Abstract
Microwave synthesis has been applied to prepare stable silver nanofluids in ethanol by reduction of AgNO3 with polyvinylpyrrolidone (PVP), used as stabilizing agent, having Ag concentrations of 1% by volume. The nanofluids were characterized by UV-vis spectroscopy, Fourier transform infrared, energy-dispersive X-ray spectroscopy, and transmission electron microscopy and systematically investigated for refractive index, electrical and thermal conductivity, and viscosity for different polymer concentrations. The size of nanoparticles was found to be in the range of 30–60 nm for two different salt-to-PVP ratios. For higher concentration of polymer in nanofluid, nanoparticles were 30 nm in size showing increase in thermal conductivity but a decrease in viscosity and refractive index, which is due to the polymer structure around nanoparticles. Thermal conductivity measurements of nanofluids show substantial increment in the thermal conductivity of nanofluid relative to the base fluid and nonlinear enhancement over the 283–323 K temperature range. Rheology of nanofluids was studied at room temperature showing effect of polymer on viscosity and confirming the Newtonian behavior of nanofluid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The addition of nanoparticles in liquid shows remarkable effect on physical, chemical, and transport properties of the base fluid [1, 2]. Pioneer works of Choi et al. [1], Lee et al. [3], Masuda et al. [4], and Eastman et al. [5] introduced the thermal conductivity enhancement of nanofluids to the scientific community. A large number of experimental and theoretical studies have been carried out by numerous researchers [6–11] on thermal conductivity of nanofluids. Patel et al. [12] have shown that, even with the same surface-to-volume ratio, different conductivities were obtained when the materials are different, indicating that the quantum nature of transport phenomenon is significant. Similar trends have been seen with gold particles (4 nm) in toluene. At higher particle volume fraction, the conductivity enhancement varies more like temperature. Several authors [13–14] have argued that large thermal conductivity increase is due to hydrodynamic effect of Brownian motion of nanoparticles.
Nanofluids have shown several distinct properties with large enhancements in thermal conductivity as compared to the base liquid [15], temperature and particle size dependence [16, 17], reduced friction coefficient [18], and significant increase in critical heat flux [19].
Even though a large number of experiments have been conducted for measuring thermal conductivity, only a few have been performed for evaluating the other transport coefficients such as viscosity. Viscosity is as critical as thermal conductivity in engineering systems that employ fluid flow. It is evident that the effects of viscosity and thermal conductivity should be considered together. There are many variables involved, including nanoparticle size, surface area, base fluid materials, type and concentration of surfactant, concentration of nanoparticles, dispersion method, etc. [20, 21]. The nanofluids have also shown that they do not follow the Einstein Batchelor Theory and exhibit a significantly rapid increase in viscosity than predicted. There have been few studies on the rheological properties of nanofluids. Since nanofluids are expected to be used under flow conditions and the flow of suspension is sometimes drastically different from that of most common heat transfer fluids that have Newtonian characteristics, it is essential to understand the rheological properties of nanofluid for practical use.
In most studies, sample sizes have been limited to less than a few hundred milliliters of nanofluid. Larger samples will be needed to test many properties of nanofluids in the future, particularly in assessing their potential for use in various applications. Robust techniques for large-scale production of stable nanofluids are needed. Recently, microwave irradiation has been widely applied to material science. Due to intense friction and the collision of molecules created by microwave irradiation, microwave irradiation not only provides the energy for heating but also greatly accelerates the nucleation. With microwave irradiation on the reactant solution, temperature and concentration gradients can be avoided leading to uniform nucleation and the obtained nanofluids have good stabilization. Microwave-based synthesis method is one of the easiest, energy-saving, and quick methods for large-scale production of nanofluids.
A single-step approach using chemical wet technology [22, 23] is a powerful method for preparing nanofluids of different metals and semiconductors. Many preparation methods of silver nanocolloids have been reported. One of the popular deoxidizing routes is to protect the silver nanoparticles in reaction with the help of protecting polymers. To get dispersed silver nanoparticles in solvents, PVP is most useful. This polar polymer is protective and has been employed as a stabilizer of colloidal silver. PVP also acts as a reducing agent for silver in solution, thus producing metallic silver in solution. The ability of PVP as reducing agent has been reported by various researchers [24].
The dispersion and diameters of silver particles is greatly influenced by the ratio between reactants, amount of PVP, the reaction time, and reaction temperature. Synthesis of silver nanoparticles–colloids using PVP in ethylene glycol has been reported recently [24, 25].
In this study, PVP-protected Ag ethanol-based nanofluids have been synthesized by chemical reduction using microwave radiation. For preventing particle aggregation and controlling particle size, PVP has been used in the weight ratio of 1:1, 1:5, and 1:10 (AgNO3 to PVP). The nanofluids have been characterized by UV-vis spectroscopy (uses spectrophotometer and deals with the measurement of absorbance of energy), energy-dispersive X-ray spectroscopy (EDX), transmission electron microscopy (TEM), and Fourier transform infrared (FTIR) and systematically investigated for refractive index, electrical and thermal conductivity, and rheological behavior for different polymer concentrations.
Experimental
Synthesis of nanofluids
Microwave-assisted chemical reduction of silver nitrate has been done with ethanol as base fluid using PVP as stabilizing and reducing agent. In order to prepare Ag nanocolloids of different particle size, different metal salt-to-PVP ratios have been selected and experimental parameters are reported in Table 1.
The nanofluid with AgNO3 to PVP weight ratio of 1:10 (vol. conc. = 0.0114) is denoted by “H”, AgNO3 to PVP weight ratio 1:5 (vol. conc. = 0.0112) by “L” hereafter.
Characterization of nanofluids
UV-vis absorption spectrum has been recorded on Nanodrop 1000 under 1-mm column in the wavelength range of 300–900 nm at room temperature. An FTIR spectrum has been taken with a Perkin Elmer (Spectrum One) spectrometer. For this purpose, nanofluid is spread on NaCl crystal to form a film and subsequently dried in an oven for 15 min under low power allowing ethanol to evaporate. EDX of silver nanofluids on glass substrate is taken using JEOL JSM 6360A. TEM images have been taken on a JEOL JEM 100 CX2 by placing the nanofluid directly on copper grid and allowing the sample to evaporate naturally. Leica ABBE MARK II Refractometer has been used to measure refractive index of silver nanofluids in the temperature range of 302 to 323 K. Electrical conductivity measurements have been done using microprocessor-based conductivity meter (ESICO model 1601) at room temperature. Transient hot wire method [26] is used to measure the thermal conductivity of nanofluids. The thermal conductivity can be expressed as
Where q can be taken as the input power per meter length of heating wire; T 1 and T 2 are temperatures at time t 1 and t 2, respectively. The thermal conductivity to be calculated is in direct proportion to the average input power q and difference in temperature dT.
For measurement of thermal conductivity, the container is filled with sample and the line heat source is inserted at the center of the double-walled container connected to constant temperature bath (Julabo F32 model) and allowed to equilibrate to bath temperature. After the initial temperature is recorded, the heater is activated and heated at a constant rate of input energy. The temperature-versus-time data are acquired using Data Acquisition System from which thermal conductivity is estimated. A rheological study of nanofluids is done using Rheologica Nova Rheometer with cone and plate having 40-mm diameter and 1° angle.
Results and discussion
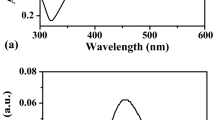
The position of the surface plasmon absorption peak of silver particles depends on the refractive index of the surrounding medium, particle size, adsorbed polymer on the particle surface, etc. Figure 1 shows the UV-vis spectra of the polymer-stabilized silver nanofluid in the wavelength range of 300 to 600 nm and Fig. 2 shows the variation of refractive index of nanofluid with temperature. Sample L shows higher refractive index because of less capping of nanoparticle surface area as polymer concentration is low while for sample H nanoparticles the high capping results in low refractive index.
The dark yellow to dark brown color in different solutions indicates the presence of silver nanoparticles in the solutions. Strong absorption is observed at 408 and 413 nm for samples H and L as shown in Fig. 1 indicating the particle size dependence and presence of silver particles in PVP. The slight shift of curve for sample H at 406 nm (Fig. 1) shows further reduction in particle size at temperature 323 K. For sample 1:1, we do not get any peak (not shown in the figure) revealing that nanoparticles have not formed in this case. Stabilization of nanofluids has been studied at room temperature and it has been found that the obtained nanofluids are stable for more than 1 month in the stationary state and more than 10 h under centrifugation at 3,000 rpm without sedimentation. Excellent stability of the obtained nanofluid is due to the protective role of PVP as it retards the growth and agglomeration of nanoparticles by steric effect [27]. Measured electrical conductivity of nanofluid is 174 and 153 μS for samples H and L, respectively. The pure ethanol is dielectric in nature having very low electrical conductivity (0.00135 μS). The presence of silver nanoparticles in ethanol increases electrical conductivity by a factor of 105.
The FTIR spectra of sample H is shown in Fig. 3 showing strong band due to amide carbonyl (present in PVP monomer) stretch vibration but shifts to 1,670 cm−1 [24]. Band displacement indicates attachment of amide carbonyl oxygen with silver. Absence of AgNO3 band at 1,384 cm−1 in FTIR spectra further confirms the reduction of AgNO3 to Ag. [24].
Figure 4 shows the EDX of silver nanofluids taken by preparing a film on a glass substrate [28], confirming the presence of Ag nanoparticle in the nanofluid. EDX analysis shows five peaks located between 2 and 4 keV [29]. These peaks are directly related to the silver characteristic lines K and L. The carbon and oxygen spots in the examined samples confirm the presence of stabilizer PVP composed of alkyl chains. The optical absorption peak is observed approximately at 3 keV, which is typical for the absorption of metallic silver nanocrystallites due to surface plasmon resonance [30].
TEM images of nanofluids are presented in Fig. 5. Observation of figure shows that Ag nanoparticles are spherical in shape and embedded in PVP polymer. Average particle size is about 60 nm for sample L and 30 nm for sample H (Fig. 5).
Measured and reported thermal conductivity of ethanol with temperature are presented in Fig. 6. There is a close agreement between the observed and reported values of ethanol as can be seen from the figure. It is observed that the thermal conductivity of ethanol decreases with temperature as reported [31]. Measurement system has been calibrated, before use, by measuring the thermal conductivity of deionized water, benzene, and glycerol and measured thermal conductivities are 0.56, 0.16, and 0.289, respectively, at room temperature, exhibiting standard deviation from the literature values by 0.035, 0.071, and 0.050, respectively. Measurements have been recorded ten times for each sample and results have been averaged. These results demonstrate that the experimental setup used in the present study can provide reliable thermal conductivity measurement.
Figure 7 presents the thermal conductivity ratio for Ag nanofluid (thermal conductivity normalized by that of base fluid) with temperature. The variation of thermal conductivity ratio(K eff/K) with temperature seems to be sigmoidal in nature showing slow to sharp increase with increase in temperature. This variation is similar to the trend variation of K eff/K b with volume percent of particles [32]. This nonlinear behavior in K eff/K b can be explained based on stochastic motion (Brownian motion) [10] of the nanoparticles freed from PVP binding as the temperature increases. For temperature above 303 K, it may be due to loss of polymer binding and increase in the diffusion rate of nanoparticles. At temperature 323 K, thermal conductivity enhancement greater than 1.55 has been observed for sample H. With increase in temperature, nanoparticles diffuse through the polymer matrix. Thermal vibrations cause polymer to loose its structure allowing greater surface area of nanoparticles to take part in heat transfer. This also adds to increase in Brownian motion of nanoparticles causing convection to increase significantly with temperature causing thermal conductivity enhancement [10]. Reynolds number (Brownian velocity) that represents the mobility of nanoparticles shows dominating temperature dependence whereas Prandtl number shows slightly decreasing dependency [10, 33]. Enhancement in thermal conductivity is the net result of contradictory processes as temperature increases.
Figure 7 also shows estimated thermal conductivity values of silver nanofluids using Maxwell model [34]. It clearly indicates that the experimental results are significantly higher than that predicted by Maxwell model and enhancement in thermal conductivity of nanofluid cannot be explained by classical models, as this model is developed for millimeter- or micrometer-sized particle colloids without taking into account the possible influences of particle size, Brownian motion, interfacial layer at the particle–liquid interface and clustering of nanoparticles, and polymer ligand binding which are considered important mechanisms for enhancing the thermal conductivity of nanofluid. Similar differences between the experimental and classical model estimation has been reported earlier by many researchers [35].
The viscosity of nanofluids depends upon shear rate, nanoparticle size, surface area, surface potential, polymer dispersed, and temperature [36]. Figure 8 shows the variation of shear rate with stress for sample H and sample L at room temperature (296 K). Observation of figure shows that sample L having low polymer yields low shear rate indicating that silver particles are large in size (≈60 nm) or in slightly agglomerated condition. The high shear rate shown in Fig. 7 for sample H reveals that the nanoparticles are well separated and small in size (≈30 nm) and there is a formation of a cage of polymer surrounding the nanoparticles as can be seen from TEM images (Fig. 5). The overlapping of polymer on nanoparticles is uniform yielding high shear rate.
The sample L shows low shear rate and higher viscosity (see Fig. 9) as compared to sample H. Shear-dependent viscosity of nanofluids is shown in Fig. 9 indicating slight increase in the viscosity with shear rate due to presence of polymer. Polymer-capped nanofluids have shown enhancement in viscosity, 1.5 mPa s for sample H and 1.7 mPa s for sample L, as compared to viscosity of ethanol, i.e., 1.2 mPa s, being thin in nature. For sample H, the large numbers of void spaces are responsible for low viscosity showing the uniform dispersion and stability of nanofluids.
The high shear and low viscosity for sample H reveal that the nanoparticles sterically stabilized by the repulsion of charges of polymer surrounding the nanoparticles and there is only small amount of electrostatic attraction present. In case of sample L, as the polymer concentration is small, the particles are less sterically stabilized and there are more electrostatic forces yielding high viscosity and low shear. In general, for colloids without polymer, small particle size has large surface area and large electrostatic forces causing increase in the viscosity [37] whereas our nanoparticles being polymer-capped have shown low viscosity.
Conclusions
Stable nanofluids containing silver nanoparticles of 30 and 60 nm have been prepared by microwave-assisted one-step method. Microwave method being fast is suitable for large-scale production of nanofluids. Nanofluids thermal study shows that a small amount (1% by vol.) of Ag enhances the thermal conductivity of the base fluids substantially (1.55 times at temperature 323 K). The experimental results have also been compared with Maxwell’s classical model of thermal conductivity. It is shown that the macroscopic model is inadequate for the prediction of the effective thermal conductivity of nanofluids. A result presented here makes nanofluids more attractive as cooling fluid for devices with high energy density where the cooling fluid is likely to work at a temperature higher than the room temperature. Higher polymer concentration increases electrical and thermal conductivity and decreases refractive index and viscosity.
References
Choi S, Siginer D, Wang H (1995) ASME (NewYork) 231:99–105
Phelan E, Bhattacharya P, Prasher S, Prasad V, Jaluria Y, Chen G (2005) Annu Rev Heat Transf 14:255–275
Lee S, Choi S, Li S, Eastman A (1999) J Heat Transfer 121:280–289
Masuda H, Ebata A, Teramae K, Hishinuma N, Bussei N (1993) Japan 7(4):227–233
Eastman A, Choi S, Li S, Thompson J, Lee S (1997) Material Research Society Symposium Proceedings, Pittsburgh, PA, vol. 457, pp 3–11
Xie H, Wang J, Xi T, Liu Y, Ai F, Wu Q (2002) J Appl Phys 91(7):4568–4572
Xuan Y, Le Q (2000) Int J Heat Fluid Flow 21:58–64
Keblinski P, Phillpot R, Choi S, Eastman A (2002) Int J Heat Mass Transfer 45:855–863
Chopkar M, Kumar S, Bhandar R, Das K, Manna I (2007) Mat Sci Engg B 139:141–148
Das K, Putra N, Thiesen P, Roetzel W (2003) J Heat Transfer 125:567–574
Patel E, Das K, Sundararajan T, Pradeep T (2005) Pramana-J Phys 65(5):863–869
Patel E, Das K, Sundararajan T, Sreekumaran A, George B, Pradeep T (2003) Appl Phys Lett 83(14):2931–2933
Jang SP, Choi SUS (2004) Appl Phys Lett 84(21):4316–4318
Prasher S, Bhattacharya P, Phelan E (2006) Trans ASME 28:588–595
Gandhi S (2007) Current Science 92(6):717–718
Chon CH, Kihm KD, Lee SP, Choi SUS (2005) Appl Phys Lett 87:153107
Yang B, Han ZH (2006) Appl Phys Lett 89:083111
Kostic M (2006) Multifunctional nanocomposites. In: Proc. MN2006, vol. 17036, pp 1–9
Yulong D, Alias H, Wen D, Williams A (2006) Int J Heat Mass Transf 49:240–250
Chen H, Ding Y, Tan C (2007) New J Phys 9:367–391
Lu K, Kessler C (2006) J Mat Sci 41:5613–5618
Zhu H, Lin Y, Yin Y (2007) J Colloid Int Sci 277:100–103
Liu M, Lin MC, Tsai CY, Wang CC (2006) Int J Heat Mass Transf 49:3028–3033
Grijalva A, Urbina R, Silva J, Borja M, Barraza F, Amarillas A (2007) Mat Res Bull 43:90–96
Ayyappan S, Gopalan RS, Subbanna GN, Rao CNR (1997) J Mater Res 12:398–401
Nagasaka Y, Nagashima A (1981) J Phys E: Sci Instrum 14:1435–1439
Shervani Z, Ikushima Y, Sato M, Kawanami H, Hakuta Y, Yokoyama T, Nagase T, Aramaki K (2007) Colloid Polym Sci 007:1784–1788
Yan Y, Kang S, Mu J (2007) Appl Surface Sci 253:4677–4679
Puchalski M, Dąbrowski P, Olejniczak W, Krukowski P, Kowalczyk P, Polański P (2007) Materials Science-Poland 25(2):473–478
Kalimuthu K, Babu R, Venkataraman D, Bilal M, Gurunathan S (2008) Colloids Surf B: Biointerfaces 65:150–153
Petravic J (2005) J Chem Phys 123:174503
Chopkar M, Das P, Manna I (2006) Scripta Materialia 55:549–552
Chon HC, Kihm K, Lee SP, Choi SUS (2005) Appl Phys Lett 87:153107–153111
Maxwell JC (1891) A treatise on electricity and magnetism. Clarendon, UK
Wang X, Mujumdar A (2007) Int J Thermal Sci 46:1–19
Kathy L, Kessler C (2006) J Mater Sci 41:5613–5618
Chang H, Jwo C, Lo C, Tsung T, Kao M, lin H (2006) Rev Adv Mater Sci 10:128–132
Acknowledgement
Authors are thankful to Vice Chancellor, Defense Institute of Advanced Technology (DIAT), Deemed University, Girinagar, Pune, India, for granting permission to publish this work. The authors sincerely appreciate the keen interest of Shree V.C. Janu Chemistry Department (DIAT) in the present work. Authors are also thankful to AFMC Pune, India, for TEM of samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.K., Raykar, V.S. Microwave synthesis of silver nanofluids with polyvinylpyrrolidone (PVP) and their transport properties. Colloid Polym Sci 286, 1667–1673 (2008). https://doi.org/10.1007/s00396-008-1932-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-008-1932-9