Abstract
Simple analytic expressions are derived for the electrostatic interaction energy between two charged colloidal particles in an electrolyte solution. The obtained expressions are based on an approximate form of the modified Poisson-Boltzmann equation taking into account the ion size effects through Carnahan-Starling activity coefficients of electrolyte ions. We derive the electrostatic interaction energy between two parallel plates on the basis of the linear superposition approximation. We further employ Derjaguin’s approximation to derive the corresponding expressions for the electrostatic interaction energy between two spheres, two parallel cylinders, or two crossed cylinders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory of colloid stability, the calculation of the electrostatic double-layer interaction force and energy between two charged particles in an electrolyte solution is based on the solution to the Poisson-Boltzmann equation for the electric potential distribution around the interacting particles [1–9]. The standard Poisson-Boltzmann equation, however, assumes that ions behave like point-charges and neglects the effects of ionic size. There are many theoretical studies on the modified Poisson-Boltzmann equation [10–16], which takes into account the effect of ionic size by introducing the activity coefficients of electrolyte ions [10, 17–19]. In a previous paper [20], on the basis of the equation for the ionic activity coefficients given by Carnahan and Starling [19], which is the most accurate among existing theories, we presented a simple algorithm for solving the modified Poisson-Boltzmann equation and derived a simple approximate analytic expression for the surface charge density/surface potential relationship for a planar charged surface.
In the present paper, we present a simple algorithm for the calculation of the interaction energy between two charged colloidal particles, that is, two parallel plates, spheres, and parallel or crossed cylinders on the basis of the modified Poisson-Boltzmann equation taking into account the ion size effects through Carnahan-Starling activity coefficients of electrolyte ions [20]. In this modified Poisson-Boltzmann equation [20], we consider only the steric interactions among ions of finite size and do not consider other effects relating to the dielectric permittivity of the electrolyte solution. We employ the linear superposition approximation for the electric potential between the two interacting plates [1, 2, 7, 8, 21–23] and Derjaguin’s approximation [24–26], which enables the calculation of the interaction energy between two spheres or cylinders from the corresponding interaction energy between two parallel plates. These approximations are widely used in theories of colloid stability including the original DLVO theory. Expressions for the interaction energy obtained with the help of the linear superposition approximation, which do not depend on the type of the electrostatic double-layer interaction, can be applied irrespective of whether the surface potential or the surface charge density of the interacting particles remains constant during interaction.
Potential distribution around a single planar plate based on the modified Poisson-Boltzmann equation
The linear superposition approximation uses a potential distribution for a single plate. Before considering two parallel plates, we thus treat a single charged plate immersed in a symmetrical electrolyte solution of valence z and bulk concentration (number density) n (in units of m−3) and take an x-axis perpendicular to the plate surface with its origin 0 at the plate surface so that the region x > 0 corresponds to the electrolyte solution. We denote the surface potential of the plate by ψ o. The electric potential distribution ψ(x) obeys the Poisson equation for the region x > 0, viz.,
Here, ε r is the relative permittivity of the electrolyte solution, ε o is the permittivity of a vacuum, and ρ el(x) is the space charge density resulting from the electrolyte ions and is given by
where n +(x) and n −(x) are, respectively, the concentrations of cations and anions at position x and e is the elementary electric charge. The boundary conditions are
We assume that the activity coefficients of cations and anions at position x have the same value γ(x). The electrochemical potential μ +(x) of cations and that of anions μ −(x) are thus given by
where \( {\mu}_{\pm}^{\mathrm{o}} \) are the constant terms, k is the Boltzmann’s constant, and T is the absolute temperature. The values of μ ±(x) must be the same as those in the bulk solution phase, where ψ(x) = 0, viz.,
where γ ∞ = γ(∞). By equating μ ±(x) = μ ±(∞), we obtain
so that Eq. (2) gives
Thus Eq. (1) becomes the following modified Poisson-Boltzmann equation:
When γ(x) = γ ∞ = 1, Eq. (8) becomes the following standard Poisson-Boltzmann equation:
We now assume that cations and anions have the same radius a. We introduce the volume fraction ϕ + of cations and that of anions ϕ − at position x. Then we have
The total ion volume fraction ϕ(x) at position x is thus given by
From Eq. (11), we obtain
where ϕ B ≡ ϕ(∞) = (4πa 3/3)2n is the total ion volume fraction in the bulk solution phase. By substituting Eq. (6) into Eq. (12), we obtain
The modified Poisson-Boltzmann equation (8) becomes, by using Eq. (13)
Now, we employ the expression for γ(x) derived by Carnahan and Starling [19], viz.,
Then Eq. (13) becomes
which is an equation for ϕ(x) for the given values of ϕ B and ψ(x).
In a previous paper [20], we have shown that Eq. (16) can be approximated well by
or equivalently
which results from Eq. (13). By substituting Eq. (17) into Eq. (14), we obtain the following approximation to the modified Poisson-Boltzmann equation [20]:
The above approximation (Eq. (19)) is a good approximation with negligible errors for low ϕ B (ϕ B < 0.1) and low-to-moderate potentials (|zeψ o/kT| ≤ 3). It is also to be noted that Eq. (19), which uses the Carnahan-Starling equation, differs from the so far proposed modified Poisson-Boltzmann equation based on the less precise Bikerman equation [17], in which “2ϕ B” appears instead of “16ϕ B/(1 + 8ϕ B)” on the right-hand side of Eq. (19).
The modified Poisson-Boltzmann equation (19) can be integrated to give
where sgn(ψ o) = 1 if ψ o > 0 and −1 if ψ o < 0, y(x) = zeψ(x)/kT is the scaled potential, y o = zeψ o/kT is the scaled surface potential, and
is the Debye-Hückel parameter. Equation (20) gives y(x) as a function of the scaled distance κx. It can be shown that at large distances x compared with the Debye length 1/κ, the asymptotic form of ψ(x) must be
where ψ eff is called the effective surface potential and is related to the surface potential ψ o by [23]
with
Equation (23) as combined with Eq. (24) will be used later. Note that at ϕ B = 0, Eqs. (23) and (24) give
which agrees with the result obtained via the standard Poisson-Boltzmann equation (8).
Electrostatic interaction between two parallel plates
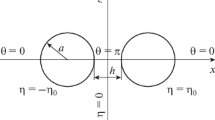
Consider two parallel plates 1 and 2 having surface potentials ψ 1 and ψ 2, respectively, in the absence of interaction, separated by h between their surfaces in a symmetrical electrolyte solution of valence z and bulk concentration (number density) n (in units of m−3) and take an x-axis perpendicular to the plate surface with its origin 0 of plate 1 (Fig. 1a). The electrostatic interaction force P pl(h) per unit area between plates 1 and 2 can be calculated by integrating the osmotic pressure and the Maxwell stress over an arbitrary closed surface Σ enclosing either one of the two interacting plates. As Σ, we choose two planes located at x = −∞ (in the bulk solution far from the plates) and x = x′ (0 < x′ < h) enclosing plate 1. Here, x′ is an arbitrary point near the midpoint in the region 0 < x < h between plates 1 and 2.
At equilibrium, the gradient of the pressure p(x) and the electric force ρ el(x)dψ(x)/dx acting on the space charge ρ el(x) must balance each other, viz.,
By substituting the Poisson equation (1) into Eq. (26), we obtain
which gives
The second term on the left-hand side of Eq. (28) corresponds to the Maxwell stress. The pressure p(x) at position x can be obtained from the Gibbs-Duhem relation [11], viz.,
By substituting Eq. (4) into Eq. (29), we obtain
By comparing Eqs. (26) and (30), we find [11]
which can be integrated to give
The first term on the right-hand side of Eq. (32) is the usual osmotic pressure obtained via the standard Poisson-Boltzmann equation (9) and the second term results from the effect of ionic size. The sum of the first and second terms on the right-hand side of Eq. (32) corresponds to the osmotic pressure of electrolyte ions taking into account the ionic size effect. Now, we substitute Eqs. (17) and (18) into Eq. (32) to obtain
where p o is the pressure in the absence of electrolyte ions. The electrostatic interaction force per unit area between plates 1 and 2 is thus given by
By substituting Eq. (33) into Eq. (34), we obtain
Here, we have used the fact that p(−∞) = 2nkT + p o and dψ/dx = 0 at x = − ∞. Since x′ is an arbitrary point near the midpoint in the region 0 < x < h between plates 1 and 2, ψ(x′) can be considered to be small so that
Eq. (35) can be linearized with respect to ψ(x′) to give
Here, P pl(h) > 0 corresponds to repulsion and P pl(h) < 0 to attraction. It must be stressed that the above linearization is not for the surface potentials ψ 1 and ψ 2 but only for ψ(x′). That is, this linearization approximation holds good even when the surface potentials are arbitrary, provided that the particle separation h is large compared with the Debye length 1/κ. Also in this region, ψ(x′) can be approximated as the sum of the asymptotic forms of the unperturbed potentials ψ 1(x′) and ψ 2(x′) produced by plates 1 and 2, respectively, in the absence of interaction, which are given by Eq. (22), viz.,
with
where ψ eff1 and ψ eff2 are, respectively, the effective surface potentials of plates 1 and 2. These potentials are given by Eq. (23) as combined with Eq. (24). By substituting Eqs. (37)–(39) into Eq. (36), we obtain
Note that P(h) is independent of the value of x′. The electrostatic interaction energy V pl(h) per unit area between plates 1 and 2 can thus be derived by integrating Eq. (40), viz.,
Equation (41) as combined with Eqs. (23) and (24) is the required expression for the electrostatic interaction energy between two parallel plates 1 and 2 based on the modified Poisson-Boltzmann equation (19) taking into account the ion size effect.
Results and discussion
We have derived an approximate expression (41) for the interaction energy per unit area between two parallel plates. This expression, which is based on the linear superposition approximation, involves the effective surface potential calculated with Eqs. (23) and (24). It is to be noted that the expressions for the interaction force and energy based on the modified Poisson-Boltzmann equation (8) takes the same form as those based on the standard Poisson-Boltzmann equation (9). The difference is that the effective surface potentials appearing in these expressions are given by Eqs. (23) and (24) for the modified Poisson-Boltzmann equation case but they are given by Eq. (25) for the standard Poisson-Boltzmann equation case.
With the help of Derjaguin’s approximation [24], one can calculate the electrostatic interaction energy V sp(H) between two spheres 1 and 2 having radii a 1 and a 2 and effective surface potentials ψ eff1 and ψ eff2, respectively, separated by a distance H between their surfaces (Fig. 1b) via the corresponding electrostatic interaction energy V pl(h) between two parallel dissimilar plates, viz.,
By substituting Eq. (41) into Eq. (42), we obtain
Similarly, by using Derjaguin’s approximation for the electrostatic interaction energy V cy//(H) per unit length between two parallel cylinders having radii a 1 and a 2 and effective surface potentials ψ eff1 and ψ eff2, respectively, separated by a distance H between their surfaces [25, 26], that is,
we obtain
For the case of two crossed cylinders having radii a 1 and a 2 and effective surface potentials ψ eff1 and ψ eff2, respectively, at separation H between their closest surfaces, Derjaguin’s approximation for the interaction energy V cy⊥(H) is given by [25, 26]
By substituting Eq. (42) into Eq. (46), we obtain
The scaled interaction energies for the above four cases take the same functional form, that is, V * (h) = y eff1 y eff2 e −κh for two parallel plates and V * (H) = y eff1 y eff2 e −κH for two spheres and two parallel or crossed cylinders. That is,
and
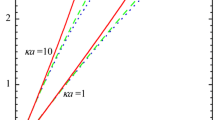
In Fig. 2, we plot the scaled electrostatic interaction energies \( {V}^{\ast }(h)={y}_{\mathrm{eff}}^2{e}^{-\kappa h} \)or \( {V}^{\ast }(H)={y}_{\mathrm{eff}}^2{e}^{-\kappa H} \)for two similar parallel plates, two similar spheres, or two similar cylinders having scaled surface potential y o = zeψ o/kT and scaled effective surface potential y eff = zeψ eff/kT as a function of κh or κH for y o = 1, 2, and 3 at ϕ B = 0, 0.05, and 0.1. Figure 2 shows how the effects of ionic size on the electrostatic interaction energy become appreciable for a higher surface potential y o and a higher total ion volume fraction ϕ B. For y o = 2 and ϕ B = 0.05 for example, the modified Poisson-Boltzmann equation (Eq. (8)) gives V*(κh) (or V*(κH)) = 1.449, while the standard Poisson-Boltzmann equation (Eq. (9)) gives V*(κh) (or V*(κH)) = 1.257, the difference being 15%. The ionic size effect always gives rise to an increase in the interaction energy V*(κh) (or V*(κH)). This is because the ionic concentration becomes lower due to the ionic size effect, leading to a decrease in the ionic shielding effects so that the magnitude of V*(κh) (or V*(κH)) increases.
Scaled electrostatic interaction energy \( {V}^{\ast }(h)={y}_{\mathrm{eff}}^2{e}^{-\kappa h} \) for two parallel similar plates and \( {V}^{\ast }(H)={y}_{\mathrm{eff}}^2{e}^{-\kappa H} \) for two similar spheres of radius a and for two parallel or crossed similar cylinders of radius a. Calculated with Eqs. (48)–(51) with a 1 = a 2 = a and y eff1 = y eff2 = y eff for the scaled unperturbed surface potential y o = 1, 2, and 3 at the total ion volume fraction ϕ B = 0, 0.05, and 0.1. The lines for ϕ B = 0 correspond to the results obtained via the standard Poisson-Boltzmann model neglecting the ionic size effect. For small κh or κH, the linear superposition approximation (LSA) is no longer good approximation and thus the energy curves are given by dotted lines κh ≤ 1 or κH ≤ 1. Note that the scaled interaction energy takes the same functional form \( V*(h)={y}_{\mathrm{eff}}^2{e}^{-\kappa h} \) for plates or \( V*(H)={y}_{\mathrm{eff}}^2{e}^{-\kappa h} \) for spheres and cylinders
It is to be noted that the expressions for the interaction energy V (κh) or V (κH) between the two particles obtained in the present paper are derived on the basis of the linear superposition approximation (LSA). This approximation is no longer a good approximation for small particle separations h or H as compared with the Debye length 1/κ and thus the energy curves are given by dotted lines in Fig. 2. The interaction force and energy between two particles for small particle separations depend on the interaction models. Most generally employed models are the constant surface potential model and the constant surface charge density model, where the surface potential ψ o or surface charge density σ of the particles remain constant during interaction. In the appendix we give the exact expressions for the interaction force between two parallel similar plates obtained from the constant surface potential model and the constant surface charge density model. It is known that the magnitude of the interaction energy or force obtained from the linear superposition approximation lies between those obtained from the above two interaction models and a good approximation for κh > 1 or κH > 1 [7]. For this reason, the linear superposition approximation is employed in the original DLVO theory of the stability of colloidal suspensions [1, 2]. Indeed, Eq. (43) with a 1 = a 2 and y eff1 = y eff2 for the case ϕ B = 0 is employed in the original DLVO theory [1, 2]. Equation (43) is thus a generalization of the DLVO electrostatic interaction energy by taking into account the ion size effects.
Conclusion
We have presented a simple algorithm for calculating the electrostatic interaction energy between two charged particles (plates, spheres, and cylinders) in an electrolyte solution based on the modified Poisson-Boltzmann equation (8) for the electric potential around the interacting particles, which takes into account the effects of ionic size on the basis of ionic activity coefficient given by Carnahan and Starling. It is to be noted that expressions for the interaction force and energy based on the modified Poisson-Boltzmann equation [8] takes the same form as those based on the standard Poisson-Boltzmann equation [9]. The difference is that the effective surface potentials ψ eff1 and ψ eff2 appearing in these expressions are different for these two cases. In a previous paper [27], we derived a simple expression for the stability ratio of colloidal dispersions by considering the sum of the electrostatic and van der Waals interaction energies between two spherical particles based on the standard Poisson-Boltzmann equation [9]. By replacing the expressions for the effective surface potentials appearing in the electrostatic interaction energy with those based on the modified Poisson-Boltzmann equation (8), the ionic size effect is taken into account in the resultant electrostatic energy expression.
References
Derjaguin BV, Landau L (1941) Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim USSR 14:633–662
Verwey EJW, Overbeek JTG (1948) Theory of the stability of lyophobic colloids. Elsevier/Academic Press, Amsterdam
Dukhin SS (1993) Non-equilibrium electric surface phenomena. Adv Colloid Interf Sci 44:1–134
Ohshima H, Furusawa K (eds) (1998) Electrical phenomena at interfaces, fundamentals, measurements, and applications, 2nd edition, revised and expanded. Dekker, New York
Delgado AV (ed) (2000) Electrokinetics and electrophoresis. Dekker, New York
Lyklema J (2005) Fundamentals of interface and colloid science, volume IV, chapter 3. Elsevier/Academic Press, Amsterdam
Ohshima H (2006) Theory of colloid and interfacial electric phenomena. Elsevier/Academic Press, Amsterdam
Ohshima H (2010) Biophysical chemistry of biointerfaces. Wiley, Hoboken
Ohshima H (ed) (2012) Electrical phenomena at interfaces and biointerfaces: fundamentals and applications in nano-, bio-, and environmental sciences. Wiley, Hoboken
Sparnaay MJ (1972) Ion-size corrections of the Poisson-Boltzmann equation. J Electroanal Chem 37:65–70
Adamczyk Z, Warszyński P (1996) Role of electrostatic interactions in particle adsorption. Adv in Colloid Interface Sci 63:41–149
Biesheuvel PW, van Soestbergen M (2007) Counterion volume effects in mixed electrical double layers. J Colloid Interface Sci 316:490–499
Lopez-Garcia JJ, Horno J, Grosse C (2011) Poisson-Boltzmann description of the electrical double layer including ion size effects. Langmuir 27:13970–13974
Lopez-Garcia JJ, Horno J, Grosse C (2012) Equilibrium properties of charged spherical colloidal particles suspended in aqueous electrolytes: finite ion size and effective ion permittivity effects. J Colloid Interface Sci 380:213–221
Giera B, Henson N, Kober EM, Shell MS, Squires TM (2015) Electric double-layer structure in primitive model electrolytes: comparing molecular dynamics with local-density approximations. Langmuir 31:3553–3562
Lopez-Garcia JJ, Horno J, Grosse C (2016) Ion size effects on the dielectric and electrokinetic properties in aqueous colloidal suspensions. Curr Opinion in Colloid Interface 24:23–31
Bikerman JJ (1942) Structure and capacity of electrical double layer. Philos Mag 33:384–397
Hill TL (1962) Statistical thermodynamics. Addison-Westley, Reading, MA
Carnahan NF, Starling KE (1969) Equation of state for nonattracting rigid spheres. J Chem Phys 51:635–636
Ohshima H (2016) An approximate analytic solution to the modified Poisson-Boltzmann equation. Effects of ionic size. Colloid Polym Sci 294:2121–2125
Ohshima H, Healy TW, White LR (1984) Accurate analytic expressions for the surface charge density/surface potential relationship and double-layer potential. J Colloid Interface Sci 90:17–26
Wilemski G (1982) Weak repulsive interactions between dissimilar electrical double layers. J Colloid Interface Sci 88:111–116
Ohshima H (1995) Effective surface potential and double-layer interaction of colloidal particles. J Colloid Interface Sci 174:45–52
Derjaguin BV (1934) Untersuchungen über die Reibung und Adhäsion, IV. Kolloid Z 69:155–164
Sparnaay MJ (1959) The interaction between two cylinder shaped colloidal particles. Recueil 78:680–709
Ohshima H, Hyono A (2009) Electrostatic interaction between two cylindrical soft particles. J Colloid Interface Sci 333:202–208
Ohshima H (2014) Approximate analytic expression for the stability ratio of colloidal dispersions. Colloid Polym Sci 292:2269–2274
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Source of funding
The author declares no sources of funding.
Appendix
Appendix
Analytic expressions for the interaction between two parallel similar plates at separation h can be derived on the basis of the modified Poisson-Boltzmann equation (19). The interaction force P pl(h) per unit area between the two plates is given by
with
where y m is the scaled potential at the midpoint x = h/2 between the two plates. Eq. (52) can be derived from Eq. (35) by choosing h/2 as x′. Note that Eq. (52) can be applied for both of the constant surface potential and surface charge density models. For the constant surface potential model, y m is related to the scaled surface potential y o = zeψ o /kT, viz.,
which is derived by applying Eq. (20) for the system of two parallel plates. For the constant surface charge density model, on the other hand, we have
Note that the scaled surface potential y (0), which is a function of the plate separation h, is related to the scaled unperturbed surface potential y o = zeψ o /kT in the absence of interaction (h = ∞) by
which can be derived by integrating Eq. (19) once and applying the boundary condition: dψ/dx| x=0 + = − σ/ε r ε o and dψ/dx| x = h/2 = 0 (from symmetry of the system). The surface charge density σ of the plates is related to the scaled unperturbed surface potential y o = zeψ o /kT (in the absence of interaction) by
By combining Eqs. (56) and (57), we obtain
Equations (55) and (58) form coupled equations for y m for the given values of y o and κh.
Once the value of y m is obtained from Eq. (54) for the constant potential model (in which y(0) is always equal to the unperturbed surface potential y o) or the coupled Eqs. (55) and (58) for the constant surface charge density model, one can calculate the interaction force P pl(h) via Eq. (52).
Rights and permissions
About this article
Cite this article
Ohshima, H. Approximate analytic expressions for the electrostatic interaction energy between two colloidal particles based on the modified Poisson-Boltzmann equation. Colloid Polym Sci 295, 289–296 (2017). https://doi.org/10.1007/s00396-016-4005-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-4005-5