Abstract
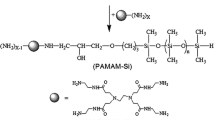
Polysiloxane containing tertiary amino group (P(SiO)m(SiOTA)n) with different molecular weight was synthesized through equilibrium polymerization of octamethylcyclotetrasiloxane (D4) and hydrolysis of N,N-diethyl-aminopropyl-methyldimethoxysilane (TAMDESi) in the presence of potassium hydroxide (KOH) as catalyst. After that, polysiloxane quaternary ammonium salt containing epoxy group (P(SiO)m(SiOQAEp)n) with different molecular weight and cationic concentration was obtained from P(SiO)m(SiOTA)n and epichlorohydrin by quaternization. It was shown that P(SiO)m(SiOQAEp)n surfactant exhibited low surface tension (γ CMC < 30.00 mN/m) at the critical micellization concentration. The thermodynamic parameters of micellization (ΔG 0 m, ΔH 0 m, ΔS 0 m) indicated that the micellization of P(SiO)m(SiOQAEp)n surfactant was enthalpy-driven. In particular, it was found that the glass transition temperature (T g) of P(SiO)m(SiOQAEp)n varied in the range of −15 ∼ –70 °C according to the molecular weights, and the thermal decomposition temperature was higher than 220 °C. This type of surfactant had excellent low temperature flexibility as well as good chemical and thermal stability. The antimicrobial activity was evaluated against Gram-negative (Escherichia coli) and Gram-positive bacteria (Staphylococcus aureus), respectively. The minimum bactericidal concentration (MBC) values against S. aureus and E. coli were below 1.5–2.8 × 10−5 mol/L, which indicated that P(SiO)m(SiOQAEp)n had strong antibacterial activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Silicone surfactants play a crucial role such as wetting, dispersing, emulsifying, solubilizing, foaming, anti-foaming, and decontaminating in many practical applications [1–4]. Their marvelous features and functions have attracted great interest of many scientists. Typically, trisiloxane surfactants perform outstanding surface activity, which can decrease the surface tension of water to 20 mN/m and have low degree of counterion binding [5–8].
Currently, a new type of cationic surfactant, polysiloxane quaternary ammonium salt, has greatly broadened the perspective in colloid and surface science because of its good surface activity, antibacterial activity, spreadability, and excellent thermal stability [9–12]. A novel polysiloxane quaternary ammonium salt containing polycation and anions (I−) (PSQAS) was synthesized. That kind of PSQAS could maintain stability below 250 °C by thermogravimetric analysis, which had been used in dye-sensitized solar cells and a relatively good result was obtained [13].
Huang et al. synthesized polysiloxane ionomers bearing pendant quaternary ammonium groups through the Menshutkin reaction of poly(-chloropropylmethylsiloxane-co-dimethylsiloxane) with N,N-dimethylbenzylamine. It showed that the quaternary ammonium chloride units improved the compatibility of polysiloxane with organic polymers which was useful for fabric-conditioning agents, bacteriocides, textile finishing, and plastic processing to bring out fabric antimicrobial and antielectrostatic properties [14].
Sauvetg et al. synthesized polysiloxanes with 3-(alkyldimethylammonio)propyl pendant groups and found that they are active in aqueous solution. The polysiloxanes bearing quaternary ammonium salts (QAS) showed excellent bactericidal activity against bacteria such as Escherichia coli and Aeromonas hydrophila when incorporated in a polysiloxane network, and the activity was retained after 66 days of immersion in water [15].
It is considered that polymers containing quaternary ammonium groups in side chain or in main chain are a kind of effective antimicrobial materials to kill bacteria that are resistant to other types of cationic antibacterials [16–18]. In particular, polymeric antimicrobial agents have the advantages of nonvolatile, chemically stable, and not to permeate through the skin [19, 20].
Lu et al. [21] synthesized four polymeric quaternary ammonium materials with different lengths of alkyl chain and evaluated their bactericidal activities by minimum bactericidal concentration (MBC) values and inhibitory zone diameters against Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (E. coli), respectively. The tested polymers showed more antimicrobial activity against Gram-negative bacteria, whereas they exhibited less activity against Gram-positive bacteria.
It is noteworthy that the silicone surfactant has a high chemical reactivity owing to the existence of epoxy group [22]. It can be used as an important intermediate for organic synthesis to introduce quaternary ammonium group into the natural macromolecules [23] (e.g., gelatin, cellulose, starch, silk, chitosan, etc.), which improves the properties of self-emulsifying and antibiosis [24], so as to greatly improve the physical and chemical properties of the natural macromolecules.
In the present work, a new type of functional polysiloxane quaternary ammonium salts containing epoxy group P(SiO)m(SiOQAEp)n with different molecular weight and cationic concentration has been successfully synthesized. The aggregation behaviors of P(SiO)m(SiOQAEp)n in aqueous solution were investigated by surface tension and electrical conductivity. The antibacterial activities of P(SiO)m(SiOQAEp)n against Gram-negative and Gram-positive bacteria were investigated by MBC value method and inhibitory zone method. The thermal stability of P(SiO)m(SiOQAEp)n had also been measured by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) methods.
Experimental procedures
Materials
γ-Chloropropylmethyldimethoxysilane (CPMDMS) Analytical reagent (A.R.) (A.R. ≥ 98.0 %) was obtained from Shanghai Chemical Reagent Co., Ltd. Octamethylcyclotetrasiloxane (D4) was purchased from Aldrich Chemical Co. Hexamethyldisiloxane (HMDS), ethanol (A.R. ≥ 99.7 %), diethylamine (A.R. ≥ 99.0 %), ether (A.R. ≥ 99.5 %), epichlorohydrin (A.R. ≥ 99.0 %), potassium hydroxide (KOH) (A.R. ≥ 85.0 %), tetrahydrofuran (THF) (A.R. ≥ 99.0 %), acetic acid (A.R. ≥ 99.5 %), yeast extract powder Biochemical reagent (B.R.), peptone form fish (B.R.), and agar powder (B.R.) were all supplied by Sinopharm Chemical Reagent Co., Ltd. All organic reagents were of analytical grade and were purified by distillation. The solutions of the quaternary ammonium-based surfactants were prepared using triple distilled water.
Methods
Synthesis of P(SiO)m(SiOTA)n
P(SiO)m(SiOTA)n was synthesized through ring-opening polymerization of octamethylcyclotetrasiloxane (D4) and the hydrolyzate of N,N-diethyl-aminopropyl-methyldimethoxysilane in the presence of HMDS as terminal blocking agent and KOH as catalyst. The polycondensations were run in anhydrous conditions at nitrogen atmosphere.
A 100-mL four-neck round-bottom flask was equipped with a thermometer, a magnetic stirrer, a reflux condenser, and a nitrogen inlet/outlet. Then, the desired amount of D4 (0.050 mol, 14.80 g), the hydrolyzate of N,N-diethyl-aminopropyl-methyldimethoxysilane (0.052 mol, 9.24 g), HMDS (0.027 mol, 4.37 g), and potassium hydroxide were placed in the reactor. After raising the temperature to 90 °C, the reaction mixture was stirred under nitrogen atmosphere for 8 h. Then, the stoichiometric amount of acetic acid was added to neutralize the catalyst in the reaction mixture, and the solid particles were separated by filtration to obtain a viscous transparent liquid mixture. The liquid portion was then treated by vacuum distillation in order to remove low weight oligomers and any unreacted starting materials. The product was obtained as a light yellow liquid. The primary amine content of the polymer was determined by non-aqueous titration as reported earlier.
The synthesis route was depicted in Scheme 1.
Synthesis of P(SiO)m(SiOQAEp)n
The solution of P(SiO)m(SiOTA)n (0.025 mol, 7.02 g) in anhydrous ethanol (10 mL) was placed in a dry, 100-mL, four-necked, round-bottomed flask equipped with a nitrogen stream, a thermometer, a reflux condenser, and an addition funnel. The fresh solution of epichlorohydrin (0.030 mol, 2.78 g) in anhydrous ethanol (6 mL) was added dropwise to the reaction flask. After that, the reaction mixture was heated to 45 °C for 8 h with magnetic stirring. When the reaction was completed, the mixture was cooled to room temperature. The ethanol was removed by vacuum distillation. Finally, the final product was washed five times with ether and dried in a vacuum oven at 40 °C for 24 h.
The synthesis route was depicted in Scheme 2.
By changing the amount of HMDS, we synthesized four P(SiO)m(SiOTA)n with different molecular weight. Then, four P(SiO)m(SiOQAEp)n with different molecular weight have been successfully quaternized with epichlorohydrin.
1H NMR (400 Hz, D2O): δppm = 0.08–0.14 (s, 3H, SiCH 3 ), 0.502 (t, 2H, SiCH 2 ), 1.7 (m, 2H, Si–CH2–CH 2 –), 3.24 (d, 2H, N+ CH 2 CH2CH2Si), 3.28 (q, 4H, N+(CH 2 CH3)2), 1.25 (t, 6H, N+(CH2 CH 3 )2), 3.209 (m, 1H, epoxy ring), and 2.892 (d, 2H, epoxy ring).
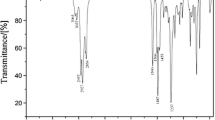
IR (KBr smear): 1,022.27–1,091.71 cm−1 (Si–O–Si symmetry stretching vibration), 800.64 cm−1 (Si–C symmetry stretching vibration), 1,645.28 cm−1 (C–N absorption), 1,259.52 cm−1 (Si–CH3 symmetry stretching vibration), 1,375.66 cm−1, 1,483.26 cm−1 (–CH3 bending vibration and shearing vibration), 1,087.85 cm−1, 896.90 cm−1 (epoxy functional group stretching vibration and shearing vibration), 856.1 cm−1 (–C–O–C asymmetry stretching vibration, epoxy ring) 2,962.87 cm−1, and 2,887.44 cm−1 (–CH2– asymmetry stretching vibration and symmetry stretching vibration).
Structural characterization
1H NMR spectra were recorded on a Bruker Advance 400 spectrometer in deuterium water-d (D2O). Fourier transform infrared spectroscopy (FT-IR) was recorded on a Nicolet NEXUS 470 FT-IR spectrometer. Measurement was performed by dispersing samples in anhydrous KBr pellets.
Molecular weight determination
The weight average molecular weight (M w), number average molecular weight (M n), and M w/M n of the polysiloxane containing tertiary amino group (P(SiO)m(SiOTA)n) were determined by gel permeation chromatography (GPC). It was performed on Waters 515 HPLC pump.
DSC
DSC analysis was carried out with a DSCQ10 differential scanning calorimeter (TA Instruments) under nitrogen atmosphere (50 mL/min).
TGA
TGA was conducted with Q600 Simultaneous under nitrogen atmosphere at a rate of 10 °C/min.
Surface activity determination
Surface tension was measured by the Du Noüy ring method on Processor Tensiometer-K100 (Krüss Company, Germany). All of the prepared surfactant solutions were standing at least 24 h before determination and every sample balanced at testing temperature (25, 30, 35, and 40 °C) no less than 30 min. All measurements were repeated until the values were stable [25]. Surfactant solutions were prepared with distilled water, and its surface tension was measured for comparison, which was determined in the range of 72.0 ± 0.3 mN/m.
Specific conductivity measurements on the aqueous solutions were performed using a low-frequency conductivity analyzer (model DDS-11A, Shanghai Precision & Scientific Instrument Co., Ltd., accuracy ±1 %). The conductivity cell was calibrated with KCl solutions, and the cell constants were determined at different temperatures using KCl solutions. Electrical conductivity measurements were performed for different concentrations of quaternary ammonium salt solutions in the temperature range of 25–40 °C. The solutions were continuously stirred and thermostated at ±1 °C.
Measurement of antimicrobial activity
In order to study the relationship between antibacterial activities of polysiloxane quaternary ammonium salts with epoxy group and its molecular weight, we have used four different molecular weight samples to prepare a series of water-soluble quaternary ammonium salts. Their bactericidal activities were evaluated by the MBC values and inhibitory zone diameters against Gram-positive bacteria (Bacillus subtilis and S. aureus) and Gram-negative bacteria (E. coli), respectively.
-
1.
MBC test: The MBC value was determined as the lowest concentration which produced no colonies on the solid agar media after 24 h. Each sample was tested in triplicate.
-
2.
Inhibitory zone method: Fifteen milliliters of the sterilized melted agar medium was left to solidify on agar plate at room temperature, and then, 10 μL of the suspension of cells was distributed over the surface of the agar plate. Wells were made in agar plates with different organisms, and each one was filled with 8 μL of the tested antibacterial samples. All plates were incubated at a proper temperature for 2 days, and then, the inhibition zone diameters were measured. Growth inhibitory tests for three types of bacteria, B. subtilis, S. aureus, and E. coli, were made on polysiloxane quaternary ammonium salts with epoxy group.
Results and discussion
Determination of molecular weight and m and n of P(SiO)m(SiOQAEp)n
P(SiO)m(SiOQAEp)n is a cationic surfactant containing amine functional group which can greatly adsorb on the GPC column, and the solubility of the P(SiO)m(SiOQAEp)n in THF exerts great influence on the measurement of molecular weight [26]. To overcome those drawbacks, the average M w of P(SiO)m(SiOQAEp)n was calculated by measuring the average molecular weight (M w0) of P(SiO)m(SiOTA)n and the numbers of quaternary ammonium groups. The average molecular weight (M w0) of polysiloxane with tertiary amino group (P(SiO)m(SiOTA)n) was measured by GPC firstly, and then, the n value of P(SiO)m(SiOTA)n was determined by non-aqueous titration. Finally, the numbers of epoxy moieties based on the quaternary ammonium conversion rate were added, and the average M w of P(SiO)m(SiOQAEp)n was obtained.
Furthermore, non-aqueous titration method was performed with glacial acetic acid as the solvent and with perchloric acid, glacial acetic acid, and acetic anhydride as the titrant (0.1 N perchloric acid standard solution, GB4612-84). The crystal violet indicator was used to indicate the end point [27].
The P(SiO)m(SiOTA)n (1 g) was dissolved in glacial acetic acid (20 mL), and two drops of crystal violet indicator were added and then titrated with 0.1 N perchloric acid standard solution. The crystal violet indicator was used to indicate the end point from purple to blue green. The amino value of P(SiO)m(SiOTA)n (A 0) is given as the equivalent of perchloric acid standard solution used in the titration per gram of P(SiO)m(SiOTA)n.
The average M w of P(SiO)m(SiOQAEp)n can be calculated from the following equations:
where 162, 74, 173, and 92.5 are the unit weights of capping group, dimethylsiloxane, tertiary aminosiloxane, and epichlorohydrin, respectively. Also, A 0, M w0, M w, and Conv. (%) are the amino values of P(SiO)m(SiOTA)n, the average molecular weight of P(SiO)m(SiOTA)n, the average molecular weight of P(SiO)m(SiOQAEp)n, and quaternary ammonium conversion rate, respectively [28]. The weight average molecular weight (M w0), number average molecular weight (M n0), and the polydispersity index (PDI) of the P(SiO)m(SiOTA)n were measured by GPC, which were listed in Table 1. The M n of the P(SiO)m(SiOTA)n are in the range of 2,000–10,000 g/mol and PDI are less than 1.4. As is known, the polymerization was not well-controlled, and the molecular weight distribution or the molecular weight polydispersity index (PDI) (<1.4) of the synthesized polymer was suitable for the theoretical analysis [29]. This phenomenon has been observed at most cases of polymerization [30, 31]. On the whole, the measurement data are consistent with the design of experiment. The results of m and n values and the average M w of P(SiO)m(SiOQAEp)n were listed in Table 2. According to the structure of the P(SiO)m(SiOQAEp)n molecule, m means the length of hydrophobic (organosilicon group) chains, and n means the length of hydrophilic (quaternary ammonium group) chains.
Thermal properties of P(SiO)m(SiOQAEp)n
DSC is used widely for the examination of polymeric materials to determine their thermal transitions, such as glass transitions of an amorphous polymer [32]. The DSC curves of P(SiO)m(SiOQAEp)n samples are presented in Fig. 1. The glass transitions appear as an inflection point in the baseline of the DSC signal due to the sample undergoing a change in heat capacity, yet no formal phase change occurs. The glass transition temperature (T g) of P(SiO)m(SiOQAEp)n is shown in Fig. 1. The result indicates that P(SiO)m(SiOQAEp)n surfactant has excellent low temperature flexibility as well as chemical stability. T g value of the P(SiO)m(SiOQAEp)n decreases with the expansion of polymer chain, from P1 (−16.35 °C) to P2 (−33.85 °C) to P3 (−46.75 °C) to P4 (−66.42 °C). It is reported that the intermolecular forces and high molecular compliant state result in a low glass transition temperature and low surface energy [9, 33]. Hence, the value T g of P(SiO)m(SiOQAEp)n is affected by polymer chain flexibility and the length of siloxane chain. Siloxane chain has free rotation of –Si–O–Si– bond (bond angle 130.5°), which makes siloxane linkage low cohesive energy and very flexible, even at low temperature [33]. The longer the siloxane chain of P(SiO)m(SiOQAEp)n is, the lower is T g [34]. That effect also has been observed in polyurethane [11] and new polysiloxanes [35].
TGA is an especially useful technique for the thermal stability study of polymeric materials. The changes in physical and chemical properties of materials are measured as a function of increasing temperature (with constant heating rate) and can provide information about chemical phenomena of decomposition. It can be seen from Fig. 2 that the P(SiO)m(SiOQAEp)n weight keeps on decreasing from the beginning, that is to say the P(SiO)m(SiOQAEp)n are hygroscopic, which is attributed to the hydrophilic quaternary ammonium ion group [13]. Obviously, the thermogram of P(SiO)m(SiOQAEp)n has divided into two decrease stages: the first stage of degradation is attributed to the dehydration and the decomposition of the QAS groups, and the second stage is related to the silicone polymer chain degradation [36, 37]. The first stage decomposition temperature of P(SiO)m(SiOQAEp)n is 230 °C or so, which outdistances the decomposition temperature of phenolic resin containing quaternary ammonium halide groups (160 °C) [28]. The siloxane moiety has partial ionic nature and high bond dissociation energy of Si–O bond [9, 38], so the presence of siloxane skeleton in the organosilicon quaternary P(SiO)m(SiOQAEp)n delays the degradation process, and the thermal stability is strengthened by the siloxane chain lengths [39]. It is shown in Table 3 that the residual content of P(SiO)m(SiOQAEp)n depends on the molecular weight. The reason is that the residual ash of up to 700 °C comes from Si–O bond. Higher molecular weight means more SiO2 residue.
Adsorption and surface behavior of P(SiO)m(SiOQAEp)n
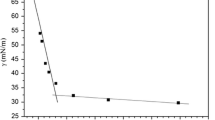
P(SiO)m(SiOQAEp)n is a kind of polymeric organosilicon surfactant and has all the properties that surfactants possessed. In colloidal and surface chemistry, the critical micelle concentration (CMC) is an important characteristic of a surfactant. It is defined as the concentration of surfactants above which micelles form. Before reaching the CMC, the surface tension changes strongly with the concentration of the surfactant, and after the CMC, the surface tension remains relatively constant or changes with a lower slope, so the CMC can be determined at the intersection point of the plots of the surface tension against the logarithm of the surfactant concentration [8].
Generally, various surface active parameters such as adsorption efficiency, pC20, surface excess concentration Γ, and area per adsorbed molecule A min are used to evaluate the surface activities of the surfactants. They can be calculated from the Gibbs equation [6, 40]. The pC20 is defined as Eq. 4:
The value of pC20 represents the efficiency of the negative logarithm of the surfactant concentration, which characterizes the reduction of the surface tension or interfacial tension of water by 20 mN/m [41]. The larger is the pC20 value, the higher is the adsorption efficiency of the surfactant.
The maximum surface excess concentration of surfactant at the air/liquid interface, Γ max, which depends on the molecular structures of surfactants, also called adsorption capacity, can be estimated by applying the Gibbs adsorption isotherm to the surface tension data:
where R is a gas constant (8.314 J mol−1 K−1), T is the absolute temperature, and the Gibbs pre-factor “n” takes the value 2 for an ionic surfactant where the surfactant ion and the counterion are univalent in the absence of added electrolyte in aqueous solution [42].
The surface area per molecule can be calculated from Eq. 6:
where N A is Avogadro’s number.
The parameters calculated from Eq. (4) to (6) are listed in Table 4.
As shown in Fig. 3 and Table 4, the P(SiO)m(SiOQAEp)n (P2) with the molecular weight of 4,665 g/mol have lower CMC (1.03 × 10−4 mol/L) and can effectively reduce the surface tension of water low to 26.92 mN/m at CMC because the polysiloxane has super hydrophobility and low surface energy. Furthermore, the CMC values of P2 increase with the rise of the temperature because it enhances the motion of molecules or ions of the surfactant, which results in the breakage or recombination of micelles through weakening water–water hydrogen bonds within the micelle interface easily [22]. The γ CMC of P2 solution decreases from 26.92 to 23.31 mN/m when the temperature increased from 298.15 to 318.15 K. As the temperature is rising, the liquid molecular thermal motion accelerates, the kinetic energy enlarges, and the intermolecular attraction weakens so that the efficiency of reducing the surface tension of water is gradually enhanced [43]. A min increased also with an increase of temperature, and correspondingly, Γ max have the opposite trend. These phenomena can be ascribed to enhance molecular motion at increasing temperature; thus, fewer surfactant molecules may be adsorbed at the air–water interface. Thus, the adsorption capacity becomes weaker with the rise of temperature, and the arrangement of surfactants molecules at the surface becomes looser [41].
Surfactants containing long hydrophobic chain tail have better surface activity than the short ones. P(SiO)m(SiOQAEp)n is a kind of polymer quaternary ammonium salt, which leads to the surface active parameters and also varies with the molecular weight at the same temperature. Take 25 °C as an example, as shown in Fig. 4 and Table 5, the CMC values of P(SiO)m(SiOQAEp)n decrease with the increase of the molecular weight. The γ CMC of the P(SiO)m(SiOQAEp)n solutions decreased from 29.32 to 23.42 mN/m with the increase of molecular weight from 3,147 to 12,996 g/mol, respectively. It is suggested that the low surface tension of P(SiO)m(SiOQAEp)n has been attributed to both the preponderant methyl substituents and siloxane chain. The flexible Si–O–Si backbone lies on the water surface, exposing the methyl groups to the air like an “umbrella” [44], which allows the methyl groups to orient in low energy configurations and show its high surface activity. A greater value of m means the more hydrophobic “umbrella” units, which exhibits the better surface active properties to form micelles at a low concentration in solution. Α min of P(SiO)m(SiOQAEp)n increases with the rise of the molecular weight and Γ max have the opposite trend, which means the connection of spacer in the high molecular weight surfactant is arranged looser at the water–air interface than that of corresponding lower molecule weight surfactant [45]. In the molecule of P(SiO)m(SiOQAEp)n, the so-called umbrella conformation with the siloxanyl group orienting parallel to the water surface causes a greater distance between the surfactant molecules and looser arrangement of the molecules at the air/water interface [46]. Greater values of m and n mean more groups loosely orienting to the water surface to occupy surface area, leading to Α min increase and Γ max decrease.
Thermodynamics of micellization
Similarly, the conductivity measurements were often used to determine the CMC. Figure 5 is the plot of electric conductivity versus concentration of P2 solution at various temperatures. Clearly, the electric conductivity value has two different increase rates with the rise of concentration. The intersection point of the two straight lines of different slope corresponds to the CMC [41]. Thus, the CMCs of P1, P3, and P4 were obtained and listed in Table 7.
The CMCs are almost equal to the one determined by surface tension measurements. From the plot of electric conductivity versus concentration of P(SiO)m(SiOQAEp)n solution, the ionization degree α can be calculated from the slopes of the two lines before and after the CMC according to Eq. 7, and the counterion binding degree β was calculated by β = 1 − α [22].
The thermodynamic parameters of micellization, such as the standard Gibbs energy (ΔG 0 m), the standard entropy (ΔS 0 m), and the standard enthalpy (ΔH 0 m), are calculated by Gibbs equation with the mass action model [47]. The standard Gibbs free energy of micellization is calculated with the following equation:
where R is the gas constant (8.314 J mol−1 K−1); T is the absolute temperature; n is the number of quaternary ammonium group units and the value was determined by non-aqueous titration; β is the counterion binding degree; X CMC is the mole fraction of silicone surfactant at CMC; and X CMC = CMC/55.4, 55.4 is the mole number of 1 L water at 25 °C.
Therefore, the standard enthalpy of the micellization can be obtained from the Gibbs-Helmholtz equation:
The entropy of micellization is calculated with the following equation:
Table 6 shows thermodynamic parameters of micellization of P2 at various temperatures, and Table 7 shows thermodynamic parameters of micellization of P(SiO)m(SiOQAEp)n at 25 °C. As shown in Table 6, it can be seen that the β values of P2 at different temperatures are all pretty low, which indicates that the self-repulsion of the hydrophilic head groups and the counterions is stronger than the attraction between the hydrophilic head groups and the counterions [22, 48]. The molecule has a lower preference in binding counter ions at the micelle surface, thus increasing the electrostatic repulsion between hydrophilic head groups of the surfactant molecules [49], and the values of β change small with the rise of temperature, that is, the temperature has little effect on the β values.
As shown in Tables 6 and 7, all the standard Gibbs free energy (ΔG 0 m) of micellization is negative, which indicates that micelle formation of the aqueous solutions of P(SiO)m(SiOQAEp)n is a spontaneous process. The standard free energy decreases with increasing hydrophobic chain length m, indicating that the aggregation forms more easily. It is known that the repulsion of hydrophobic chains and water molecules is the motivating factor in micelle formation [50]. With the increasing hydrophobic chain length, the hydrophobic chain water molecule repulsion is larger than the electrostatic attraction interactions between molecules, which enforces the surfactant molecules to aggregate to micelle by their van der Waals interaction to pass into a less energetic stable state [51, 52]. The standard enthalpy (ΔH 0 m) of the micellization is negative, which suggests that the formation of micelles is an exothermic process, and the London dispersion interaction is the major attractive force for micellization [53]. That can explain why the transference of the silicone chain from the aqueous environment to the interior of the micelle is an exothermic process [54]. It is worth noting that the value of ΔG 0 m is mainly contributed by ΔH 0 m, which indicates that the micellization process of P(SiO)m(SiOQAEp)n in aqueous solution is enthalpy-driven. Furthermore, the ΔS 0 m values of P(SiO)m(SiOQAEp)n decrease with temperature, which is due to the breakage of hydrogen bonds in water with temperature. When the P(SiO)m(SiOQAEp)n dissolves in water, the hydrophobic group of the surfactant in some way damages the “iceberg structure” of water molecules.
Overall, the micelle formation in aqueous solutions of P(SiO)m(SiOQAEp)n is relevant to the molecular weight. This can be explained that the surfactant with higher molecular weight has the longer Si–O–Si chain length. And the molecular chain length can increase its softness, so the molecular chain is easy to curl and the surfactant is easy to form a single molecule or molecular micelle. It can be concluded that the structures of the hydrophobic groups of the cationic silicone surfactants have a significant influence on the driving force of the aggregate formation [41].
Antibacterial activity
MBC means the minimum bactericidal concentration to kill all the bacterial. Therefore, the lower is the MBC value of an agent, the higher is the antimicrobial activity [55].
The MBC values of P(SiO)m(SiOQAEp)n are shown in Fig. 6. It can be seen that P(SiO)m(SiOQAEp)n exhibit an excellent antibacterial activity against B. subtilis, S. aureus, and E. coli. Furthermore, the increase of molecular weight of P(SiO)m(SiOQAEp)n leads to high antimicrobial activity and wide antimicrobial spectrum by the comparison of MBC. It is suggested that the antibacterial activity is due to the positively charged nitrogen atom in polymers [56]. The P(SiO)m(SiOQAEp)n are long chain quaternary ammonium salts, higher molecular weight means more number of cationic groups, which makes the antibacterial activity stronger. The reason is that long quaternary group chain with high molecular weight can dissolve the fat cell of the bacteria easily [57]. In addition, the bacterial species also influence the antibacterial activity of agents [20, 58]. B. subtilis is most sensitive to the P(SiO)m(SiOQAEp)n, followed by S. aureus, and E. coli was most insensitive. As shown in Fig. 7, P(SiO)m(SiOQAEp)n exhibits a large and clear inhibition zone on solid agar media against B. subtilis and S. aureus in contrast to E. coli.
Generally, there are three elementary processes to inhibit or kill the bacteria: (1) adsorption onto the negatively charged bacterial cell surface; (2) penetrating the cell wall; (3) and binding to the cytoplasmic membrane and damaging the cytoplasmic membrane [22, 57]. Bacteria can be divided into two classes, Gram-positive bacteria (i.e., S. aureus) and Gram-negative bacteria (i.e., E. coli). Gram-positive bacteria tend to have a loose cell wall, while Gram-negative bacteria have an outer membrane structure in the cell wall and form an additional barrier against foreign molecules. QACs are typically more easily absorbed on the loose cell wall and diffused to the cytoplasmic membrane and then damaged the cell structure [56, 59]. Clearly, the schematic mechanism for killing the bacteria and bacteria structure is shown in Fig. 8.
Conclusions
The polysiloxane quaternary ammonium salts containing epoxy group (P(SiO)m(SiOQAEp)n) with different molecular weight and cationic concentration had been synthesized. The aggregation behaviors of in aqueous solution, antibacterial activities, and thermal stability of P(SiO)m(SiOQAEp)n were investigated. It is shown that P(SiO)m(SiOQAEp)n gives unusual properties at the air/water interface and in bulk solution. As expected, the surfactants show fairly low CMC and high efficiency in lowering surface tension. The molecule weight has significant impacts on the properties of the surfactants’ properties. The negative standard Gibbs free energy (ΔG 0 m) and standard enthalpy (ΔH 0 m) of micellization suggest that micelle formation of P(SiO)m(SiOQAEp)n in an aqueous solution is a spontaneous and exothermic process. The enthalpy of micellization ΔH 0 m is the main contribution to ΔG 0 m so that the micellization process is an enthalpy-driven process. It is also found that the P(SiO)m(SiOQAEp)n exhibits an excellent antibacterial activities against B. subtilis, S. aureus, and E. coli. In addition, P(SiO)m(SiOQAEp)n has excellent low temperature flexibility as well as chemical and thermal stability. From a practical point of view, it is interesting that those surfactants, when incorporated in polymers with epoxy quaternary ammonium salt group, would work as antibacterial agents in the medical field, wastewater treatment, and so on.
References
Hill RM (2002) Silicone surfactants—new developments. Curr Opin Colloid Interface Sci 7:255–261
Hill RM, He M, Davis HT, Scriven LE (1994) Comparison of the liquid crystal phase behavior of four trisiloxane superwetter surfactants. Langmuir 10:1724–1734
Majumdar P, Lee E, Patel N, Stafslien SJ, Daniels J, Chisholm BJ (2008) Development of environmentally friendly, antifouling coatings based on tethered quaternary ammonium salts in a crosslinked polydimethylsiloxane matrix. J Coat Technol Res 5:405–417
Deng XB, Luo R, Chen HL, Liu BL, Feng YJ, Sun YH (2007) Synthesis and surface properties of PDMS–acrylate emulsion with gemini surfactant as co-emulsifier. Colloid Polym Sci 285:923–930
Tan JL, Ma DP, Feng SY, Zhang CQ (2013) Effect of headgroups on the aggregation behavior of cationic silicone surfactants in aqueous solution. Colloid Surface A 417:146–153
Tan JL, Zhao PJ, Ma DP, Feng SY, Zhang CQ (2013) Effect of hydrophobic chains on the aggregation behavior of cationic silicone surfactants in aqueous solution. Colloid Polym Sci 291:1487–1494
Peng ZL, Huang JF, Chen FR, Ye QH, Li QY (2011) Syntheses and properties of ethoxylated double-tail trisiloxane surfactants containing a propanetrioxy spacer. Appl Organometal Chem 25:383–389
Luo RX, Liu PP, Chen YB (2013) Synthesis and properties of a hydrolysis resistant cationic trisiloxane surfactant. J Surfact Deterg 16:33–38
Schmaucks G, Sonnek G, Wustneck R, Herbst M, Ramms M (1992) Effect of siloxanyl groups on the interfacial behavior of quaternary ammonium compounds. Langmuir 8:1724–1730
Nigmatullin R, Gao FG (2012) Onium-functionalised polymers in the design of non-leaching antimicrobial surfaces. Macromol Mater Eng 297:1038–1074
Askari F, Barikani M, Barmar M (2013) Study on thermal stability of polyurethane-urea based on polysiloxane and polycaprolactone diols. Korean J Chem Eng 30:2093–2099
Zhou WJ, Yang H, Guo XZ, Lu JJ (2006) Thermal degradation behaviors of some branched and linear polysiloxanes. Polym Degrad Stabil 91:1471–1475
Kang JJ, Li WY, Lin Y, Li XP, Xiao XR, Fang SB (2004) Synthesis and ionic conductivity of a polysiloxane containing quaternary ammonium groups. Polym Adv Technol 15:61–64
Huang ZX, Yu YZ, Huang Y (2002) Ion aggregation in the polysiloxane ionomers bearing pendant quaternary ammonium groups. J Appl Polym Sci 83:3099–3104
Sauvet G, Dupond S, Kazmierski K, Chojnowski J (2000) Biocidal polymers active by contact. V. Synthesis of polysiloxanes with biocidal activity. J Appl Polym Sci 75:1005–1012
Dizman B, Elasri MO, Mathias LJ (2006) Synthesis and antibacterial activities of water-soluble methacrylate polymers containing quaternary ammonium compounds. J Polym Sci Pol Chem 44:5965–5973
Kenawy ER, Abdel-Hay FI, El-Shanshoury AERR, El-Newehy MH (1998) Biologically active polymers: synthesis and antimicrobial activity of modified glycidyl methacrylate polymers having a quaternary ammonium and phosphonium groups. J Control Release 50:145–152
Kanazawa A, Ikeda T, Endo T (1994) Polymeric phosphonium salts as a novel class of cationic biocides VIII. Synergistic effect on antibacterial activity of polymeric phosphonium and ammonium salts. J Appl Polym Sci 53:1245–1249
Muñoz-Bonilla A, Fernández-García M (2012) Polymeric materials with antimicrobial activity. Prog Polym Sci 37:281–339
Chen CZ, Beck-Tan NC, Dhurjati P, Van Dyk TK, LaRossa RA, Cooper SL (2000) Quaternary ammonium functionalized poly (propylene imine) dendrimers as effective antimicrobials: structure-activity studies. Biomacromolecules 1:473–480
Lu GQ, Wu DC, Fu RW (2007) Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React Funct Polym 67:355–366
Liu YM, Xu J, Cui YQ, Liu LB, Li JY (2014) Synthesis, surface properties and antibacterial activity of quaternary ammonium salts containing epoxide group. J Disper Sci Technol 35:1460–1467
Liu YM, Cui X, Hao CM, Tao FR, Li JY (2014) Modified gelatin with quaternary ammonium salts containing epoxide groups. Chinese Chem Lett 25:1193–1197
Negm NA, Mohamed AS (2008) Synthesis, characterization and biological activity of sugar-based gemini cationic amphiphiles. J Surfact Deterg 11:215–221
Wang GY, Du ZP, Li QX, Zhang W (2010) Carbohydrate-modified siloxane surfactants and their adsorption and aggregation behavior in aqueous solution. J Phys Chem B 114:6872–6877
Li YJ, Zhang BY, Hoskins JN, Grayson SM (2012) Synthesis, purification, and characterization of “perfect” star polymers via “click” coupling. J Polym Sci Pol Chem 50:1086–1101
Xie HQ, Chen Y, Guan JG, Xie D (2006) Novel method for preparation of quaternary ammonium ionomer from epoxidized styrene–butadiene–styrene triblock copolymer and its use as compatibilizer for blending of styrene–butadiene–styrene and chlorosulfonated polyethylene. J Appl Polym Sci 99:1975–1980
Xie HQ, Gao Y, Zhan XG, Xie D (2004) Selection of quaternary ammonium groups for optimizing properties of water-soluble, photosensitive phenolic resins and study of their postcuring mechanism. J Appl Polym Sci 91:2914–2922
Xu JX, Wang XH, Zhang YY, Zhang WQ, Sun PC (2012) RAFT-mediated batch emulsion polymerization of styrene using poly[N-(4-vinylbenzyl)-N, N-dibutylaminehydrochloride] trithiocarbonate as both surfactant and macro-RAFT agent. J Polym Sci Pol Chem 50:2484–2498
Rajendran PB, Raghavachari D (2012) Synthesis of graft copolymers onto styrenic polymer backbone via “grafting from” RAFT process. J Polym Sci Pol Chem 50:4772–4782
Hauffman G, Rolland J, Bourgeois JP, Vlad A, Gohy JF (2013) Synthesis of nitroxide-containing block copolymers for the formation of organic cathodes. J Polym Sci Pol Chem 51:101–108
Amass W, Amass A, Tighe B (1998) A review of biodegradable polymers: uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym Int 47:89–144
Soni SS, Sastry NV, Aswal VK, Goyal PS (2002) Micellar structure of silicone surfactants in water from surface activity, SANS and viscosity studies. J Phys Chem B 106:2606–2617
Zheng Y, Tan YX, Dai L, Lv Z, Zhang XZ, Xie ZM, Zhang ZJ (2012) Synthesis, characterization, and thermal properties of new polysiloxanes containing 1,3-bis(silyl)-2,4-dimethyl-2,4-diphenylcyclodisilazane. Polym Degrad Stabil 97:2449–2459
Kumar RS, Alagar M (2006) Studies on mechanical, thermal, and morphology of diglycidylether-terminated polydimethylsiloxane-modified epoxybismaleimide matrices. J App Polym Sci 101:668–674
Kweon JO, Lee YK, Noh ST (2001) Synthesis and thermal behavior of poly(ethylene oxide) poly(N-substituted urethane). J Polym Sci Pol Chem 39:4129–4138
Sreedhar B, Sairam M, Chattopadhyay DK, Mitra PP, Mohan Rao DV (2006) Thermal and XPS studies on polyaniline salts prepared by inverted emulsion polymerization. J Appl Polym Sci 101:499–508
Walsh R (1981) Bond dissociation energy values in silicon-containing compounds and some of their implications. Acc Chem Res 14:246–252
Filippou AC, Baars B, Chernov O, Lebedev YN, Schnakenburg G (2014) Silicon–oxygen double bonds: a stable silanone with a trigonal-planar coordinated silicon center. Angew Chem Int Ed 53:565–570
Badawi AM, Mekawi MA, Mohamed AS, Mohamed MZ, Khowdairy MM (2007) Surface and biological activity of some novel cationic surfactants. J Surfact Deterg 10:243–255
Asadov ZH, Rahimov RA, Ahmadova GA, Mammadova KA (2012) Synthesis, surface active and thermodynamic parameters of novel quaternary ammonium salts. J Surfact Deterg 15:721–727
Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv Colloid Interfac 97:205–253
Hsu CT, Chang CH, Lin SY (1999) A study of surfactant adsorption kinetics: effect of intermolecular interaction between adsorbed molecules. Langmuir 15:1952–1959
Snow SA, Fenton WN, Owen MJ (1990) Synthesis and characterization of zwitterionic silicone sulfobetaine surfactants. Langmuir 6:385–391
Mohamed AS, Mohamed MZ (2010) Preparation of novel cationic surfactants from epichlorohydrin: their surface properties and biological activities. J Surfact Deterg 13:159–163
Snow SA, Fenton WN, Owen MJ (1991) Zwitterionic organofunctional siloxanes as aqueous surfactants: synthesis and characterization of betaine functional siloxanes and their comparison to sulfobetaine functional siloxanes. Langmuir 7:868–871
Shimizu S, Pires PAR, Seoud OAEI (2004) Thermodynamics of micellization of benzyl(2-acylaminoethyl)dimethylammonium chloride surfactants in aqueous solutions: a conductivity and titration calorimetry study. Langmuir 20:9551–9559
Shi LJ, Li N, Yan H, Gao YA, Zheng LQ (2011) Aggregation behavior of long-chain N-aryl imidazolium bromide in aqueous solution. Langmuir 27:1618–1625
Li XW, Gao YA, Liu J, Zheng LQ, Chen B, Wu LZ, Tung CH (2010) Aggregation behavior of a chiral long-chain ionic liquid in aqueous solution. J Colloid Interface Sci 343:94–101
Wettig SD, Nowak P, Verrall RE (2002) Thermodynamic and aggregation properties of gemini surfactants with hydroxyl substituted spacers in aqueous solution. Langmuir 18:5354–5359
Wang JJ, Wang HY, Zhang SL, Zhang HC, Zhao Y (2007) Conductivities, volumes, fluorescence, and aggregation behavior of ionic liquids [C4mim][BF4] and [Cnmim]Br (n = 4, 6, 8, 10, 12) in aqueous solutions. J Phys Chem B 111:6181–6188
Wettig SD, Li XF, Verrall RE (2003) Thermodynamic and aggregation properties of gemini surfactants with ethoxylated spacers in aqueous solution. Langmuir 19:3666–3670
Ao MQ, Huang PP, Xu GY, Yang XD, Wang YJ (2009) Aggregation and thermodynamic properties of ionic liquid-type gemini imidazolium surfactants with different spacer length. Colloid Polym Sci 287:395–402
Zhang L, Lv X, Zhu YJ, Zhang J, Wang H, Tan YB (2011) Synthesis and properties of new polymeric surfactant with quaternary ammonium salt. Colloid Polym Sci 289:1579–1587
Dizman B, Elasri MO, Mathias LJ (2004) Synthesis and antimicrobial activities of new water-soluble bis-quaternary ammonium methacrylate polymers. J Appl Polym Sci 94:635–642
Rózga-Wijas K, Mizerska U, Fortuniak W, Chojnowski J, Hałasa R, Werel W (2007) Quaternary ammonium salts (QAS) modified polysiloxane biocide supported on silica materials. J Inorg Organomet Polym 17:605–613
Cloete TE (2003) Resistance mechanisms of bacteria to antimicrobial compounds. Int Biodeterior Biodegrad 51:277–282
Tiller JC, Lee SB, Lewis K, Klibanov AM (2002) Polymer surfaces derivatized with poly (viny-N-hexylpyridinium) kill airborne and waterborne bacteria. Biotechnol Bioeng 79:465–471
Temiz A, Özmen Toğay S, Sener A, Güven G, Rzaev ZMO, Piskin E (2006) Antimicrobial poly(N-vinyl-2-pyrrolidone-alt-maleic anhydride)/poly(ethylene imine) macrocomplexes. J Appl Polym Sci 102:5841–5847
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (No. 21406121 and No. 21376125), the Foundation for the Excellent Middle-aged and Young Scientist of Shandong Province (No. BS2013CL037), and the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, X., Qiao, C., Wang, S. et al. Synthesis, surface properties, and antibacterial activity of polysiloxane quaternary ammonium salts containing epoxy group. Colloid Polym Sci 293, 1971–1981 (2015). https://doi.org/10.1007/s00396-015-3588-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3588-6