Abstract
Four artificial soils (AS) were prepared based on a mixture of humus, bentonite, kaolinite, and polyvinyl chloride (PVC), as inert matter, in the following proportion: 0%, 12.44%, 37.50%, 78.55% of humus, 10.5% of bentonite, 10.5% of kaolinite, and 78.92%, 66.26%, and 41.46% of PVC. The AS were prepared with variable content of organic matter (OM) in order to evaluate the retention of lead (II) due solely to the content of OM. The results indicated that retention capacity of Pb+2 increases (19.74 mg/g, 20.89 mg/g, 61.61 mg/g, and 79.48 mg/g) as OM increases (0%, 1%, 5%, and 10%); however, this retention is not proportional to the OM increment. An increase of background solution concentration of 0.01 M to 0.1 M resulted in a 50% decrease in the lead retention capacity. The fitting of lead adsorption was performed by the regression coefficient (R2). All R2 of the Langmuir model fit successfully to all types of AS (0.973 for 10-OM, 0.9845 for 5-OM, 0.999 for 1-OM, 0.994 for 0-OM). The adsorption kinetics also fits well to the pseudo-second-order model (R210-OM = 0.989, R25-OM = 0.999, R21-OM = 0.999, and R20-OM = 0.999). The thermodynamic values of the Gibbs free energy (ΔG010-OM = − 10.62, ΔG05-OM = − 11.50, ΔG01-OM = − 14.23, and ΔG00-OM = − 17.06) indicated that it was a spontaneous process, and the energy of the process suggests a retention mechanism by ion exchange. A soil with high content of OM does not guarantee high retention of lead, even more so when the adsorption mechanism is given by ion exchange.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A considerable number of products widely used in agriculture, such as fertilizers, pesticides, raw materials, and growth factors, among others, are regularly contaminated with metal ions that present toxic effects, e.g., lead, mercury, cadmium (Abdullah et al., 2020; Abdullahi et al., 2021; Magri et al., 2021; Morozova et al., 2020; Munishi et al., 2021; Nizamutdinov et al., 2021; Tesser et al., 2021; Zoffoli et al., 2013). The continuous use of these products in farming activities has increased the likelihood of environmental pollution with metals by retention, leaching, runoff, migration to underground waters, and, finally, their distribution to the food chain (Kaurin et al., 2018; Wang et al., 2016), affecting the economy and human health (Fahr et al., 2013; Kushwaha et al., 2018; Sharma & Dubey, 2005). Therefore, the accumulation and behavior of metals in soils is an issue of scientific interest.
The retention of metals in soils is determined by their adsorption and desorption capacity at the surface of the particles that make up the soil. These two processes have already been studied with several metals in soils from different parts of the world (Fonseca et al., 2009; Park et al., 2018; Pokrovsky et al., 2012; Prado et al., 2010). The soils differ by the concentration of their components, especially OM, clay minerals, and metallic oxides, among others (Momčilović et al., 2011; Pontoni et al., 2016; Qu et al., 2018). Moreover, some studies suggest that the adsorption process is the best method to understand the retention of metals in soils (Zhang et al., 2017; Chakraborty et al., 2020).
The magnitude of the metal adsorption/desorption in soils depends on the properties such as texture, cation exchange capacity, moisture, and pH. These properties also regulate the mobility (distribution and mass transfer) of metals to the environment (Uchimiya et al., 2011; Zeng et al., 2011). However, there are two parameters that have the most influence on the adsorption process: pH and OM (measured as organic carbon). pH in soils determine the speciation, solubility, and bioavailability of metals (Li et al., 2003; Oste et al., 2002; Tan et al., 2008), such as the case of soils contaminated with Pb, Cd, and Zn, where soil acidic conditions (pH < 7) do not facilitate the adsorption of metals, favoring their desorption (Oste et al., 2002; Zhang et al., 2005).
On the other hand, OM has been considered the primary cause of metal retention in soils (including lead), due to the great number of functional groups of the organic compounds that may interact with metals generating retention and immobilization (Zeng et al., 2011). In soils at pH > 6 but below at pH < 7, the adsorption process is affected by negatively charged functional groups (carboxylic, phenolic, and hydroxyl groups) from different organic and inorganic compounds present in the AS, especially humic and fulvic acids. Above pH > 7, the precipitation of lead must be taken into account (Gustafsson et al., 2003; Escudero-García et al., 2013; Shi et al., 2016; Gankhurel et al., 2020).
The capacity of soil to retain metals can be determined if the adsorption mechanism is established (Chakraborty et al., 2020). In an adsorption process, there are three fundamental steps: first, mass transfer from the solution to the surface of the adsorbent; second, diffusion of the adsorbate to the sorption sites; and third, the adsorption itself which is obtained by adsorbate-adsorbent equilibrium (Largitte & Pasquier, 2016). Some models consider that the limiting step is sorption, while others consider diffusion as the limiting step. The model that best fits the experimental data allows describing the adsorption profile, which could be by chemisorption or physisorption (Limousin et al., 2007).
The prediction, modeling, and thermodynamics of lead retention in different types of soils have been extensively investigated using kinetic and adsorption models (Fu et al., 2015; Li et al., 2003; Zhang et al., 2005). However, there is not enough information in the literature on the effect of different concentrations of OM in the same soil for the adsorption and mobility of lead, mainly due to the difficulty of obtaining soils that differ only in OM content.
In this study, four AS were prepared with different amounts of OM, bentonite, kaolinite, and PVC as inert matter, in order to determine its effect on lead retention in soils.
2 Materials and Methods
2.1 Preparation of AS and Characterization
All experiments were carried out with AS samples where the concentrations of OM were controlled. The AS were prepared in the laboratory by mixing humus (purchased in an agrochemical store), bentonite, kaolin, (purchased in a chemical store), and inert matter (polyvinyl chloride, PVC) to obtain samples with different OM contents as follows: 0% OM, 1% OM, 5% OM, and 10% OM (called 0-OM, 1-OM, 5-OM, and 10-OM, respectively), as it can be seen in Fig. 1. All raw materials were commercial grade and purchased from regular stores. Before mixing, the components were dried, finely ground, and sieved to 88 µm (W.S Tyler, Incorporated, USA) in order to assess the lead adsorption at a larger sorption surface. Afterward, they were stored 3 months before being used in the experiments. The AS were characterized by the analysis of oxidizable organic carbon (redox titration after the oxidation of the OM with potassium dichromate solution 1 N), cation exchange capacity (acidimetric titration after mixing the soil with an excess of ammonium acetate 1 N at pH 7), water retention capacity (gravimetric method based on the amount of water held by the dried sample soil), pH (1:1 soil–water ratio), carbonates (reaction of the carbonates with an excess of HCl 1 N followed by back titration of the remaining acid with NaOH 1 N), sulfates (gravimetric method based on the mass of the barium sulfate resulting of the reaction of the sulfates and barium chloride), phosphates (spectrophotometric method at 400 nm of the complex of the phosphate and molybdovanadate reagent), and humic (determination of oxidizable organic carbon from the acid-insoluble fraction of the alkaline soil extract), fulvic acids (determination of oxidizable organic carbon of the soluble fraction of the alkaline soil extract), and lead (digestion of the sample in HCl:HNO3 (3:1) and analyzed by atomic absorption spectroscopy). These parameters were analyzed by following normalized methods of the Colombian Technical Standard, NTC-5167 (ICONTEC, 2011).
2.2 Chemicals
The lead stock solution was prepared from lead (II) nitrate (Pb(NO3)2) analytical grade salt (Sigma-Aldrich, USA). Working solutions, used in all the experiments, were prepared by dilutions of the Pb+2 stock solution plus 0.01 M NaNO3 as a background solution at 298 K ± 1 K and adjusted at pH 7 by adding small volumes of 0.01 M HNO3 solution (Sigma-Aldrich, USA). All the solutions were diluted with deionized water (type I).
2.3 Batch Sorption Experiments
Batch sorption experiments were carried out following the procedure reported by Liu (Liu & Gonzalez, 2000). In a stoppered 50-mL polyethylene test tube, 0.5 g ± 0.0001 g of AS and 25.00 mL of lead (II) working solution (from 0 up to 3000 mg/L) were added. This mixture was mechanically stirred for 24 h at room temperature (298 K). Afterward, the solutions were centrifuged at 3000 rpm for 30 min, filtered in a black band Whatman filter paper (No. 42), and collected into a volumetric flask. The non-adsorbed lead was determined in the filtrates by atomic absorption spectroscopy (PerkinElmer AAnalyst 100).
2.4 Sorption Kinetic Experiments
The kinetic experiments were conducted by batch sorption experiments using a 500-mg/L lead (II) working solution and kept in stirring at different times (3, 5, 10, 15, 20, 30, 60, 90, 180, 270, and 360 min). Once the samples were centrifuged and filtered, the non-adsorbed lead in solution was determined by atomic absorption spectroscopy.
2.5 Effect of the Organic Matter Content
The effect of OM on lead retention was studied in the four AS prepared previously. The procedure for the sorption and kinetic experiments was described previously. In order to determine the effect of the ionic strength of the medium, the experiment was repeated with AS 10-OM adding an aliquot of 0.01 M NaNO3 solution (as background solution) to one sample and 0.1 M NaNO3 to another; afterward, the sorption calculations were performed. All experiments were performed in triplicate.
The simultaneous effect of two factors (OM concentration and initial lead (II) concentration) on lead adsorption capacity (response variable) was evaluated using a multilevel factorial experimental design (central composite design) and analyzed by a two-way ANOVA and a response surface methodology (RSM). The behavior of the response variable due to the effect of the factors was studied by the second-order polynomial model (Eq. 1):
where Y is the response of the model, xi, xj, … xk are the factors affecting the response Y, x2i, x2j, x2k, are the quadratic effects xi xj, xi xk, and xj xk are the interaction effects, β0 is the intercept term, βi is the linear effect coefficient, βii is the squared effect coefficient, βij is the interaction effect coefficient, and ɛ is the random error (Gutiérrez & De la Vara, 2004).
2.6 Adsorption Isotherms and Kinetic Models
In order to calculate the equilibrium Pb+2 concentration and the adsorption kinetics in the AS, the volume was considered constant during the process, that is, the volatilization losses were negligible. The amount of Pb+2 adsorbed (mg/g) or the retention capacity (qe) at equilibrium time was calculated by Eq. 2.
where Ci and Ce are the initial and equilibrium concentrations of Pb+2 (mg/L), M is the mass of AS (g), and V is the volume of solution (L) (Pokrovsky et al., 2012).
2.7 Adsorption Equilibrium
The interaction between adsorbate (Pb+2) and adsorbent (AS) in equilibrium can be described by the adsorption isotherm. This provides information on the capacity of the AS to retain Pb+2. In addition, it helps to understand the adsorption mechanism. Three models were studied for this system: Langmuir, Freundlich, and Dubinin–Radushkevich (D–R), whose linearized equations (Eq. 3–5) are shown in Table 1 (Momčilović et al., 2011; Al-Ghouti & Da'ana, 2020; Karim, 2020).
2.8 Kinetic Theory Models
Mathematical models that describe adsorption kinetics can be classified into two categories, depending on the limiting step of the adsorption process: adsorption models and diffusional models (Qiu et al., 2009). In this study, two adsorption models (Largergren’s first order and Ho’s pseudo-second order) and two diffusional models (intraparticle diffusion and liquid-film diffusion) were applied. The linearized equations of the kinetic models (Eq. 6–9) are presented in Table 2 (Das et al., 2014; Gupta et al., 2011; Ho & McKay, 1999; Qiu et al., 2009).
2.9 Quality Control
All data were acquired in triplicate and reported as the mean values. Precision was determined based on the relative standard deviation (RSD), and the Horwitz equation was used for acceptable repeatability. When the RSD was higher than Horwitz’s RSD, the analysis was repeated. The accuracy was calculated based on the recovery percentage (% R) giving a 99.5% of recovery of lead (p-value < 0.05). The instrumental limit of detection (LOD) was calculated as the signal of the blank (B) plus 3 times the standard deviation (B + 3 s) which the value for the lead was 0.1 mg/L. The LOD of the method was determined with soil with no lead content and calculated as described above giving 0.41 mg/kg. The linearity was evaluated through the correlation coefficient (r) giving 0.999 (p-value < 0.05).
2.9.1 Statistical Analysis
The effect of the initial lead concentration and the OM content on the adsorption of the metal in the AS was determined by a completely randomized experimental design in factorial arrangement 32. The statistical analysis of these results (two-way ANOVA and RSM) was performed using Statgraphics Centurion 18 software (Statgraphics Technologies, Inc., Virginia). The normality of the data was checked prior to the use of ANOVA and RSM. Excel® was also used for calculation and graphical software when necessary.
3 Results and Discussion
3.1 AS Characterization
The physical–chemical properties of the studied AS are important to help elucidate the adsorption and retention results of Pb+2, as well as to predict the behavior of the process. The AS were designed to maintain kaolin and bentonite concentrations fixed (ca 11.5% w/w), so their effect on lead retention is considered constant. The inert matter (PVC) content is variable, but, because the adsorption of Pb+2 is negligible (0.001 mg*g−1, RSD = 0.13%), there is no significant effect on lead retention. The OM (from the compost) content is variable, and it is assumed to be the only component responsible for the changes in the physicochemical properties of the AS and, hence, for the retention of the metal. Table 3 summarizes the results of the characterization of the AS.
The variation in the OM concentration influences the physical–chemical properties of AS by changing the chemical composition; thus, the water retention capacity and humic and fulvic acids increase linearly with the increase in the concentration of total organic carbon, while cation exchange capacity increases quadratically. This drives to the assumption that AS with high OM concentrations have a greater capacity for sorption and desorption of cations on the surface of the particles, from which it follows that the mobility or retention of metallic ions depends on the cation exchange capacity (Vega et al., 2009). Since the 0-OM AS has no added OM, the value obtained from the reported oxidizable organic carbon is due to the reduction of chromium VI, based on the Walkley–Black method (ICONTEC, 2011) and was taken as a blank. This outcome is corroborated by the values of humic and fulvic acids, which are directly involved in the adsorption and retention of lead (Liu & Gonzalez, 2000; Shi et al., 2016). The original concentration of Pb in the AS was found to be less than the detection limit of the analytical method (< 0.41 mg/kg). Furthermore, the concentration of sulfates, carbonates, and phosphates was not detectable (LOD of the methods: 13.7 mg/kg, 50 mg/kg, and 0.8 mg/kg, respectively), which avoids losses due to interaction and precipitation with Pb+2 in the retention experiments (Nejad et al., 2018).
3.2 Study of the Retention of Pb +2 Based on the Adsorption Isotherms
The retention of Pb+2 in the AS was determined by adsorption isotherms (Ce vs. qe). The data corresponding to each AS were adjusted to the mathematical models of the isotherms (Langmuir, Freundlich, and D–R). A detailed analysis of the parameters, obtained from the linearization of the adsorption equations (see Table 4) determines which model is the most suitable to describe the lead adsorption process (Chotpantarat et al., 2011). The plots of the isotherms (Fig. 2) show that 1-OM AS presents slightly higher lead retention (taken as qmax) than 0-OM (AS with only bentonite and kaolin) at low concentrations of Pb+2 (10 to 400 mg/L), while at high concentrations (400 to 3000 mg/L), the retention becomes very similar. In AS with higher OM content (5-OM and 10-OM) a stronger effect on lead retention was observed. However, the qmax/OM ratio decreases as OM increases as follows: 7.948 for 10-OM, 12.2 for 5-OM, and 20.89 for 1-OM. This means that, although a AS with a high concentration of OM has a high capacity to retain the lead (II), this increase is not proportional to the increase in OM.
The results of fitting the data to the mathematical adsorption models are shown in Table 4, and the corresponding graphs to the linearization of Langmuir isotherms are shown in Fig. 3.
The simultaneous effect of the factors (OM content and initial Pb+2 concentration), taking qe as the response variable, showed (two-way ANOVA) the existence of statistical differences of both factors (both had a p-value < 0.05), as well as the existence of interaction between the two (p-value < 0.05). In the RSM plot (shown in Fig. 4), it can be seen that the two studied factors in relation to the response variable present curvatures, that is, quadratic effects. A quadratic effect suggests that the simultaneous response to the two factors is not linear (Gutiérrez & De la Vara, 2004). In this sense, it corroborates the fact that for high OM concentrations, the adsorption is not as high as expected. The results of the analysis of variance of the factors showed statistical significance (p < 0.05). The quadratic model fitting of the RSM was evaluated by the determination coefficient (R2). The closer R2 is to 1, the stronger the model and the better the model predicts the response variable (Amini et al., 2008). The value of R2 obtained was 0.9470 with a standard deviation of 5.2, indicating that there is statistical evidence that only 5.30% of the variables are not explained by the mathematical expression (Eq. 12).
where Y corresponds to the lead adsorption capacity (qe), x1 is the initial lead concentration, and x2 is the OM concentration. The comparison between the experimental and the model data was made by means of a regression analysis (Fig. 5). Note that the quadratic effect of the OM is higher (− 0.2380) than that of Pb+2 initial concentration (− 0.00001) which signifies the strong effect of OM on the adsorption of the metal in the soils. The regression (R2) and correlation (r) coefficients obtained were 0.9469 and 0.9731, respectively, meaning that the data fit appropriately to a linear model and show a good correlation between the experimental and the modeled data. The plot shows that in the middle zone (30 to 60 mg/g), there is a smaller deviation, which suggests a better prediction of qe.
In soil with high OM content (above 15%), humic and fulvic acids (main components) have a considerable number of functional groups (− COOH, − OH, − NH2, etc.) that produce a negative layer on the soil particles able to interact with the cations, under suitable conditions of humidity and pH (Wu et al., 2003). Nevertheless, there are two factors that influence the reduction in the retention capacity. First, in soil with a high OM content, the aggregation of particles reduces the active surface and, hence, the negative layer available for metal adsorption becomes less (Khan et al., 2018). And second, in soils with high OM content (above 2.1% and 0.88% of humic acids), the solubility of organic carbon facilitates the interaction between humic and fulvic acids with Pb+2 in solution, avoiding retention in the solid fraction of the soil (Huang et al., 2021; Khokhotva & Waara, 2010; Rais et al., 2006). In the organic carbon solubilization, two chemical equilibriums can occur, represented by Eq. 10 and Eq. 11:
where \({K}_{\mathrm{Pb}{(\mathrm{S})}_{x}^{{(x-2)}^{-}}}\) and \({K}_{\mathrm{Pb}{(\mathrm{OC})}_{x}^{{(x-2)}^{-}}}\) are the thermodynamic equilibrium constants, \({\gamma }_{y}\) is the activity coefficient of the specie y and, \(\left[Y\right]\) is the concentration of the specie Y \(\left(Y=\mathrm{Pb}^{2+}\;\mathrm{or}\;\left(\mathrm S\right)^-\;\mathrm{or}\;\mathrm{Pb}\;\left(\mathrm S\right)_x^{{(x-2)}^-}\;\mathrm{or}\;\left(\mathrm{OC}\right)^-\;\mathrm{or}\;\mathrm{Pb}\;\left(\mathrm{OC}\right)_x^{{(x-2)}^-}\right)\), being \({\left(S\right)}^{-}\) the interaction site in the soil and, \({(\mathrm{OC})}^{-}\) the soluble organic carbon. If the soluble metal–carbon equilibrium constant is higher than the metal-interaction site equilibrium constant \(\left(\begin{array}{cc}{K}_{\mathrm{Pb}{(\mathrm{OC})}_{x}^{{(x-2)}^{-}}}\gg & {K}_{\mathrm{Pb}{\left(\mathrm{S}\right)}_{x}^{{\left(x-2\right)}^{-}}}\end{array}\right)\), there is a high probability that the metal interacts with the soluble carbon and subsequently leaches with no interaction with the adsorption or retention sites in the soil. Despite (and unfortunately) that we didn’t perform the quantification of the dissolution of organic carbon, its solubilization is evident in soils with high OM content by the dark color of the solution, as were observed in our results. On the contrary, in soils with lower OM content (1%), there was considerable retention of the metal in the soil. In soil with 1% OM, there is less amount of organic carbon in solution; therefore, there is more likely that the Pb+2 will react with the insoluble organic phase, increasing the probability of being retained (Cao et al., 1999).
From Fig. 3, it is easy to deduce that, qualitatively, all experimental data is fitted properly to the Langmuir isotherm, which is corroborated by the highest values of the regression coefficient (0.973 to 0.999) for all AS. This model assumes the formation of a monolayer on the surface of soil particles, which qmáx is the concentration necessary to saturate the soil particles, i.e., maximum adsorption capacity (Limousin et al., 2007). The adsorption parameters (Table 4) corroborate the adsorption in monolayers, that is, the data fits very well to the Langmuir model. There are several parameters that confirm that the Langmuir isotherm fits well: 1/nF values, obtained from the slope of linearized Freundlich equation, greater than 1, indicate that the adsorption in multilayers is unfavorable (Al-Ghouti & Da'ana, 2020). In this case, the 1/nF values were above 1 (2.88 and 4.20), which confirms that monolayer adsorption (described by the Langmuir model) is favorable. Free energy, ∆Go, \(\left(\Delta {G}^{o}= -RT\mathrm{ln}{K}_{\mathrm{L}}\right)\), gives negative values (Table 4) which can be attributed that a spontaneous and thermodynamically favorable process (Mishra et al., 2012). The adsorption intensity, RL \(\left({R}_{\mathrm{L}}=\frac{1}{1+{K}_{\mathrm{L}}{C}_{0}}\right)\), also confirms a favorable Langmuir adsorption, since the RL values are less than 0.1 (0.02 to 0.70) (Nanta et al., 2018). The qmáx values, obtained from the slope of the linearized Langmuir model, increase as the OM increases (19.74 to 79.48 mg/g). The adsorption strength can be inferred from the KL coefficient and calculated from the intercept of the linearized Freundlich equation (Karim, 2020). The outcomes show that the KL value of the 1-OM AS has an appreciable difference with respect to the 0-OM AS (p-value = 0.00047) caused by the increase in OM. Although there is a statistical difference between AS with 5-OM and 10-OM (p-value = 0.00035), this difference is not as high as expected.
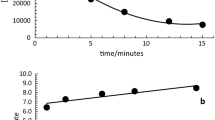
Lead retention in the 10-OM AS at two different concentrations (0.01 M and 0.1 M) of NaNO3 (background solution) was carried out. The outcomes show that the increasing tenfold of background solution (from 0.01 to 0.1 M) leads to lower Pb+2 retention of ca. 50% (Fig. 6). This fact indicates that lead retention in soils is strongly affected by ionic strength (Tan et al., 2008; Wang et al., 2010). The D–R adsorption model has been used to describe the adsorption mechanism based on the concept of adsorption free energy, where a homogeneous surface or constant sorption potential is not assumed (Lasheen et al., 2012), and to differentiate between physical, chemical, or ion exchange adsorption of metal ions (Al-Ghouti & Da'ana, 2020; Wang et al., 2016). The monolayer adsorption capacity (qDR) obtained by D–R isotherm increases from 33.46 for 0-OM to 114.27 mg/g for 10-OM (Table 4); this behavior is in accordance with the adsorption capacity calculated by the Langmuir isotherm. The free adsorption energy, E, \(\left(E=\frac{1}{\sqrt{2{K}_{\mathrm{DR}}}}\right)\) is the energy necessary to adsorb 1 mol of Pb+2 from the bulk solution onto the soils particles (Sen & Bhattacharyya, 2011). According to literature, it is considered that the process corresponds to physical adsorption when E is between 1 and 8 kJ/mol, ion exchange with E is between 8 and 16 kJ/mol, and chemical adsorption when E is greater than 16 kJ/mol (Sari et al., 2007; Wang et al., 2016). In this investigation, very similar E values were obtained for all AS (Table 4), which ranged between 12.30 and 14.74 kJ/mol, indicating that the ion exchange is the mechanism that explains the adsorption process in the AS of this experiment. These results are congruent with those obtained by other researchers who have determined that the cation exchange is the mechanism used to explain the metal adsorption in soil with OM (Gustafsson et al., 2003).
3.3 Adsorption Kinetics
The rate at which the analyte reaches equilibrium helps to determine the ability of soils to retain or leach metals in solution. Retention or leaching depends mainly on the physical characteristics of the material and the mechanism of interaction between soil and cations (Sen & Bhattacharyya, 2011; Tan et al., 2015). Thus, a soil that takes a shorter time to reach the equilibrium can retain the metal more easily, while in those systems that take longer, leaching can occur. This rate transfer of Pb+2 from the aqueous to the solid phase can occur by several processes such as chemical interaction, diffusion, and mass transfer that could be determined by the adsorption kinetics (Liu & Liu, 2008). Figure 7 shows the behavior of lead adsorption in equilibrium (qe) as a function of time, t (from 3 to 360 min) of the AS with different OM concentrations. In the four studied AS, the amount of adsorbed lead increases with time until reaching the equilibrium state. The time needed to get the equilibrium was 10 min for the 0-OM AS, 15 min for 1-OM, 20 min for 5-OM, and 60 min for the 10-OM AS. Although the amount of OM in the 1-OM AS is very low, it is enough to show the difference in time at the equilibrium point with 0-OM AS; however, the adsorption capacity is very similar at the end of the plots. In 1-OM and 5-OM AS, there is not much difference in time that it takes to reach equilibrium (5 min); nevertheless, the difference in the qe value is greater. These observations agree with the k2 value (rate constant) of the pseudo-second-order model. Despite the fact that the AS with 10-OM has twice the OM content, the adsorption capacity at equilibrium is not that much. The fast lead adsorption on the different AS at the beginning may be due to the availability of the binding sites in the OM, which allows a quick interaction with lead until reaching equilibrium (Gupta et al., 2009). This fact leads to deduce that the interaction between Pb+2 and binding sites is due to chemical interactions rather than physical adsorption (Tan et al., 2008). The aggregation of the particles in the soil with higher OM content generates electrostatic and steric repulsions between the Pb+2 ions in the adsorption process (Litniewski & Ciach, 2019) and could be the reason why this type of soil takes longer to reach equilibrium. This fact is evidenced when the ionic strength is increased using background solutions from 0.01 to 0.1 M, in which the repulsion and competition between the Pb+2 and Na+ cations for the adsorption sites cause a decrease in the capacity of retention of ca 50%. In other words, the Na + helps to displace Pb+2 of the adsorption sites because of the raise of the sodium adsorption ratio (not calculated).
Four kinetic models were applied to the results in order to help to derive the possible mechanism of the adsorption process (Sen & Bhattacharyya, 2011). Table 5 shows the kinetic parameters and regression coefficients (R2) of the Pb+2 adsorption obtained by the four models for the tested AS.
Taking the regression coefficient as an adjustment parameter to a linear model, it is observed that the highest R2 values are those of the pseudo-second-order model, which are 0.999 for 0-OM, 0.999 for 1-OM, 0.999 for 5-OM, and 0.989 for 10-OM. Table 2 shows that all the models display regression coefficients above 70%; therefore, all models present a considerable degree of fitting to the kinetics of lead-soil interaction. Soils are composed of different materials, generating a high degree of heterogeneity, where each of the components can exhibit different sorts of interaction with the cation, either attraction or repulsion (Limousin et al., 2007), which explains why all the models present some adjustment. However, the model with the highest regression coefficient is Ho’s pseudo-second-order model. Furthermore, it is the only one that shows a notable variation of the rate constant (k2), in the following sequence: OM-10 (6 × 10−4 g/(mg*min)) < OM-5 (5.9 × 10−3 g/(mg*min)) < OM-1; (0.0625 g/(mg*min)), with p-values: OM-10 < OM-5 (0.00002); OM-5 < OM-1 (0.0005). From the above, it can be concluded that the equilibrium constant has a tendency to increase with the decrease in OM; in other words, the rate of interaction is higher at low OM concentrations. Ho & McKay (1999) assume that the pseudo-second-order model “is based on the assumption that the rate-limiting step may be a chemical surprise or chemisorption involving valency forces through sharing or exchange of electrons between sorbent and sorbate,” which is consistent with the E values found from the Langmuir model, from which it is concluded that lead interacts with carboxylic and phenolic groups from OM by ion exchange (Mohapatra et al., 2009; Özcan et al., 2009; Qiu et al., 2009). The importance of these groups in the adsorption of metals, including lead, was observed by Shi et al. (2016). Note that the value of R2 for 10-OM is not as high as in the other AS, meaning a higher deviation of the adsorption process. This deviation may occur for the decrease of the adsorption of lead at high OM content. A conclusion that can be drawn from this work is that a high OM concentration does not guarantee proportional retention of lead that, from an environmental point of view, could cause leaching and runoff of the metal.
On the other hand, the thin-film diffusion model assumes the flow of cations on the adsorbent surface, this being the limiting step in adsorption kinetics (Momčilović et al., 2011). The diffusion coefficient values found (Table 5) are high in contrast to values found in the literature of 10−10 to 10−3 (Sen & Bhattacharyya, 2011), which suggests that there is a high flux of Pb2+ ions on the surface of the soil particles. In this way, it is possible to discard that the limiting step in the adsorption kinetics is the transference of the ion to the surface of the adsorbent.
4 Conclusions
The results showed that lead retention increased with increasing OM concentration. This difference in lead retention can be attributed to the effect of OM. The increase in lead retention does not occur in the same proportion as the increase in OM. The application of the mathematical models of adsorption allows concluding that the Langmuir model is the one that best fits the experimental data, which suggests adsorption on the surface of the soil particles in a monolayer.
The findings showed that the process was thermodynamically spontaneous and exothermic. The application of the response surface model allowed determining the quadratic effects of the two evaluated factors that led to corroborate that lead adsorption in soils is not proportional with OM concentration. The adsorption of Pb+2 in the AS followed the pseudo-second-order kinetic model. This model allowed deducing that the adsorption mechanism occurs by ion exchange, which is confirmed by the decrease in the lead retention when a background solution increases the concentration by ten-fold. As a final consideration, in soils contaminated with Pb+2, high content of OM does not guarantee the retention of the metal by adsorption, which is diminished by the ionic strength of soluble salts in the soil, allowing the leaching into groundwater.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code Availability
Not applicable.
References
Abdullah, M. Z., Sulaiman, F. R., & Nadzir, N. S. C. (2020). The impact of the application of agrochemicals on heavy metal pollution in plantation area. Gading Journal of Science and Technology (e-ISSN: 2637–0018), 3(01), 1–9.
Abdullahi, N., Igwe, E. C., & Dandago, M. A. (2021). Heavy metals contamination sources in Kano, Nigeria and their concentrations along Jakara River and its agricultural produce: A review. Moroccan Journal of Agricultural Sciences, 2(2).
Al-Ghouti, M. A., & Da'ana, D. A. (2020). Guidelines for the use and interpretation of adsorption isotherm models: A review. Journal of Hazardous Materials, 122383. https://doi.org/10.1016/j.jhazmat.2020.122383
Amini, M., Younesi, H., Bahramifar, N., Lorestani, A. A. Z., Ghorbani, F., Daneshi, A., & Sharifzadeh, M. (2008). Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. Journal of Hazardous Materials, 154(1–3), 694–702. https://doi.org/10.1016/j.jhazmat.2007.10.114
Cao, J., Tao, S., & Li, B. G. (1999). Leaching kinetics of water soluble organic carbon (WSOC) from upland soil. Chemosphere, 39(11), 1771–1780. https://doi.org/10.1016/S0045-6535(99)00071-5
Chakraborty, R., Asthana, A., Singh, A. K., Jain, B., & Susan, A. B. H. (2020). Adsorption of heavy metal ions by various low-cost adsorbents: A review. International Journal of Environmental Analytical Chemistry, 1-38. https://doi.org/10.1080/03067319.2020.1722811
Chotpantarat, S., Ong, S. K., Sutthirat, C., & Osathaphan, K. (2011). Effect of pH on transport of Pb2+, Mn2+, Zn2+ and Ni2+ through lateritic soil: Column experiments and transport modeling. Journal of Environmental Sciences, 23(4), 640–648. https://doi.org/10.1016/S1001-0742(10)60417-2
Das, B., Mondal, N. K., Bhaumik, R., & Roy, P. (2014). Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. International Journal of Environmental Science and Technology, 11(4), 1101–1114. https://doi.org/10.1007/s13762-013-0279-z
Escudero-García, R., Espinoza-Estrada, E., & Tavera, F. (2013). Precipitation of lead species in a Pb–H2O system. Research Journal of Recent Sciences, 9(4), 1–8. https://doi.org/10.9790/2402-1010034650
Fahr, M., Laplaze, L., Bendaou, N., Hocher, V., Mzibri, M. E., Bogusz, D., & Smouni, A. (2013). Effect of lead on root growth. Frontiers in Plant Science, 4, 1–7. https://doi.org/10.3389/fpls.2013.00175
Fonseca, B., Maio, H., Quintelas, C., Teixeira, A., & Tavares, T. (2009). Retention of Cr(VI) and Pb(II) on a loamy sand soil Kinetics, Equilibria and Breakthrough. Chemical Engineering Journal, 152, 212–219. https://doi.org/10.1016/j.cej.2009.04.045
Fu, H., Chai, T., Huang, G., Gao, P., & Liu, Z. (2015). Effects of rhamnolipid on the adsorption of Pb2+ onto compost humic acid. Desalination and Water Treatment, 54, 3177–3183. https://doi.org/10.1080/19443994.2014.943059
Gankhurel, B., Fukushi, K., Akehi, A., Takahashi, Y., Zhao, X., & Kawasaki, K. (2020). Comparison of chemical speciation of lead, arsenic, and cadmium in contaminated soils from a historical mining site: Implications for different mobilities of heavy metals. ACS Earth and Space Chemistry, 4(7), 1064–1077. https://doi.org/10.1021/acsearthspacechem.0c00087
Gupta, S., Kumar, D., & Gaur, J. P. (2009). Kinetic and isotherm modeling of lead (II) sorption onto some waste plant materials. Chemical Engineering Journal, 148(2–3), 226–233. https://doi.org/10.1016/j.cej.2008.08.019
Gupta, V. K., Agarwal, S., & Saleh, T. A. (2011). Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. Journal of Hazardous Material., 185, 17–23. https://doi.org/10.1016/j.jhazmat.2010.08.053
Gustafsson, J. P., Pechová, P., & Berggren, D. (2003). Modeling metal binding to soils: The role of natural organic matter. Environmetal Science and0 Technology, 37, 2767–2774. https://doi.org/10.1021/es026249t
Gutiérrez Pulido, H., & De la Vara Salazar, R. (2012). Análisis y diseño de experimentos: (3a. ed.). McGrawHill
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34(5), 451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Huang, X., Xu, B., Zhu, S., Ma, F., & Jin, C. (2021). Overlooked contributions of biochar-derived dissolved organic matter on the adsorption of Pb (II): Impacts of fractionation and interfacial force. Journal of Hazardous Materials, 420, 126692. https://doi.org/10.1016/j.jhazmat.2021.126692
Instituto Colombiano de Normas Técnicas, ICONTEC. (2011). Agricultural industry products. Organic Products Used As Fertilizers And Soil Amendments. (NTC 5167). https://tienda.icontec.org/
Karim, K. H. (2020). Copper adsorption behavior in some calcareous soils using Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich models. Journal of Soil Sciences and Agricultural Engineering, 11(1), 27–34. https://doi.org/10.21608/jssae.2020.79168
Kaurin, A., Cernilogar, Z., & Lestan, D. (2018). Revitalisation of metal-contaminated, EDTA-washed soil by addition of unpolluted soil, compost and biochar: Effects on soil enzyme activity, microbial community composition and abundance. Chemosphere, 193, 726–736. https://doi.org/10.1016/j.chemosphere.2017.11.082
Khan, S., Ding, X., Khan, A., & Alam, M. (2018). The effects of biochar and rice husk on adsorption and desorption of cadmium on to soils with different water conditions (upland and saturated). Chemosphere, 193, 1120–1126. https://doi.org/10.1016/j.chemosphere.2017.11.110
Khokhotva, O., & Waara, S. (2010). The influence of dissolved organic carbon on sorption of heavy metals on urea-treated pine bark. Journal of Hazardous Materials, 173(1–3), 689–696. https://doi.org/10.1016/j.jhazmat.2009.08.149
Kushwaha, A., Hans, N., Kumar, S., & Rani, R. (2018). A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicology and Environmental Safety., 147, 1035–1045. https://doi.org/10.1016/j.ecoenv.2017.09.049
Largitte, L., & Pasquier, R. (2016). A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chemical Engineering Research and Design, 109, 495–504. https://doi.org/10.1016/j.cherd.2016.02.006
Lasheen, M. R., Ammar, N. S., & Ibrahim, H. S. (2012). Adsorption/desorption of Cd (II), Cu (II) and Pb+2 using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sciences, 14(2), 202–210. https://doi.org/10.1016/j.solidstatesciences.2011.11.029
Li, K., Liu, W., Xu, D., & Lee, S. (2003). Influence of organic matter and pH on bentazone sorption in soils. Journal of Agricultural and Food Chemistry., 51, 5362–5366. https://doi.org/10.1021/jf0343332
Limousin, G., Gaudet, J. P., Charlet, L., Szenknect, S., Barthès, V., & Krimissa, M. (2007). Sorption isotherms: A review on physical bases, modeling and measurement. Applied Geochemistry, 22, 249–275. https://doi.org/10.1016/j.apgeochem.2006.09.010
Litniewski, M., & Ciach, A. (2019). Effect of aggregation on adsorption phenomena. The Journal of Chemical Physics, 150(23), 234702. https://doi.org/10.1063/1.5102157
Liu, A., & Gonzalez, R. D. (2000). Modeling adsorption of copper (II), cadmium (II) and lead (II) on purified humic acid. Langmuir, 16(8), 3902–3909. https://doi.org/10.1021/la990607x
Liu, Y., & Liu, Y. J. (2008). Biosorption isotherms, kinetics and thermodynamics. Separation and Purification Technology., 61, 229–242. https://doi.org/10.1016/j.seppur.2007.10.002
Magri, E., Valduga, A. T., Gonçalves, I. L., Barbosa, J. Z., de Oliveira Rabel, D., Menezes, I. M. N. R., & Motta, A. C. V. (2021). Cadmium and lead concentrations in yerba mate leaves from agroforestry and plantation systems: An international survey in South America. Journal of Food Composition and Analysis, 96, 103702. https://doi.org/10.1016/j.jfca.2020.103702
Mishra, S., Maity, S., Bhalke, S., Pandit, G., Puranik, V., & Kushwaha, H. (2012). Thermodynamic and kinetic investigations of uranium adsorption on soil. Journal of Radioanalytical and Nuclear Chemistry, 294(1), 97–102. https://doi.org/10.1007/s10967-011-1506-z
Mohapatra, M., Khatun, S., & Anand, S. (2009). Pb(II) adsorption on Tata chromite mine overburden. Desalination, 247, 530–539. https://doi.org/10.1016/j.desal.2008.12.038
Momčilović, M., Purenović, M., Bojić, A., Zarubica, A., & Randelovid, M. (2011). Removal of lead(II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination, 276, 53–59. https://doi.org/10.1016/j.desal.2011.03.013
Morozova, T. S., Geltukhina, V. I., Manokhina, L. A., & Kolesnichenko, E. Y. (2020, August). Ecological and agrochemical assessment of the effect of fertilizers on the behavior of cadmium and lead in soil. In IOP Conference Series: Earth and Environmental Science (Vol. 548, No. 7, p. 072055). IOP Publishing. https://doi.org/10.1088/1755-1315/548/7/072055
Munishi, L. K., Ndakidemi, P. A., Blake, W., Comber, S., & Hutchinson, T. H. (2021). Toxic metals in East African agro-ecosystems: Key risks for sustainable food production. Journal of Environmental Management, 294, 112973. https://doi.org/10.1016/j.jenvman.2021.112973
Nanta, P., Kasemwong, K., & Skolpap, W. (2018). Isotherm and kinetic modeling on superparamagnetic nanoparticles adsorption of polysaccharide. Journal of Environmental Chemical Engineering., 6, 794–802. https://doi.org/10.1016/j.jece.2017.12.063
Nejad, Z. D., Jung, M. C., & Kim, K. H. (2018). Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environmental Geochemistry and Health, 40(3), 927–953. https://doi.org/10.1007/s10653-017-9964-z
Nizamutdinov, T., Abakumov, E., Morgun, E., Loktev, R., & Kolesnikov, R. (2021). Agrochemical and pollution status of urbanized agricultural soils in the central part of Yamal Region. Energies, 14(14), 4080. https://doi.org/10.3390/en14144080
Oste, L. A., Temminghoff, E. J. M., & Van Riemsdijk, W. H. (2002). Solid-solution partitioning of organic matter in soils as influenced by an increase in pH or Ca concentration. Environmental Science and Technology., 36, 208–214. https://doi.org/10.1021/es0100571
Özcan, A. S., Gök, Ö., & Özcan, A. (2009). Adsorption of lead(II) ions onto 8-hydroxy quinoline-immobilized bentonite. Journal of Hazardous Materials., 161, 499–509. https://doi.org/10.1016/j.jhazmat.2008.04.002
Park, J. H., Wang, J. J., Xiao, R., Pensky, S. M., Kongchum, M., DeLaune, R. D., & Seo, D. C. (2018). Mercury adsorption in the Mississippi River deltaic plain freshwater marsh soil of Louisiana Gulf coastal wetlands. Chemosphere, 195, 455–462. https://doi.org/10.1016/j.chemosphere.2017.12.104
Pokrovsky, O. S., Probst, A., Leviel, E., & Liao, B. (2012). Interactions between cadmium and lead with acidic soils: Experimental evidence of similar adsorption patterns for a wide range of metal concentrations and the implications of metal migration. Journal of Hazardous Materials., 199–200, 358–366. https://doi.org/10.1016/j.jhazmat.2011.11.027
Pontoni, L., van Hullebusch, E. D., Fabbricino, M., Esposito, G., & Pirozzi, F. (2016). Assessment of trace heavy metals dynamics during the interaction of aqueous solutions with the artificial OECD soil: Evaluation of the effect of soil organic matter content and colloidal mobilization. Chemosphere, 163, 382–391. https://doi.org/10.1016/j.chemosphere.2016.08.005
Prado, A. G. S., Moura, A. O., Holanda, M. S., Carvalho, T. O., Andrade, R. D. A., Pescara, I. C., de Oliveira, A. H. A., Okino, E. Y. A., Pastore, T. C. M., Silva, D. J., & Zara, L. F. (2010). Thermodynamic aspects of the Pb adsorption using Brazilian sawdust samples: Removal of metal ions from battery industry wastewater. Chemical Engineering Journal., 160, 549–555. https://doi.org/10.1016/j.cej.2010.03.066
Qiu, H., Lv, L., Pan, B. C., Zhang, Q. J., Zhang, W. M., & Zhang, Q. X. (2009). Critical review in adsorption kinetic models. Journal of Zhejiang University-Science A, 10(5), 716–724. https://doi.org/10.1631/jzus.A0820524
Qu, C., Du, H., Ma, M., Chen, W., Cai, P., & Huang, Q. (2018). Pb sorption on montmorillonite-bacteria composites: A combination study by XAFS, ITC and SCM. Chemosphere, 200, 427–436. https://doi.org/10.1016/j.chemosphere.2018.02.136
Rais, D., Nowack, B., Schulin, R., & Luster, J. (2006). Sorption of trace metals by standard and micro suction cups in the absence and presence of dissolved organic carbon. Journal of Environmental Quality, 35(1), 50–60. https://doi.org/10.2134/jeq2005.0040
Sari, A., Tuzen, M., Citak, D., & Soylak, M. (2007). Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. Journal of Hazardous Materiasl., 149, 283–291. https://doi.org/10.1016/j.jhazmat.2007.03.078
Sen, G. S., & Bhattacharyya, K. G. (2011). Kinetics of adsorption of metal ions on inorganic materials: A review. Advances in Colloid Interface Science., 162, 39–58. https://doi.org/10.1016/j.cis.2010.12.004
Sharma, P., & Dubey, R. S. (2005). Lead toxicity in plants. Brazilian Journal of Plant Physiology., 17, 35–52. https://doi.org/10.1590/S1677-04202005000100004
Shi, Z., Wang, P., Peng, L., Lin, Z., & Dang, Z. (2016). Kinetics of heavy metal dissociation from natural organic matter: Roles of the carboxylic and phenolic sites. Environmental Science and Technology., 50, 10476–10484. https://doi.org/10.1021/acs.est.6b01809
Tan, X., Liu, Y., Gu, Y., Zeng, G., Wang, X., Hu, X., & Yang, Z. (2015). Immobilization of Cd (II) in acid soil amended with different biochars with a long term of incubation. Environmental Science and Pollution Research, 22(16), 12597–12604. https://doi.org/10.1007/s11356-015-4523-6
Tan, X. L., Chang, P. P., Fan, Q. H., Zhou, X., Yu, S. M., Wu, W. S., & Wang, X. K. (2008). Sorption of Pb(II) on Na-rectorite: Effects of pH, ionic strength, temperature, soil humic acid and fulvic acid. Colloids and Surfaces a: Physicochemical and Engineering Aspects., 328, 8–14. https://doi.org/10.1016/j.colsurfa.2008.06.022
Tesser, T. T., Rocha, C. D., & Castro, D. (2021). Metal contamination in omnivores, carnivores and detritivores fish along the tramandaí river basin, rs, brazil. Environmental Nanotechnology, Monitoring & Management, 100496https://doi.org/10.1016/j.enmm.2021.100496
Uchimiya, M., Chang, S., & Klasson, K. T. (2011). Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. Journal of Hazardous Materials., 190, 432–441. https://doi.org/10.1016/j.jhazmat.2011.03.063
Vega, F. A., Covelo, E. F., & Andrade, M. L. (2009). The role of cation exchange in the sorption of cadmium, copper and lead by soils saturated with magnesium. Journal of Hazardous Materials, 171(1–3), 262–267. https://doi.org/10.1016/j.jhazmat.2009.05.137
Wang, L., Zhang, J., Zhao, R., Li, Y., Li, C., & Zhang, C. (2010). Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresource technology, 101(15), 5808–5814. https://doi.org/10.1016/j.biortech.2010.02.099
Wang, Y., Chen, Y., Xie, H., Zhang, C., & Zhan, L. (2016). Lead adsorption and transport in loess-amended soil-bentonite cut-off wall. Engineering Geology., 215, 69–80. https://doi.org/10.1016/j.enggeo.2016.11.002
Wu, Z., Gu, Z., Wang, X., Evans, L., & Guo, H. (2003). Effects of organic acids on adsorption of lead onto montmorillonite, goethite and humic acid. Environmental Pollution, 121(3), 469–475. https://doi.org/10.1016/S0269-7491(02)00272-5.Khan,M.A
Zeng, F., Ali, S., Zhang, H., Ouyang, Y., Qiu, B., Wu, F., & Zhang, G. (2011). The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environmental Pollution., 159, 84–91. https://doi.org/10.1016/j.envpol.2010.09.019
Zhang, M., Li, W., Yang, Y., Chen, B., & Song, F. (2005). Effects of readily dispersible colloid on adsorption and transport of Zn, Cu, and Pb in soils. Environmental. International., 31, 840–844. https://doi.org/10.1016/j.envint.2005.05.037
Zhang, C., Su, J., Zhu, H., Xiong, J., Liu, X., Li, D., & Li, Y. (2017). The removal of heavy metal ions from aqueous solutions by amine functionalized cellulose pretreated with microwave-H 2 O 2. RSC Advances, 7(54), 34182–34191. https://doi.org/10.1039/c7ra03056h
Zoffoli, H. J. O., do Amaral-Sobrinho, N. M. B., Zonta, E., Luisi, M. V., Marcon, G., & Tolón-Becerra, A. (2013). Inputs of heavy metals due to agrochemical use in tobacco fields in Brazil’s southern region. Environmental monitoring and assessment, 185(3), 2423–2437. https://doi.org/10.1007/s10661-012-2721-y
Acknowledgements
The authors gratefully acknowledge the Department of Chemistry of the Universidad del Valle for allowing the use of the equipment.
Author information
Authors and Affiliations
Contributions
Rubén Albeiro Sánchez-Andica: conceptualization, methodology, supervision, writing, reviewing, and editing. Andrés Felipe Chamorro-Rengifo: investigation, validation, and writing the original draft. Martha Isabel Páez-Melo: resources and supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez-Andica, R.A., Chamorro-Rengifo, A.F. & Páez-Melo, M.I. Assessment of the Effect of Organic Matter on the Retention of Pb+2 in Artificial Soils. Water Air Soil Pollut 232, 426 (2021). https://doi.org/10.1007/s11270-021-05361-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05361-3