be successfully applied to measure particle motions in large-scale suspension-coated films during drying.
Conclusions
The effects of spherical and ellipsoidal PS particles on particle motions in a suspension drop while drying and on final coffee ring patterns were investigated using MSDWS and image analysis. The pinned contact line was determined by observing the change in contact angle of suspension drops during drying, considering the known coffee ring mechanism in which particles in a suspension liquid move toward the edge of the drop from the center. Final coffee ring patterns under various conditions demonstrated that the coffee ring formed by spherical particles was thicker and higher than rings formed by ellipsoidal particles, directly suggesting that coffee ring formation is suppressed by the ellipsoidal particles in a suspension drop. The relationship between coffee ring formation and particle dynamics in suspension drops during drying was examined using MSDWS in real time. The fast Brownian motions of spherical or ellipsoidal particles during drying were quantified from autocorrelation data obtained by a line scan camera. The characteristic times for β-relaxation of particle motion indicate that the correlation between ellipsoidal particles was less relaxed than the correlation between spherical particles under the same conditions, because ellipsoidal particles move more slowly than the spherical particles. MSWDW will be a useful non-contact method that can be utilized to determine the particle dynamics within large-scale suspension-coated films in coating and drying processes.
References
Abramchuk SS, Khokhlov AR, Iwataki T, Oana H, Yoshikawa K (2001) Direct observation of DNA molecules in a convection flow of a drying droplet. Europhys Lett 55(2):294–300
Smalyukh II, Zribi OV, Butler JC, Lavrentovich OD, Wong GCL (2006) Structure and dynamics of liquid crystalline pattern formation in drying droplets of DNA. Phys Rev Lett 96:177801
Fang X, Li B, Petersen E, Seo Y-S, Samuilov VA, Chen Y, Sokolov JC, Shew C-Y, Rafailovich MH (2006) Drying of DNA droplets. Langmuir 22(14):6308–6312
Kim MH, Im SH, Park OO (2005) Rapid fabrication of two and three dimensional colloidal crystal films via confined convective assembly. Adv Funct Mater 15(8):1329–1335
Lin Z, Granick S (2004) Patterns formed by droplet evaporation from a restricted geometry. J Am Chem Soc 127(9):2816–2817
Xu J, Xia J, Hong SW, Lin Z, Qiu F, Yang Y (2006) Self-assembly of gradient concentric rings via solvent evaporation from a capillary bridge. Phys Rev Lett 96:066104
Ahn BY, Duoss EB, Motala MJ, Guo X, Park S-I, Xiong Y, Yoon J, Nuzzo RG, Rogers JA, Lewis JA (2009) Omnidirectional printing of flexible, stretchable, and spanning silver microelectrodes. Science 323(5921):1590–1593
Magdassi S, Grouchko M, Toker D, Kamyshny A, Balberg I, Millo O (2005) Ring stain effect at room temperature in silver nanoparticles yields high electrical conductivity. Langmuir 21(23):10264–10267
Park J, Moon J (2006) Control of colloidal particle deposit patterns within picoliter droplets ejected by ink-jet printing. Langmuir 22(8):3506–3513
Soltman D, Subramanian V (2008) Inkjet-printed line morphologies and temperature control of the coffee ring effect. Langmuir 24(5):2224–2231
Zhang L, Liu H, Zhao Y, Sun X, Wen Y, Guo Y, Gao X, Di C, Yu G, Liu Y (2012) Inkjet printing high-resolution, large-area graphene patterns by coffee-ring lithography. Adv Mater 24(3):436–440
Watanabe S, Mino Y, Ichikawa Y, Miyahara MT (2012) Spontaneous formation of cluster array of gold particles by convective self-assembly. Langmuir 28(36):12982–12988
Hu H, Larson RG (2001) Evaporation of a sessile droplet on a substrate. J Phys Chem B 106(6):1334–1344
Deegan RD, Bakajin O, Dupont TF, Huber G, Nagel SR, Witten TA (1997) Capillary flow as the cause of ring stains from dried liquid drops. Nature 389(6653):827–829
Deegan RD (2000) Pattern formation in drying drops. Phys Rev E 61(1):475–485
Deegan RD, Bakajin O, Dupont TF, Huber G, Nagel SR, Witten TA (2000) Contact line deposits in an evaporating drop. Phys Rev E 62(1):756–765
Weon BM, Je JH (2010) Capillary force repels coffee-ring effect. Phys Rev E 82(1):015305(R)
Kralchevsky PA, Denkov ND (2001) Capillary forces and structuring in layers of colloid particles. Curr Opin Colloid Interface Sci 6(4):383–401
Li Y-F, Sheng Y-J, Tsao H-K (2013) Evaporation stains: suppressing the coffee-ring effect by contact angle hysteresis. Langmuir 29(23):7802–7811
Majumder M, Rendall CS, Eukel JA, Wang JYL, Behabtu N, Pint CL, Liu T-Y, Orbaek AW, Mirri F, Nam J, Barron AR, Hauge RH, Schmidt HK, Pasquali M (2012) Overcoming the “coffee-stain” effect by compositional Marangoni-flow-assisted drop-drying. J Phys Chem B 116(22):6536–6542
Yunker PJ, Still T, Lohr MA, Yodh AG (2011) Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 476(7360):308–311
Kim D-O, Pack M, Hu H, Kim H, Sun Y (2016) Deposition of colloidal drops containing ellipsoidal particles: competition between capillary and hydrodrynamic forces. Langmuir 32:11899–11906
Dugyala VR, Basavaraj MG (2014) Control over coffee-ring formation in evaporating liquid drops containing ellipsoids. Langmuir 30(29):8680–8686
Malaquin L, Kraus T, Schmid H, Delamarche E, Wolf H (2007) Controlled particle placement through convective and capillary assembly. Langmuir 23(23):11513–11521
Madivala B, Fransaer J, Vermanta J (2009) Self-assembly and rheology of ellipsoidal particles at interfaces. Langmuir 25(5):2718–2728
Xu L, Davies S, Schofield AB, Weitz DA (2008) Dynamics of drying in 3D porous media. Phys Rev Lett 101(9):094502
Zakharov P, Scheffold F (2010) Monitoring spatially heterogeneous dynamics in a drying colloidal thin film. Soft Mater 8(2):102–113
Wyss HM, Romer S, Scheffold F, Schurtenberger P, Gauckler LJ (2001) Diffusing-wave spectroscopy of concentrated alumina suspensions during gelation. J Colloid Interface Sci 241(1):89–97
Harden JL, Viasnoff V (2001) Recent advances in DWS-based micro-rheology. Curr Opin Colloid Interface Sci 6(5–6):438–445
Lee JY, Hwang JW, Jung HW, Kim SH, Lee SJ, Yoon K, Weitz DA (2013) Fast dynamics and relaxation of colloidal drops during the drying process using multispeckle diffusing wave spectroscopy. Langmuir 29(3):861–866
Oh GJ, Hwang JW, Bong JW, Jung HW (2017) Particle dynamics and relaxation in bimodal suspensions during drying using multispeckle diffusing wave spectroscopy. AICHE J 63(3):1114–1121
Knaebel A, Bellour M, Munch J-P, Viasnoff V, Lequeux F, Harden JL (2000) Aging behavior of Laponite clay particle suspensions. Europhys Lett 52(1):73–79
Cohen-Addad S, Höhler R (2001) Bubble dynamics relaxation in aqueous foam probed by multispeckle diffusing-wave spectroscopy. Phys Rev Lett 86(2):4700–4703
Bellour M, Knaebel A, Harden JL, Lequeux F, Munch J-P (2003) Aging processes and scale dependence in soft glassy colloidal suspensions. Phys Rev E 67(3):031405
Viasnoff V, Jurine S, Lequeux F (2003) How are colloidal suspensions that age rejuvenated by strain application? Faraday Discuss 123:253–266
Narita T, Beauvais B, Hébraud P, Lequeux F (2004) Dynamics of concentrated colloidal suspensions during drying—aging, rejuvenation and overaging. Eur Phys J E 14(3):287–292
Viasnoff V, Lequeux F, Pine DJ (2002) Multispeckle diffusing-wave spectroscopy: a tool to study slow relaxation and time-dependent dynamics. Rev Sci Instrum 73(6):2336–2344
Ahn SJ, Ahn KH, Lee SJ (2016) Film squeezing process for generating oblate spheroidal particles with high yield and uniform sizes. Colloid Polym Sci 294:859–867
Zastawny M, Mallouppas G, Zhao F, Wachem BV (2012) Derivation of drag and lift force and torque coefficients for non-spherical particles in flows. Int J Multiphase Flow 39:227–239
Madivala B, Vandebril S, Fransaer J, Vermant J (2009) Exploiting particle shape in solid stabilized emulsions. Soft Matter 5(8):1717–1727
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2016R1A5A1009592 and NRF-2017R1E1A1A01075107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 8222 kb)
Rights and permissions
About this article
Cite this article
Park, B.S., Jung, K.I., Lee, S.J. et al. Effect of particle shape on drying dynamics in suspension drops using multi-speckle diffusing wave spectroscopy. Colloid Polym Sci 296, 971–979 (2018). https://doi.org/10.1007/s00396-018-4315-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4315-x