Abstract
The effects of dispersant ammonium poly(methacrylic acid) (PMAA-NH4) and poly(acrylic acid) (PAA) on nano-Al2O3 particle dispersion have been investigated. Under the same dispersion viscosity, Al2O3 of 38 nm size requires 20 times more PAA dispersant than Al2O3 of 0.2 μm size but with only 9 times specific surface area increase. For the same carboxylic acid group to Al2O3 mole ratio, the PAA dispersant adsorbs more readily onto nano-Al2O3 particles than the PMAA-NH4 dispersant and has better dispersion efficiency. Rheology measurements confirm the better dispersion and higher dispersion efficiency when PAA is used. Maximum solids loading has been predicted for each suspension based on the rheological data; this predication capability can serve as the important guidance for future dispersion designs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoceramics can be densified at much lower sintering temperatures and provide excellent structural properties as well as unique functional properties. Because of these processing and performance advantages, there has been continuous research effort in nanoceramic forming and sintering areas, such as pressure infiltration of yttria stabilized zirconia and hot pressing of Al2O3 [1–3]. However, nano-oxides are more susceptible to agglomeration than the micron-sized counterparts due to extremely high specific surface area. Undesired agglomerates can be created during powder synthesis, drying, or even storage. To avoid agglomeration and form uniform components, colloidal processing of nanopowders is often preferred [4, 5].
Like numerous other species, ceramic particles exhibit a net electrodynamic attraction to one another due to oscillating dipoles, commonly referred to as van der Waals forces. A net surface charge results when the particles are immersed in H2O. For Al2O3, the net surface charge is positive at low pH and negative at high pH with isoelectric point (IEP) at 8.7 in pure H2O [6, 7] There are three colloidal stabilization mechanisms. When the pH of the solution is adjusted according to the surface charge of Al2O3, the stabilization mechanism is called electrostatic stabilization. When one end of a polymer species adsorbs onto the particles and the other end of the polymer species extends into the liquid to physically repel one another; this method of stabilization is called steric stabilization. If the adsorbed polymer is ionic, it generates strong electrostatic forces while polymer strands repel one another [8]; this method of colloidal stabilization is called electrosteric stabilization [9].
Complex chemistry of a dispersion system
Although stable suspensions can be created, a delicate balance must be maintained in the amount of dispersant in the system. The adsorbed polymer must be thick enough to prevent particle close contacts and counteract van der Waals forces. This means that there must be enough polymer present to provide complete coverage of the ceramic particles. Incomplete coverage will cause polymer strands to “bridge” the gap between particles, resulting in bridging flocculation [10]. However, as the adsorption of polymer dispersant increases, the net charge of the system decreases. This results in a barrier for further adsorption. A suitable amount of excessive dispersant is needed to attain saturation adsorption. If the dispersant amount is increased further, the non-adsorbed free polymer will flocculate the suspension [11]. The amount of adsorbed polymer needed to attain Al2O3 surface saturation and stable dispersion is dependent on the particle size, the polymer dispersant, the solids loading, and the pH of the system. The saturation level of PMAA-Na in sub-micron Al2O3 suspension decreases by a factor of 2 when pH is increased from 8.2 to 9.8 [8]. Electrosterically stabilized Al2O3 suspensions generally have IEP around 8.8. At pH higher than IEP, excessive dispersant hinders steric stabilization. At pH lower than IEP, polymer adsorption onto Al2O3 particles is promoted and this condition is usually used as a desirable intermediate state for dispersion stabilization [6], but this generally occurs at low dispersant and solids loading levels.
For most colloidal forming processes, a suspension with a high solids loading level (≥50 vol%) is desirable. A polymer dispersant allows for well-dispersed suspensions to be created. However, the polymer necessarily occupies space, which decreases the maximum solids loading of the suspension. The exact achievable solids loading is dependent upon the length and morphology of the polymer chain. Sigmund et al. [10] calculated the effect of interparticle repulsion on relative packing density. Too short of a polymer chain will yield a thin adsorption layer, resulting in flocculation due to van der Waals attraction, while too long a chain will result in a dramatically decreased maximum solids loading. The polymer dispersant type is also of critical importance for effective dispersion and the maximum solids loading. The pH of the suspension must be controlled to prevent imparting an attractive force between particles since the dispersant efficiency is dependent on steric mechanism as well as electrostatic mechanism for high solids loading suspensions. Singh et al. [12] found that use of ammonium poly(methacrylic acid) (PMAA-NH4) as a dispersant shifts IEP of Al2O3 system from 9.14 to 5.65. The positive surface charge of the Al2O3 particles attracts negative ions from the polymer. The net charge of the system is reversed due to the adsorption of the negatively-charged polymer. Since the negative surface charge of the PMAA-NH4 polymer dominates the electrical charge of the suspension, the pH needs to be purposely adjusted to alkaline condition for a well dispersed suspension.
The work reported here is focused on understanding PMAA-NH4 and poly(acrylic acid) (PAA) dispersant effects on the stability and rheology of nano-Al2O3 suspensions. By measuring the suspension rheology under different dispersion conditions, the maximum solids loading for suspensions of different dispersants has been predicted.
Experimental
Al2O3 nanopowder with average particle size of 38 nm and specific surface area of 45 m2/g was used in this study (Nanophase Technologies, Romeoville, IL). A TEM image of the particle size distribution was shown in Fig. 1. Even though the average particle size is much less than 100 nm, the particle size distribution is wide and there is a small percent of large particles close to 100 nm. In this study, the average particle size was used in comparing with micron size powders. Detailed particle size distribution data would be beneficial but not necessary due to the five times difference in the particle sizes studied. This Al2O3 powder was also reported to have 70:30 of δ:γ phases from the vendor. PMAA-NH4 (M w 15,000, Vanderbilt Co., Inc., Norwalk, CT) and PAA (M W 1,800, Aldrich, St Louis, MO) were used as polymer dispersants. The polymer segment for PMAA-NH4 is [–CH2C(CH3)(CO2H)–] and for PAA is [–CH2CH(CO2H)–]. These two dispersants have different chain length and functional groups to affect nano-Al2O3 particle dispersion.
To prepare Al2O3 suspensions, glycerol (water basis, Fisher Chemicals, Fairlawn, NJ) and water were mixed at 1:10 weight ratio and homogenized for 5 min using a ball mill. The reason to use glycerol is for solid compact forming, which will be reported in future publication [K. Lu, C.S. Kessler, and R. M. Davis, to be published]. The Al2O3 powder was added for a specific solids loading in 10 g increments along with an appropriate amount of PMAA-NH4 or PAA dispersant. Since low pH promotes dispersant adsorption onto nano-Al2O3 particles, HCl solution was added to lower pH to 1.5 [6]. The suspension was ball milled for overnight with periodic adjustment of pH to 1.5. This procedure makes suspensions of approximately 20 vol% Al2O3. NH4OH was then used to adjust the suspension pH to 9.5. High pH induces full dissociation of the dispersants PMAA-NH4 and PAA and creates mutual repulsion among the particles, resulting in better dispersion. Depending on the desired final solids loading, nano-Al2O3 was again added in 10 g increments, along with an appropriate amount of the PMAA-NH4 or PAA dispersant. The suspension was then mixed for 24 h for complete homogenization.
Potentiometric titration was used to determine the amount of dispersant adsorbed onto the Al2O3 particles in a suspension. pH was measured and adjusted to 9.5 ± 0.05 before titrant HCl solution was added in order to ascertain the dissociation of the dispersants in H2O. Adsorption curve was developed for blank suspensions of PMAA-NH4 and PAA. To measure the adsorption of each dispersant in an actual suspension, suspensions with PMAA-NH4 or PAA dispersant at 20 vol% solids loading were centrifuged at 2500 rpm for 45 min before collecting the resulting supernatant. A known volume of the supernatant was titrated and the amount of un-adsorbed dispersant was determined using the standard curve from the blank polymer suspensions.
For suspension characterization, the pH of the suspensions was measured by a pH meter (Denver Instrument, Arvada, CO). The zeta potentials were measured using Malvern 3000 Zetasizer (Malvern Instruments, UK). The viscosity was measured using an AR2000 rheometer (TA Instruments, New Castle, DE).
Results and discussion
Zeta potential
Zeta potential measures the electrical potential at the surface of moving nano-Al2O3 particles in a suspension and is an important guide to the suspension stability. In general, the absolute value of zeta potential should be greater than 25 mV in order to obtain highly stabilized suspensions. Even though it is best not to alter the solids loading of the suspensions during zeta potential measurement, the most widely used method for measuring the zeta potential is microelectrophoretic technique and requires very dilute suspensions. In this study, the suspensions were diluted at 1:250 ratio in order to examine the zeta potentials of the suspensions with PMAA-NH4 and PAA dispersants. As shown in Table 1, all the suspensions have negative zeta potentials at less than −40 mV and the measurements are very close to each other. This result indicates that all the suspensions have similar stability and provides the basis for the comparisons of other parameters in evaluating the suspensions.
It should be noted that even though the starting dispersants are PMAA-NH4 and PAA, the adjustment of the suspension pH to 9.5 using NH4OH has essentially converted PAA and PMAA-NH4 into the similar dissociated format with polymer segments [–CH2C(CH3)(CO2)–]− and [–CH2CH(CO2)–]−. Here, we discuss the results by continuously using dispersant PMAA-NH4 and PAA for consistency.
Dispersion efficiency
As stated in the Experimental section, the PMAA-NH4 dispersant has M w 15,000 and approximately 146 carboxylic acid groups per molecule. The PAA dispersant has M w 1800 and approximately 25 carboxylic acid groups per molecule. In this study, the amount of the dispersants was purposely controlled so that the carboxylic acid groups per mole of Al2O3 are the same at 0.028 mol. This equals to 2.78 wt% of Al2O3 for the PMAA-NH4 dispersant and 2.2 wt% of Al2O3 for the PAA dispersant. The difference in the dispersion efficiency should mainly come from the polymer–particle interaction, which is a function of polymer chain length and polymer molecular structure. For the PMAA-NH4 dispersant, the polymer chain is longer and there is a methyl group for each polymer segment; thus it should be more difficult for all the carboxylic acid groups to attach to the particle surface while still providing the steric stabilization; so lower dispersion efficiency is expected. For the lower molecular weight and shorter chain PAA, it is expected that more carboxylic acid groups will adsorb onto the nano-Al2O3 particle surface while the polymer chains extend into the suspension; better dispersion should result. These predictions were verified by the polymer adsorption results through potentiometric titration. For the 20 vol% solids loading suspension with the PMAA-NH4 dispersant, only 72.84% PMAA-NH4 adsorbed onto nano-Al2O3 particles. For the 20 vol% solids loading suspension with the PAA dispersant, 75.73% PAA adsorbed onto nano-Al2O3. It can be predicted that as the suspension solids loading increases, the dispersant adsorption difference will become more obvious. The quantitative comparison will be reported in future studies.
Comparison can also be made regarding the dispersion efficiency between the PMAA-NH4 and PAA suspensions using viscosity results at the same solids loading. Figure 2 shows the viscosity results at 100 s−1 shear rate and different solids loading levels. Clearly, PAA has higher dispersion efficiency than PMAA-NH4 at all solids loading levels, manifested by the lower suspension viscosity. At 20 vol% solids loading, the viscosity of the PMAA-NH4 suspension is 62.5% higher than that of the PAA suspension. As solids loading increases to 30 and 35 vol%, the viscosity difference increases to 213% and 295%. This clearly indicates the higher the solids loading, the higher impact a specific polymer has. This is extremely important for high solids loading systems that many nanoceramic forming processes demand and should be carefully evaluated.
The dispersant amount needed to achieve the same viscosity can be used as another parameter to evaluate the dispersion efficiency. Here, the data from Cesarano and Aksay [6] on 0.2 μm Al2O3 were included to compare the suspensions of different particle sizes. The comparison was shown in Table 2. At the same 0.06 Pa s viscosity, the PAA amount needed increases more dramatically than the particle specific surface area does. Al2O3 of 38 nm size requires 20 times more PAA dispersant than Al2O3 of 0.2 μm size but with only 9 times specific surface area increase. This means the smaller the particle size and the higher the specific surface area, the more PAA is needed and the lower the PAA dispersion efficiency is. For the 0.2 μm Al2O3, 50 vol% solids loading can be achieved with PAA amount at only 0.10 wt% of Al2O3 [8]. For the 38 nm Al2O3 used in this study, the PAA amount used is 2.0 wt% of Al2O3 to achieve only 20 vol% solids loading.
Rheology
The rheological behavior of a colloidal suspension is critical for the flow properties and the subsequent forming process. The rheology of nano-Al2O3 dispersions depends on many parameters, such as shear rate, solids loading, particle size, and surface potential, and can be measured by monitoring changes in flow behaviors in response to an applied stress (or strain). Ogawa et al. [13] theorized that viscosity increases with the decrease of the particle size due to the increase in the overlapping area of the electrical double layer around each particle and verified that with experimental data. For the present study, it is shown in Table 2 that the nano-Al2O3 suspensions will have much higher viscosity than the 0.2 μm Al2O3 suspensions under the same solids loading, most likely due to the increase of the overlapping areas between the particles. For the dispersant effects on the suspension viscosity, the PMAA-NH4 dispersant has longer chains but poorer adsorption onto Al2O3 nanoparticles than the PAA dispersant; it is more likely that the longer PMAA-NH4 chains will extend into the suspension around each Al2O3 nanoparticle. Based on the steric interaction theory, these long chains will result in thicker electrical double layer and larger overlapping area; the PMAA-NH4 suspension will have higher viscosity compared to the PAA suspension. These trends are clearly shown by the measurements in Figs. 3 and 4.
Figure 3 shows the shear stress ∼ shear rate correlation for the PMAA-NH4 suspension at 20–35 vol% solids loading (a) and the PAA suspension at 20–40 vol% solids loading (b). The shear rate for both suspensions is from 10 to 200 s−1. Shear stress difference can be easily observed for the PMAA-NH4 and PAA suspensions at all shear rates. For example, at 35 vol% solids loading, the shear stress approaches 100 Pa for the PMAA-NH4 suspension while for the PAA suspension the shear stress is only 37 Pa. Also, suspension solids loading has a direct effect on the shear stress. Figure 3 shows that the higher the solids loading, the higher the shear stress under a constant shear rate. Another important observation from Fig. 3(b) is the rapid shear stress increase from 35 to 40 vol% solids loading. This means that there is a critical solids loading between 35 and 40 vol% for the PAA dispersant. When the particle–particle distance reaches such critical state, it is very difficult to overcome the inter-particle force for the suspension to flow. For the PMAA-NH4 dispersant, such transition is less clear.
As shown in Fig. 4, pseudoplastic or shear thinning behavior occurs for all the dispersions. Shear thinning becomes more severe as the solids loading increases. There have been many interpretations of shear thinning based on the microstructure. It is widely believed that shear thinning is due to ordering of the particles into layers or strings which reduces the energy dissipated under shear [14]. It is also postulated that shear thinning is due to a distortion of the liquid-like structure which presumably leads to a decrease in the energy dissipation. Two orders of magnitude difference can be observed in viscosity between 20 and 40 vol% solids loading. The ability to predict flow behavior of concentrated suspensions is clearly needed.
Maximum solids loading prediction
Maximum solids loading is the solid loading under infinite viscosity for a specific suspension system. Since most of the ceramic components require a low or zero porosity, the maximum solids loading prediction during colloidal processing is of great interest. With a known maximum solids loading, effective strategies can be purposely devised to achieve the highest solids loading in a real system. For micron size particle suspension systems, random particle packing can be realized and actual solids loading can approach 60 vol% or higher. However, nano-Al2O3 has large specific surface area that adsorbs large amount of dispersant, which can quickly reduce the particle packing efficiency based on effective solids loading theory [15]:
where φ is the solids loading with no dispersant or dispersing medium influence, φeff is the effective solid loading taking into consideration of the dispersant and stabilized suspension, δ is the thickness of the adsorbed dispersant layer, As is the specific surface area of the dispersed particles, i.e., nano-Al2O3, and ρs is the particle density. If excessive dispersant is used, some polymer chains stay freely in the suspension, which further decreases the maximum solids loading. Achieving high solids loading for nano-oxides has been a challenge for numerous nanoparticle suspensions.
The viscosity of suspensions (0.3 < φ < 0.6) has been well modeled by Krieger and Dougherty [16]:
φm is the maximum solids loading of the suspension. ηr is the relative viscosity of the suspension:
η s is the viscosity of the suspension and η o is the viscosity of the dispersing media. Equation (2) is a suspension specific model which allows the viscosity of a given suspension to be predicted once the parameters are accurately determined.
In this study, a best-fit procedure was followed to predict the φm for different suspensions. By assuming that at φm viscosity η s approaches infinity, Eq. (2) can be transformed into [17]:
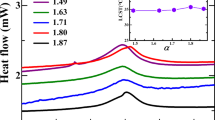
a and b are suspension specific constants. Equation (4) can be used to predict the maximum solids loading for both PMAA-NH4 and PAA suspensions. As shown in Fig. 5, the maximum solids loading for the PMAA-NH4 suspension is 41.5 vol% and for the PAA suspension is 47.5 vol%. There is a 6.0 vol% maximum solids loading difference just by using different dispersants. By obtaining these critical values, judicious decision can be made on dispersant selection in order to obtain the desired level of solids loading.
Conclusions
Lower molecular weight and shorter chain PAA has better adsorption onto nano-Al2O3 particles than higher molecular weight and longer chain PMAA-NH4. The smaller the particle size and the higher the specific surface area, the more PAA is needed for achieving stable suspension. Desired PAA content increases more dramatically than the particle specific surface area does. PAA has higher dispersion efficiency than PMAA-NH4 for Al2O3 nanoparticles at all solids loading levels. The higher the solids loading, the higher impact a specific dispersant has. Higher shear stress and viscosity are observed for the PMAA-NH4 suspensions at all shear rates when compared to the PAA suspensions. Also, shear stress and viscosity exhibit much faster increase at high solids loading. The predicted maximum solids loading for the PMAA-NH4 suspension is 41.5 vol% and for the PAA suspension is 47.5 vol% in the studied systems. These critical values are important for obtaining the desired level of solids loading in practice.
References
Zych L, Haberko K (2004) Key Eng Mater 264–268:2323
Lance D, Valdivieso F, Goeuriot P (2004) Ibid 264–268:205
Chang S, Doremus RH, Schadler LS, Siegel RW (2004) Int J Appl Ceram Tech 1:172
Wagner NJ, Bender JW (2004) MRS Bull 29:100
Tohver V, Chan A, Sakurada O, Lewis JA (2001) Langmuir 17:8414
Cesarano J III, Aksay IA (1988) J Am Ceram Soc 71:1062
Lewis JA (2000) ibid 83:2341
Cesarano J III, Aksay IA, Bleier A (1988) Ibid 71:250
Napper D (1983) Polymeric stabilization of colloidal dispersions Academic Press, London, p 8
Sigmund W, Bell N, Bergstrom L (2000) J Am Ceram Soc 83:1557
Cho J, Dogan F (2001) J Mat Sci 36:2397
Singh B, Bhattacharjee S, Besra L, Sengupta DK (2004) Ceram Int 30:939
Ogawa A, Yamada H, Matsuda S, Okajima K (1997) J Rheol 41:769
Hoffman RL (1974) J Colloid Interface Sci 46:491
Kirby GH, Harris DJ, Li Q, Lewis JA (2004) J Am Ceram Soc 87:181
Krieger IM, Dougherty M (1959) Trans Soc Rheol 3:137–152
Liu D-M (2000) J Mater Sci 35:5503
Acknowledgments
The authors are grateful for the support of Dr. Marc Edwards from Civil and Environmental Engineering Department of Virginia Tech in carrying out the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, K., Kessler, C. Colloidal dispersion and rheology study of nanoparticles. J Mater Sci 41, 5613–5618 (2006). https://doi.org/10.1007/s10853-006-0303-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0303-5