Abstract
Continuing with our previous work, in which CdSe nanoparticles were functionalized with polystyrene (PS) brushes (CdSe-PS) by the grafting through method, nanocomposites were prepared by adding them to a poly(styrene-b-butadiene-b-styrene) (SBS) triblock copolymer. After characterizing CdSe-PS nanoparticles obtained at different polymerization times of 3, 5, and 8 h by means of thermogravimetric analysis and gel permeation chromatography, CdSe-PS nanoparticles obtained after 5 h of polymerization (CdSe-PS(5h)) were chosen as the most adequate for the generation of nanocomposites. Atomic force microscopy (AFM) was used for morphological characterization of SBS/CdSe-PS(5h) nanocomposites. AFM images showed a good dispersion of the nanoparticles in the block copolymer, with the placement of the nanoparticles in the PS domains due to the improved affinity obtained by their functionalization with PS brushes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Self-assembly of block copolymers has been studied extensively for more than four decades due to the rich variety of nanostructures that can be generated depending on the nature of the blocks, molecular weight, composition, and processing characteristics [1–7]. These kinds of materials could open a way to generate different nanostructures with applications in fields such as medicine [8, 9] and electronics [10]. In that way, selective incorporation of inorganic nanoparticles into specific locations of nanophase-separated structures could enable to obtain nanocomposites with prominent features such as optic, electronic, and magnetic properties, which could lead to the generation of remarkable photonic and microelectronic devices [11–20].
On the other hand, semiconductor nanocrystals, such as CdSe quantum dots, vividly illustrate the impact of the nanoparticle size on electronic properties. In these nanoparticles, quantum confinement gives discrete electronic states, quite distinct from the classic valence–conduction band description. In the case of nanocomposites prepared with CdSe quantum dots, it is critical to preserve their optical and electronic properties (desirable for applications in electronic materials and biosensors) and to obtain a uniform dispersion of the nanoparticles in the matrix, avoiding their agglomeration. One way to overcome this problem is the functionalization of the nanoparticle surface improving their affinity with the host phase of the copolymer [21, 22].
In this work, CdSe functionalized with polystyrene brushes by the grafting through method, obtained in previous work, were added to a poly(styrene-b-butadiene-b-styrene) (SBS) block copolymer which self-assembles into a lamellar morphology. Morphology of the obtained nanocomposites was analyzed by atomic force microscopy (AFM).
Experimental section
Materials
2-Mercaptoethanol, cadmium sulfate hydrate (3CdSO4·8H2O), selenium metal powder (Se), sodium sulfite (Na2SO3), and N,N-dimethylformamide were purchased from Panreac and used without further purification. Styrene monomer, 2,2′-azobisisobutyronitrile, and methacryloxypropyltrimethoxysilane (MPS) were supplied by Aldrich and used without further purification. Styrene monomer (99 %) was distilled under reduced pressure over CaH2 before use. SBS linear triblock copolymer (Dynasol C540), with 40 wt% polystyrene, was kindly supplied by Repsol-YPF. M n of polystyrene (PS) and PB blocks was around 30,000 and 45,000 g mol−1, respectively.
Polystyrene-grafted CdSe nanoparticle synthesis
CdSe nanoparticles with polystyrene chains on the surface were prepared in a previous work [23]. In the first step, mercaptoethanol-stabilized nanoparticles, synthesized by aqueous methods [24], were modified in their surface with MPS silane group (CdSe-MPS), anchoring a double bond on the surface. In the second step, polymerization of styrene was carried out for 3, 5, and 8 h with CdSe-MPS nanoparticles in the reaction media. UV–Vis and XRD measurements demonstrated that the size of the nanoparticles did not change after the functionalization with PS chains [23].
Nanocomposite preparation
Samples for AFM were prepared by casting toluene solutions of SBS/CdSe-PS mixtures (5 and 10 wt% of nanoparticles) onto glass substrates. Samples were then annealed for 24 h at 102 °C under vacuum and 24 h at room temperature for solvent removal. Previous rheological and morphological studies of SBS block copolymer set this annealing treatment as adequate in order to obtain a self-ordered structure [25].
Characterization
Thermogravimetric analysis (TGA) was performed with TGA/SDTA851 (Mettler Toledo) under nitrogen atmosphere at a heating rate of 10 K/min from room temperature to 600 °C. Samples were prepared by drying under vacuum for solvent removal.
Molecular weight and molecular weight distribution of polystyrene chains were determined by gel permeation chromatography (GPC) with a PerkinElmer chromatograph equipped with a binary pump and a refractive index detector. The mobile phase was THF, and the separation was carried out with four Phenomenex columns with an elution rate of 1 mL/min at 30 °C. Columns were calibrated with polystyrene standards before the measurements, according to standard procedures. Polystyrene solutions were filtered through a nylon filter with a pore size of 0.20 μm.
Atomic force microscopy images were obtained in tapping mode with a scanning probe microscope (Nanoscope IIIa Multimode, Digital Instruments) equipped with an integrated phosphorous-doped tip/cantilever having a resonance frequency of approximately 75 kHz and a spring constant of about 3 N/m; the tip radius was lower than 10 nm. Images show the surface morphology in the left part and the simultaneously taken phase image in the right part.
CdSe-PS nanoparticles were also analyzed by transmission electron microscopy (TEM). A solution drop was deposited on a Formvar film Copper grid and examined in a Tecnai G2 20 Twin (FEI) microscope operating at an accelerating voltage of 200 keV in a bright-field image mode.
Results and discussion
CdSe nanoparticles functionalized with polystyrene brushes by the grafting through method were characterized in a previous work [23]. Polymerization times of 3, 5, and 8 h were used. Whereas CdSe-PS nanoparticles obtained after 3 h of polymerization (CdSe-PS(3h)) were not stable in toluene suspension and precipitated after few hours, functionalized nanoparticles obtained after 5 and 8 h (CdSe-PS(5h) and CdSe-PS(8h), respectively) were very stable with time.
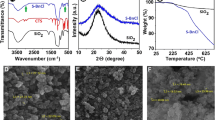
CdSe-PS nanoparticles were analyzed by TGA. Figure 1 shows the thermograms of the nanoparticles functionalized with polystyrene chains obtained after different polymerization times. CdSe-PS(3h) nanoparticles show a higher quantity of CdSe nanoparticles when comparing with those obtained after 5 and 8 h of polymerization. A value of 40 wt% CdSe was obtained for CdSe-PS(3h) nanoparticles, while values of 14 and 7.5 % were obtained for CdSe-PS(5h) and CdSe-PS(8h), respectively. There could be two reasons for this behavior. The first one could be the molecular weight of the polystyrene chains localized in the surface of the nanoparticles is lower. The second reason could be that as CdSe-PS(3h) precipitated from the suspension in toluene, the free polystyrene present in the solution was better eliminated. In addition, the temperature at which the weight loss of PS takes place has moved to higher values. As mentioned before, the higher amount of CdSe and the lower quantity of free PS could be the reason for this displacement [26].
GPC analysis was carried out to obtain the molecular weights of PS chains grafted on the nanoparticles surface. As in our previous work [23], to obtain an approximation of the molecular weight of PS chains, a polymerization at the same conditions but without the presence of nanoparticles in the media was carried out. Molecular weights obtained for the PS chains for CdSe-PS(3h), CdSe-PS(5h), and CdSe-PS(8h) were 9,000, 15,000, and 20,000, respectively. As CdSe-PS(3h) nanoparticles were not stable in toluene suspension, and taking into account the molecular weight of the PS block in the SBS block copolymer (30,000), CdSe-PS(5h) were chosen to prepare the nanocomposites. In these nanoparticles, the molecular weight of the PS chains is lower than that of the PS block chains. As in previous works, a good dispersion was obtained for nanoparticles with brushes with a molecular weight of 20,000 [27] and a better wetting could be expected for nanoparticles modified with brushes with lower molecular weight [28]; thus, CdSe-PS(5h) ones were chosen.
Once functionalized CdSe nanoparticles were chosen, they were added to the SBS block copolymer to prepare the nanocomposites. The morphology of the nanocomposites was analyzed by AFM. As it was commented above, an annealing treatment was done to obtain a self-ordered structure. As seen in previous works [27], a lamellar structure was obtained for the neat block copolymer.
For 5 wt% SBS/CdSe-PS nanocomposites (Fig. 2), the morphology of the SBS matrix was not significantly altered compared with that of neat SBS, obtaining a lamellar structure. Figure 2a shows CdSe-PS nanoparticles located in the PS phase. The value of the phase intensity has grown (brighter dots), thus indicating the presence of a harder material. These points are located in the same position both in the phase and height images. Therefore, the affinity between CdSe nanoparticles and the PS block of the copolymer has been improved. In Fig. 2b, a wider area of the nanocomposite can be seen. The good dispersion of the nanoparticles in the block copolymer is maintained, with no remarkable agglomerations. In addition, the bright dots are only located in the PS phase.
Taking into account obtained nanoparticle size values [23], it is evident that the points are not single nanoparticles. In the grafting through functionalization method, the polymerization was carried out with silane-modified nanoparticles in the media, and distinctly from the grafting from method, where the polymer chain grows only from the surface of the nanoparticle, the functional group located in the surface of the nanoparticle could join a growing polystyrene chain in which there could be more nanoparticles previously joined. As the surface of the nanoparticles is multifunctional, with several double bonds, several chains could be bonded to different nanoparticles [29]. In fact, some polystyrene chains have grown from the surface of the nanoparticles, while some others are surrounding the nanoparticles. Due to these reasons, it is supposed that CdSe nanoparticles, instead of being located individually, are bonded together with several polymer chains, creating a kind of network formed by nanoparticles and PS chains. Scheme 1 shows the creation of the network. To assure the disposition of nanoparticles, TEM measurements were carried out. Figure 3 shows the TEM image of CdSe-PS nanoparticles. As it can be seen, nanoparticles are not found alone. There are small groups (highlighted with circles) of nanoparticles and PS chains found together. These groups or networks are the ones placed in the PS phase of the block copolymer.
When CdSe-PS nanoparticle amount is increased to 10 wt%, the lamellar self-structure of the block copolymer is still maintained. As it can be seen in Fig. 4, nanoparticles are located only in the PS phase of the block copolymer. As the amount of nanoparticles is increased, the number of bright dots has also increased. Figure 4a shows that nanoparticles, due to their high amount, have started to agglomerate in the PS lamellas. Only in very few cases the size of these agglomerates is big enough to break the PS lamella. Anyway, the agglomerates seem to be small enough for being located at PS lamellas, and a good dispersion is obtained. As in the previous case, also for a wider area (Fig. 4b), a good dispersion of the nanoparticles is obtained for SBS/CdSe-PS (10 wt%) nanocomposites, with no remarkable big agglomeration of CdSe-PS nanoparticles that would break completely the lamellar structure.
Conclusions
An efficient method for obtaining nanocomposites based in semiconductive CdSe nanoparticles has been developed. After the obtention and characterization of CdSe nanoparticles functionalized with PS brushes by the grafting through method in a previous work, nanocomposites were prepared adding these nanoparticles to a SBS block copolymer that is able to organize into a lamellar morphology. Different polymerization times were analyzed by TGA and GPC measurements and finally CdSe-PS nanoparticles obtained after 5 h of polymerization were chosen for the preparation of nanocomposites. AFM measurements showed that a good dispersion of CdSe-PS nanoparticles was obtained, with them being located in the PS phase due to improvement of the affinity of the nanoparticles with this phase by the presence of PS brushes on the surface. As the size of the bright points located in the PS phase is too big for a single nanoparticle, it was supposed that the nanoparticles were creating a kind network together with PS chains. At any rate, the size of this network is small enough to be located at PS lamellas, maintaining the lamellar structure of the block copolymer without breaking it.
References
Helfand E, Wasserman ZR (1976) Block copolymer theory. 4. Narrow interphase approximation. Macromolecules 9:879–888
Leibler L (1980) Theory of microphase separation in block copolymers. Macromolecules 13:1602–1617
Matsen MW, Bates FS (1996) Unifying weak- and strong-segregation block copolymer theories. Macromolecules 29:1091–1098
Bates FS, Fredrickson GH (1999) Block copolymers—designer soft materials. Phys Today 52:32–38
Lodge TP (2003) Block copolymers: past successes and future challenges. Macrom Chem Phys 204:265–273
Zalakain I, Ramos JA, Fernandez R, Etxeberria H, Mondragon I (2011) Nanostructuration of self-assembled poly(styrene-b-isoprene-b-styrene) block copolymer thin films in a highly oriented pyrolytic graphite substrate. Thin Solid Films 519:1882–1885
Zalakain I, Ramos JA, Fernandez R, Etxeberria H, Mondragon I (2012) Silicon and carbon substrates induced arrangement changes in poly(styrene-b-isoprene-b-styrene) block copolymer thin films. J Appl Polym Sci 125:1552–1558
Gan Z, Jim TF, Li M, Yuer Z, Wang S, Wu C (1999) Enzymatic biodegradation of poly(ethylene oxide-b-ε-caprolactone) diblock copolymer and its potential biomedical applications. Macromolecules 32:590–594
Mequanint K, Patel A, Bezuidenhout D (2006) Synthesis, swelling behavior, and biocompatibility of novel physically cross-linked polyurethane-block-poly(glycerol methacrylate) hydrogels. Biomacromolecules 7:883–891
Peres LO, Gruber J (2007) The use of block copolymers containing PPV in gas sensors for electronic noses. Mater Sci Eng C 27:67–69
Bockstaller MR, Lapetnikov Y, Margel S, Thomas EL (2003) Size-selective organization of enthalpic compatibilized nanocrystals in ternary block copolymer/particle mixtures. J Am Chem Soc 125:5276–5277
Arora H, Li ZH, Sai H, Kamperman M, Warren SC, Wiesner U (2010) Block copolymer directed nanoporous metal thin films. Macromol Rapid Commun 31:1960–1964
Bockstaller MR, Mickiewicz RA, Thomas EL (2005) Block copolymer nanocomposites: perspectives for tailored functional materials. Adv Mater 17:1331–1349
Balazs AC, Emrick T, Russell TP (2006) Nanoparticle polymer composites: where two small worlds meet. Science 314:1107–1110
Glogowski E, Tangirala R, Russell TP, Emrick T (2006) Functionalization of nanoparticles for dispersion in polymers and assembly in fluids. J Polym Sci Polym Chem 44:5076–5086
Sun Z, Bai F, Wu H, Boye DM, Fan H (2012) Monodisperse fluorescent organic/inorganic composite nanoparticles: tuning full color spectrum. Chem Mater 24:3415–3419
Lin Y, Daga VK, Anderson ER, Gido SP, Watkins JJ (2011) Nanoparticle-driven assembly of block copolymers: a simple route to ordered hybrid materials. J Am Chem Soc 133:6513–6516
Lo CT, Lee B, Gao MW, Chou PW (2012) Ordering of block copolymer/nanoparticle composite thin films. Polym Int. doi:10.1002/pi.4303
Jang G, Kramer EJ, Hawker CJ (2011) Controlled supramolecular assembly of micelle-like gold nanoparticles in PS-b-P2VP diblock copolymers via hydrogen bonding. J Am Chem Soc 133:16986–16996
Jang G, Khan A, Hawker CJ, Kramer EJ (2012) Morphology evolution of PS-b-P2VP diblock copolymers via supramolecular assembly of hydroxylated gold nanoparticles. Macromolecules 45:1553–1561
Synytska A, Ionov L, Minko S, Motornov M, Elchorn K, Stamm M, Grundke K (2004) Tuning wettability by controlled roughness and surface modification using core-shell particles. Polym Mater Sci Eng 90:624–625
Wang TL, Yang CH, Shieh YT, Yeh AC (2009) Synthesis of CdSe–poly(N-vinylcarbazole) nanocomposite by atom transfer radical polymerization for potential optoelectronic applications. Macromol Rapid Commun 30:1679–1683
Etxeberria H, Zalakain I, Tercjak A, Eceiza A, Kortaberria G, Mondragon I (2012) Functionalisation of CdSe semiconductor nanoparticles with polystyrene brushes by radical polymerization. J Nanosci Nanotech. doi:10.1166/jnn.2012.6858
Etxeberria H, Kortaberria G, Zalakain I, Larrañaga A, Mondragon I (2012) Effect of different aqueous synthesis parameters on the size of CdSe nanocrystals. J Mater Sci 47:7167–7174
Garcia I, Tercjak A, Zafeiropoulos NE, Stamm M, Mondragon I (2007) Self-assembling nanomaterials using magnetic nanoparticles modified with polystyrene brushes. Macromol Rapid Commun 28:2361–2365
Yoon H, Lee J, Park DW, Hong CH, Shim SE (2010) Preparation and electrorheological characteristic of CdS/polystyrene composite particles. Colloid Polym Sci 288:613–619
Etxeberria H, Zalakain I, Fernandez R, Kortaberria G, Mondragon I (2012) Controlled placement of polystyrene-grafted CdSe nanoparticles in self-assembled block copolymers. Colloid Polym Sci. doi:10.1007/s00396-012-2765-0
Xu C, Ohno K, Ladmiral V, Composto RJ (2008) Dispersion of polymer-grafted magnetic nanoparticles in homopolymers and block copolymers. Polymer 49:3568–3577
Rahimi-Razin S, Haddadi-Asl V, Salami-Kalajahi M, Gehgoodi-Sadabad F, Roghani-Mamaqani H (2012) Matrix-grafted multiwalled carbon nanotubes/ poly(methyl methacrylate) nanocomposites synthesized by in situ raft polymerization: a kinetic study. Int J Chem Kinet 44:555–569
Acknowledgments
Financial support from the Basque Country Government (NanoIker IE11-304, SAIOTEK2012-S-PE12UN106) and Grupos Consolidados (IT-776-13) and from the Ministry of Education and Innovation (MAT 2009–06331 and MAT2012-31675) is gratefully acknowledged. H. Etxeberria thanks University of Basque Country (UPV/EHU) for the grant “Ayudas para la Contratación de Doctores Recientes hasta su Integración en Programas de Formación Postdoctoral”. Technical and human support provided by SGIker (UPV/EHU, MICINN, GV/EJ, ERDF, and ESF) is also acknowledged. In memoriam, this study is dedicated to Dr. Iñaki Mondragon Egaña.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Etxeberria, H., Zalakain, I., Mondragon, I. et al. Generation of nanocomposites based on polystyrene-grafted CdSe nanoparticles by grafting through and block copolymer. Colloid Polym Sci 291, 1881–1886 (2013). https://doi.org/10.1007/s00396-013-2927-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-013-2927-8