Abstract
Adsorption of the herbicide linuron on organo-bentonites was measured in the presence of several salts. The bentonites had been modified by cation exchange with hexadecylpyridinium and hexadecyl tributylphosphonium ions. The organo-bentonites adsorbed distinctly higher amounts of linuron than calcium bentonite and desorption was also reduced. The binding coefficient K (derived from the Freundlich adsorption isotherm) was several times higher than for calcium bentonite. The adsorption isotherms of linuron on the organo-bentonites from salt-free aqueous solutions were of S-type but of J-type from saline water. In the presence of many salts, the amount of linuron adsorbed decreased with increasing salt concentration. These salts reduced the linuron adsorption (at a salt concentration of 20 g/L) in the order NaClO4 ≈ KClO4 < NaCl < NaBr < RbCl < CsCl. Only NaI, CsBr, and CsI increased the adsorption of linuron in comparison to salt-free solutions. Desorption of linuron was also reduced in saline (NaCl) solutions. The salt effect may have environmental importance in developing environmentally friendly formulations of herbicides with reduced mobility in soil and enhanced ground water protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Adsorption of natural organic compounds from aqueous solutions can be influenced by inorganic salts when water structure effects [1] compete with the adsorption process. The influence of salt addition is expected for neutral organic compounds, which are weakly adsorbed from aqueous solutions. For instance, the adsorption of nuclein bases such as adenine and cytosine from aqueous solutions on montmorillonite was strongly enhanced by the addition of NaCl, NaNO3, NaI, and KSCN. The effect followed the Hofmeister series [2]. In general, the effect is scarcely studied. In a few papers [3–7] enhanced adsorption is explained by the salting out effect which, however, only operates at distinctly higher salt concentrations.
The effect of inorganic salts on the adsorption of neutral organic compounds is, in particular, of interest in the context of the effect of salinity on the adsorption of pesticides on soils and sediments. Many of these compounds are only weakly soluble in water. Nevertheless, their distribution in the environment produces serious problems.
Salt effects on the adsorption of pesticides, specially herbicides, can influence their immobilization or mobilization in soils or sediments. A recent study [8] showed that the adsorption of polycyclic aromatic compounds on harbor sediments increased with increasing salinity. The effect was related to changes in the solubility of these compounds in water. Adsorption of chlorpyrifos was greatly enhanced by addition of NaCl [9, 10]. Zagyi et al. [11] reported the effect of salts on the binding of some environmental pollutants to corn proteins. Ionic strength also played a role on retention and overloading behavior of ionizable compounds in reverse phase liquid chromatography [12]. El-Nahhal and Safi [13, 14] reported the effect of NaCl on the adsorption of phenanthrene on modified clay minerals.
Another field where the effect of salts has to be considered is the design of herbicide formulations. Several herbicides are strongly adsorbed by modified clays, mostly bentonites, and reduce leaching in the soil [13–29]. The bentonites were made hydrophobic by exchanging the inorganic cations (Na+, Ca2+) of the montmorillonite (the clay mineral in bentonites) by organic cations.
The effect of salinity in clay-pesticide formulations has to be considered from two points: addition of salts changes (1) the adsorption of herbicides on the bentonites and (2) can affect the leaching behavior.
For the present study, we have selected linuron (Fig. 1), a herbicide widely used in agriculture for weed control. In spite of its relatively low solubility in water and the low vapor pressure, the use of linuron provides environmental problems such as water and soil contamination. Tauler et al. [30] found linuron residues and other 19 organic pollutants in surface water in Portugal. Among other herbicides linuron was frequently detected in drinking water wells in the USA [31]. Linuron was found in potatoes, carrots, and mixed vegetables in Italy [32], in carrots grown from seed in soils spiked with [14C=O]-linuron in Canada [33].
The affinity of linuron to montmorillonite is certainly weak because of the hydrophilic nature of this mineral. To increase adsorption, the bentonite was made hydrophobic by exchanging a part of the Ca2+ ions by hexadecylpyridinium and hexadecyl tributylphosphonium ions.
2 Materials and methods
2.1 Materials
We used a Ca2+-bentonite from Greece (Milos, Prassa Carys, mined 1992, M48 in our collection). This bentonite contains about 95% Ca2+-montmorillonite with an average layer charge of 0.33 eq/(Si,Al)4O10 unit. The cation exchange capacity CEC of the raw bentonite was 96 cmol(+)/kg (=96 meq/100 g) [34, 35].
The bentonite was modified by cation exchange with N-hexadecylpyridinium bromide (HDP+Br−) and N-hexadecyl tributylphosphonium bromide (HDTBP+Br−) (from Sigma Chemical Comp., Germany).
Linuron (HPLC grade, N-(3,4-dichlorophenyl)-N-methoxy-N-methylurea, molecular weight 249.11, Fig. 1) was purchased from Fluka (Germany). It is hydrophobic with an octanol/water partitioning coefficient Kow=3.0043 [36] and weakly soluble in water (81 mg/L). The volatility is low (vapor pressure at room temperature is 2 mPa).
2.2 Preparation of the organo-bentonites
The organo-bentonites were prepared by the addition of 5 mmol of the solid organic salt to 1 L of a 1% (w/v) aqueous dispersion of Ca2+-bentonite under stirring which was continued for 3 days. The organo-bentonite was separated by centrifugation (4,000 g, 30 min). The sediments were washed three times with distilled water, freeze-dried, and ground to <50-μm aggregates. In all cases, 0.5 mmol organic salts were added per gram bentonite. The amount of organic cations bound was calculated from the carbon content (CNSHO analyzer, Euro EA 3000 Elemental Analyzer), and related to gram silicate (Table 1).
2.3 Adsorption experiments
The stock solution of linuron was prepared by dissolving 30 mg in 2–3 mL methanol and diluting to 1 L with deionised water. The low concentration of methanol in the adsorption experiments had no influence on linuron adsorption.
The adsorption of linuron on the organo-bentonite was measured at 10±2 °C. Appropriate aliquots of the aqueous stock solution of linuron were diluted with water to 25 mL and added to 5 mg organo-bentonite in 30-mL centrifuge tubes. The concentration of linuron ranged between 1.2 and 31 mg/L. The final concentration of the adsorbent was 0.2 g/L. The dispersions were kept under continuous rotary agitation during 48 h. The supernatant was separated by centrifugation at 20,000 g for 0.5 h.
The concentration of linuron in the supernatants was determined by Waters 717 HPLC with UV detector (detection wavelength 254 nm). Column: Nova-Pak C18 (inner diameter 3.9 mm, length 150 mm), flow rate: 1 mL/min. The mobile phase was methanol/water 51/49 (v/v).
The relationship between concentrations of linuron and peak areas of the HPLC chromatogram was linear for 0.03–6 mg/L linuron. Linear regression was used to determine the equilibrium concentration of linuron in the solutions. The regression showed R2 value close to unity (0.9992). The amount of linuron adsorbed (correctly the specific reduced surface excess nσ (n)/m) was calculated from the depletion of the linuron concentration by adsorption [37].
For each isotherm a reference solution with an intermediate linuron concentration was stirred without organo-bentonite to evaluate adsorption on the glass or other losses. Analysis showed that linuron was not adsorbed on the glass of the centrifuge tubes. All adsorption experiments were made in duplicate.
The influence of salts (NaCl, NaBr, NaI, RbCl, CsCl, CsBr, CsI, NaClO4, and KClO4 in concentrations 1–80 g/L) was studied at a fixed addition of linuron (6.2 mg/L). The final concentration of organo-bentonite was 0.2 g/L so that 31 mg linuron was added per gram organo-bentonite.
2.4 Desorption experiments
The sediments obtained from the adsorption experiments were immediately dispersed in 25 mL water. After stirring overnight the linuron concentration in the supernatant was determined.
3 Results
3.1 Organo-bentonites
Studies on the adsorption of several herbicides have shown that the highest amounts of herbicides are adsorbed when the inorganic exchangeable cations of the montmorillonite are not completely exchanged by organic cations [19–21, 38]. In this case, the clay mineral becomes sufficiently hydrophobic but the organic cations are not densely packed in the interlayer space and provide more adsorption sites for the herbicide. The organo-montmorillonites used in this study contain organic cations in amounts of 25–43% of CEC (Table 1).
3.2 Adsorption in the absence of salts
Ca2+-bentonite adsorbed modest amounts of linuron (Fig. 2). Hydrophobization strongly increased the adsorption. About 6% of the added amounts were adsorbed on Ca2+-bentonite and 39% on HDP+-bentonite (sample II). HDTB+-bentonite adsorbed still higher amounts (58%). The adsorption isotherm of linuron on Ca2+-bentonite was linear and S-shaped for HDP+-bentonite (Fig. 3).
Adsorption of linuron by Ca2+-bentonite (a), two HDP+-bentonites (samples I and II, see Table 1) (b, c), and HDTBP+-bentonite (d). Initial linuron concentration 6.2 mg/L; 0.2 g /L bentonite or organo-bentonite. Bars indicate deviation between parallel measurements
We fitted adsorption data by the Freundlich equation:
The term nσ (n)/m is the specific reduced surface excess of linuron adsorbed (expressed in mg/g bentonite) [37], ce the equilibrium concentration (mg/L), K and n are the experimental parameters. The adsorption coefficient K gives the affinity between adsorbent and adsorptive, 1/n is related to the surface heterogeneity [39]. However, a direct comparison of K values is difficult because the dimension of K, e.g. (mg/g)(mg/L)n, changes with n.
The adsorption isotherms of linuron on Ca2+-bentonite and the modified bentonites may be described by the Freundlich model with correlation coefficients (R2) exceeding 0.98 (Table 2). The adsorption coefficient for linuron on Ca2+ and HDP+-bentonite reveals the increased affinity of the organo-bentonite for this herbicide (Table 2). Desorption of the herbicide was also reduced (Table 3).
3.3 Adsorption in the presence of NaCl
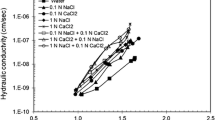
Adsorption of linuron on the HDP+-bentonite decreased with increasing concentration of NaCl (Fig. 4). At salt concentrations above 4 g NaCl/L the amount of linuron adsorbed was almost constant. However, addition of larger amounts of salts (>4 g/L) changed the shape of adsorption isotherms from S-type as in Fig. 3b to L- or J-type [40] (Fig. 5). The adsorption coefficient for HDP+-bentonite decreased at salt contents of 4 or 40 g/L NaCl (Table 2) but reached a distinctly higher value when 80 g/L NaCl was added.
Adsorption of linuron by HDP+-bentonite (sample II) as a function of salt concentration. a Sodium chloride, b rubidium chloride, c cesium chloride, d sodium bromide, e sodium iodide. Initial linuron concentration 6.2 mg/L; 0.2 g/L organo-bentonite. Bars indicate deviation between parallel measurements
3.4 Adsorption in the presence of different salts
Most salts reduced the adsorption of linuron in comparison to salt-free solutions. The effect increased in the order NaClO4 ≈ KClO4 < NaCl < NaBr < RbCl < CsCl. NaI, CsBr, and CsI increased linuron adsorption (Figs. 4, 6).
4 Discussion
At the first sight, the behavior of alkylammonium montmorillonites in water is unexpected. Alkylammonium montmorillonites intercalate larger amounts of water only when the alkyl chains are long, e.g. containing more than 15 carbon atoms [41]. The adsorption of larger amounts of water by alkylammonium ion beidellites and vermiculites starts with distinctly shorter alkyl chains (number of carbon atoms in the alkyl chain nC>6). The reason is the different alkyl chain arrangement in the dried alkylammonium clay minerals. The alkyl chains in alkylammonium montmorillonites are parallel to the silicate surface forming strong van der Waals contacts with the surface oxygen atoms because the methylene groups fit perfectly the oxygen atoms of the silicate layer [42–44]. The van der Waals interaction between the alkyl chain and the silicate layer is particularly strong for monolayers of alkyl chains when each chain is in direct contact with both silicate surfaces. In contrast, the alkyl chains in (more highly charged) vermiculites are in paraffin-type arrangements and only the chain ends are in contact with the surface [45–47]. Interlayer adsorption (intercalation) of water or other neutral solvents by alkylammonium montmorillonites requires the movement of the alkyl chains from parallel orientation into the paraffin-type arrangement [48]. For shorter alkyl chains which are in direct contact with both silicate layers the high van der Waals interaction between the flat-lying alkyl chains and the surface oxygen atoms impedes the intercalation of larger amounts of water; some water molecules only fill the pores between the alkyl chains. Longer alkyl chains which are arranged in bilayers or pseudo-trimolecular layers require a smaller energy per methylene group to move into the paraffin-type arrangement, and larger amounts of water can penetrate between the layers as indicated by the distinct increase of the basal spacing. When the alkylammonium ions in the dried samples are already in paraffin-type arrangement as in vermiculites, the adsorbing water molecules expand the interlayer space normal to the layers [41, 48–50]. In this state, the paraffin-type arrangement provides enhanced conformational freedom to the alkyl chains. The conformational entropy of the alkyl chains pointing away from the surface is considered an important contribution to the free energy change during interlayer adsorption [50, 51].
Because the swelling of the alkylammonium clay minerals in water is limited, i.e. the basal spacing does not exceed the value of expanded paraffin-type monolayers [41], the number of intercalated water molecules is restricted. These clusters of water are in an almost hydrophobic environment because not only the alkyl chains but also the siloxane groups of the silicate surface are hydrophobic [52]. A certain degree of hydrophobicity results from tetrahedral Al3+ for Si4+ substitution. The extent of such substitutions is small for montmorillonites but considerable for beidellite and vermiculites which also favors the interlayer adsorption of water.
The almost hydrophobic environment in alkylammonium montmorillonites promotes the adsorption of linuron as a hydrophobic solute. Small amounts are also adsorbed by Ca2+-montmorillonite, very likely enriched on the hydrophobic patches of the siloxane groups of the silicate surface [15]. The S-shaped isotherm for HDP+-bentonite, initially convex to the concentration axis, is indicative of weak adsorbent–adsorbate interactions. With increasing equilibrium concentration, linuron molecules adsorb more and more at sites already covered by linuron molecules [39].
There are only a few papers reporting the thermodynamics of adsorption of compounds with limited solubility in water on hydrophobized clay minerals. The adsorption of linuron from aqueous solutions on alkylammonium montmorillonite may be compared with the adsorption of butanol, hexanol, and octanol from aqueous solutions on alkylammonium montmorillonites [53–55] and of butanol on alkylammonium vermiculite [56, 57].
Because of the clustering of the water molecules between the alkyl chains [41], alkyl chain arrangement and intercalation of water are expected to be influenced by the addition of salts. The pronounced ability of anions to influence the alkyl chain conformation was recently reported [58]. The basal spacing of tetradecylammonium beidellite dispersed in water increases by salt addition, the maximum increase of 1.4 nm is observed with KSCN. Breaking of the water structure promotes an additional uptake of water. The effect of different salts shows analogy to the variation of the turbidity points of aqueous poly(ethylene oxide) solutions [49, 59]. The anions exert the dominant effect.
The strong influence of salts on the structure of water [59–69] should also influence the adsorption of a second component, in our case linuron. As the salt concentrations are relatively high, salt penetrates into the interlayer space, indicated by small concentration changes measured in some systems [70]. In most cases a very modest increase of the bulk salt concentration indicates that the salt concentration in the interlayer space is smaller than in the bulk. However, the screening effect of these ions seems to be strong enough to reduce the adsorption of hydrophobic molecules between the alkyl chains on the external surface and in the interlayer space. On the other hand, strongly water-structure breaking ions can lead to the enrichment of the hydrophobic molecules between the alkyl chains.
Many salts change the shape of the linuron adsorption isotherm (Fig. 5) and reduce the amount adsorbed (Fig. 4a–d). The opposite change of K and also of n between 40 and 80 g/L NaCl addition (Table 2) may be considered indicative of a different adsorption mechanism. The linearity of the initial section of the isotherms indicates that the addition of the salts leads to a distribution process of linuron between the bulk and the interlayer phase. In this respect the adsorption of linuron is comparable to the distribution of hexanol between the interlayer space of alkylammonium montmorillonites and the aqueous bulk solution [53].
Enhanced adsorption of linuron by salt addition is only observed (Figs. 4e, 6) when the structure-breaking power of the anion is very strong, i.e. in the presence of iodide ions. Bromide ions which are less effective structure-breaking anions only enhance linuron adsorption when the cation like the cesium ion is also structure-breaking. When only the cations are structure-breaking as in the case of RbCl and CsCl, linuron adsorption is not enhanced.
4.1 Conclusions
The adsorption of the hydrophobic molecules from aqueous solutions on hydrophobized adsorbents can be influenced by the addition of salts. This is shown here for the adsorption of the herbicide linuron on hydrophobized montmorillonite. Most salts reduce the adsorption of the herbicide between the alkyl chains on the external and internal surface. Only strongly structure-breaking salts increase the adsorption.
For practical aspects only the effect of NaCl is important. The reduced adsorption of linuron in the presence of this salt can promote leaching of the herbicide in soils at saline conditions.
References
Luck WAP (1979) In: Rowland SP (ed) Water in polymers, ACS symposium series 127:43–71
Lagaly G (1984) Philos Trans R Soc Lond A 311:315–332
Turner A, Rawling CM (2001) Water Res 35:4379–4389
Turner A (2003) Sci Total Environ 314–316:599–612
Wenhui X, Ziqin Z, Ming T, Dong LJ (1994) Chem Eng Data 39:568–571
Masterton LW, Lee PT (1970) J Phys Chem 74:1776–1782
McDevit FW, Long AF (1952) J Am Chem Soc 74:1773–1777
Hegemen WJM, van der Weijden CH, Loch JPG (1995) Environ Sci Technol 29:363–371
El-Nahhal Y (2002) In: Ottner F, Gier S (eds) Berichte der Deutschen Ton-und Tonmineralgruppe -DTTG, Wien, vol 9, pp 17–30
El-Nahhal Y (2003) Bull Environ Contam Toxicol 70:1104–1111
Zagyi M, Forgacs E, Prodan M, Cserhati T, Illes Z (2003) Environ Sci Technol 37:2836–2841
Fabric G, Guiochon G (2004) J Chromatogr A 1033:43–55
El-Nahhal Y, Safi J (2004) Appl Clay Sci 24:129–136
El-Nahhal Y, Safi J (2004) J Colloid Interface Sci 269:265–273
Laird D, Barriuso E, Dowdy RD, Koskinen WC (1992) Soil Sci Soc Am J 56:62–67
Weber JB, Swain LR (1993) Soil Sci 156:171–177
Nasser A, Gal M, Gerstl Z, Mingelgrin U, Yariv S (1997) J Therm Anal 50:257–268
El-Nahhal Y, Nir S, Polubesova T, Margulies L, Rubin B (1998) J Agric Food Chem 46:3305–3012
El-Nahhal Y, Nir S, Polubesova T, Margulies L, Rubin B (1999) Pesticide Sci 55:857–864
El-Nahhal Y, Nir S, Serban C, Rabinowich O, Rubin B (2000) J Agric Food Chem 48:4791–4801
El-Nahhal Y, Nir S, Serban C, Rabinowitz O, Rubin B (2001) J Agric Food Chem 49:5464–5371
Gerstl Z, Nasser A, Mingelgrin U (1996) J Agric Food Chem 46:3803–3809
Lagaly G (2001) Appl Clay Sci 18:205–209
Nennemann A, Kulbach S, Lagaly G (2001) Appl Clay Sci 18:285–298
Nir S, Undabeytia T, Yaron-Marcovich D, El-Nahhal Y, Polubesova T, Serban C, Rytwo G, Lagaly G, Rubin B (2000) Environ Sci Technol 34:1269–1274
Mishael YG, Undabeytia T, Rabinovitz O, Rubin B, Nir S (2003) J Agric Food Chem 51:2253–2259
Polubesova T, Epstein M, Yariv S, Lapides I, Nir S (2004) Appl Clay Sci 24:177–183
Yaron-Marcovich D, Nir S, Chen Y (2004) Appl Clay Sci 24:167–175
Rytwo G, Tavasi M, Afuta S, Nir S (2004) Appl Clay Sci 24:149–157
Tauler R, de Azevedo DA, Lacorte S, Cespedes R, Viana P, Barcelo D (2001) Environ Technol 9:1043–1054
Smith CN, Payne WR, Pope JD, Winkie JH, Parrish RS (1999) Chemosphere 38:875–889
Sannino A (1998) J Assoc Off Anal Chem 81:1048–1053
Worobey BL, Shields JB (1991) Food Addit Contam 8:193–200
Ewald W (1995) PhD Thesis, University Kiel
Donner G (2004) PhD Thesis, University Kiel
US-Department of Agriculture (1998) Agriculture Research Service. The ARS pesticide property database. Washington
Everett DH (1986) Pure Appl Chem 58:967–984
Undabeytia T, Nir S, Rubin B (2000) J Agric Food Chem 48:4767–4773
Rouquerol F, Rouquerol J, Sing K (1999) Adsorption by powders and porous solids. Academic, San Diego
Giles HC, MacEwan HT, Nakhwa NS, Smith D (1960) J Chem Soc 3973–3993
Lagaly G, Witter R, Sander H (1983) In: Ottewill HR, Rochester HC, Smith LA (eds) Adsorption from solution. Academic, London, pp 65–77
Lagaly G, Weiss A (1969) Z Naturforsch 24b:1057–1058
Lagaly G, Weiss A (1971) Kolloid ZZ Polymere 243:48–55
Lagaly G, Malberg R (1990) Colloid Surface 49:11–27
Lagaly G, Weiss A (1970) Kolloid ZZ Polymere 238:485–493
Lagaly G (1986) Solid State Ionics 22:43–51
Lagaly G (1994) In: Mermut A (ed) Charge characteristics of 2:1 clay minerals. CMS workshop lectures, vol 6. The Clay Minerals Society, Boulder, pp 1–46
Lagaly G, Witter R (1982) Ber Bunsenges Phys Chem 86:74–80
Lagaly G (1987) In: Kleeberg H (ed) Interactions of water in ionic and nonionic hydrates. Springer, Berlin Heidelberg New York, pp 229–240
Lagaly G (2005) Adv Colloid Interface Sci (in press)
Dékány I, Szánto F, Weiss A, Lagaly G (1986) Ber Bunsenges Phys Chem 90:427–431
Yariv S (1992) Intern Rev Phys Chem 11:345–375
Stul MS, Uytterhoeven JB, de Bock J (1978) Clays Clay Min 26:309–317
Stul MS, Maes A, Uytterhoeven JB (1979) Clays Clay Min 27:377–386
Stul MS, de Bock J (1985) Clays Clay Min 33:350–356
Regdon I, Dékány I, Lagaly G (1998) Colloid Polymer Sci 276:511–517
Regdon I, Király Z, Dékány I, Lagaly G (1994) Colloid Polymer Sci 272:1129–1135
Gurau MC, Lim SM, Castellana ET, Albertorio F, Kataoka S, Cremer PS (2004) J Am Chem Soc 126:10522–10523
Luck PAW (1984) In: Belford G (ed) Synthetic membrane processes. Academic, New York, pp 21–72
Wicke E (1966) Angew Chem 78:1–19
Hertz HG (1970) Angew Chem 82:91–106
Luck PAW (1978) Progr Colloid Polymer Sci 65:6–28
Kruus P, Poppe EB (1979) Can J Chem 57:538–551
Magnera FT, Caldwell G, Sunner J, Ikuda S, Kebarle P (1984) J Am Chem Soc 106:6140–6146
Ebert G (1985) Top Curr Chem 128:1–36
Kondo Y, Nakano A, Kusabayashi S (1986) J Chem Soc Faraday Trans 1 82:2141–2149
Chai-fu P (1988) J Chem Soc Faraday Trans 1:1341–1347
Holtz M, Grunder R, Sacco A, Meleleo A (1993) J Chem Soc Faraday Trans 89:1215–1222
Leontidis E (2002) Curr Opin Colloid Interface Sci 7:81–91
Sander H (1995) PhD Thesis, University Kiel
Acknowledgements
Dr. El-Nahhal thanks the Alexander von Humboldt Stiftung, grant no. IV-PAL/1104842 STP, Germany. We are very thankful to Mrs. Ute Cornelissen for the carbon content determinations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Nahhal, Y.Z., Lagaly, G. Salt effects on the adsorption of a pesticide on modified bentonites. Colloid Polym Sci 283, 968–974 (2005). https://doi.org/10.1007/s00396-004-1244-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1244-7