Abstract
In this study, temperature-/pH-responsive semi-interpenetrating polymer network (semi-IPN) hydrogels based on linear sodium alginate (SA) and cross-linked poly(N-isopropylacrylamide) (PNIPAAm) were prepared. The semi-IPN hydrogels reached an equilibrium deswelling state within 6 h in response to temperature or pH stimuli. Compared with the conventional PNIPAAm hydrogel, their dewelling rate in response to temperature was improved significantly, owing to the formation of a porous structure within the hydrogels in the presence of ionized SA during the polymerization process. Moreover, the deswelling process could be well described with a first-order kinetics equation and it is possible to design any hydrogel with the desired deswelling behavior through the control of the SA content in the semi-IPN hydrogels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intelligent hydrogels, which can change their swelling behavior and other properties in response to environmental stimuli such as temperature [1], pH [2], solvent composition [3] and electric fields [4], have attracted great interest not only because of their unique properties but also because of their potential for significant technological and biomedical applications [5, 6]. Among all intelligent hydrogels, temperature- and pH-responsive hydrogels are the most favorable members, and have been widely investigated during the last decade, because temperature and the pH value are important environmental factors in biomedical and other systems [5, 6, 7].
Poly(N-isopropylacrylamide) (PNIPAAm) hydrogel, prepared from the monomer N-isopropylacrylamide (NIPAAm) and a suitable cross-linker, is a well-known thermoresponsive hydrogel, which undergoes a volume phase transition around 32 °C (volume phase transition temperature, VPTT) in aqueous solution [8, 9]. Owing to this unique property, it has been utilized in many fields, such as controlled drug release [10], molecular separation [11], enzyme immobilization [12] and chemical valves [13]. For some of these potential applications, such as temperature-sensitive actuators and chemical valves, a high response rate is needed. However, the deswelling rate of conventional PNIPAAm hydrogel is known to be very slow owing to the formation of a dense and thick skin layer, which prevents water molecules from migrating out of the gel when the deswelling occurs [14].
In recent years, there have been considerable efforts in enhancing the swelling and deswelling rates of PNIPAAm hydrogel, and three main methods have been developed. The first approach was to prepare a PNIPAAm hydrogel with a porous structure, which is advantageous to the migration of water through the large surface area contained within the pores [5]. The second method was to introduce comb-type grafted polymer chains into the PNIPAAm network, the free mobility of which can help the expulsion of water from the gel network during collapse [15, 16]. The third means was to incorporate hydrophilic polymers, such as poly(vinyl alcohol) [14] and poly(ethylene glycol) [17], which can form water-releasing channels within the hydrogel network, into the PNIPAAm hydrogel network by interpenetrating polymer network (IPN) technology. Compared with the former two methods, the last one has some advantages. For example, an IPN hydrogel can be prepared easily, and the combination of temperature-sensitivity with other properties such as pH sensitivity can achieved by adjusting the IPN structure. Moreover, it was reported that interpenetration of the two networks might lead to much higher mechanical strength in comparison with the homopolymer network [18].
Sodium alginate (SA) is an anionic, hydrophilic polysaccharide, consisting of β-d-mannuronic acid and α-l-guluronic acid linked together in varying proportions by 1–4-linkages and has been utilized in a wide range of industrial and medical applications in both the non-cross-linked and hydrogel forms [19]. SA can also be used as a hydrophilic polymer as well as a pH-responsive component to improve the deswelling rate of the PNIPAAm hydrogel and obtain pH-/temperature-responsive hydrogels. Recently, Kim et al. [6] and Ju et al. [7] prepared two kinds of hydrogels based on an amino semitelechelic PNIPAAm and cross-linked SA with Ca2+. One is a comb-type macroporous hydrogel, in which PNIPAAm was grafted on the surface or bulk of SA. The other is a semi-IPN hydrogel, where a polyelectrolyte complex was formed via the reaction between carboxyl groups in SA and amino groups in the modified PNIPAAm. However, the hydrogels based on the cross-linked SA with Ca2+ might be unsuitable for contact with biological buffer fluid because of a loss of mechanical properties with time, which is attributed to the replacement of Ca2+ by Na+. On the other hand, the reduction of the carboxyl groups in SA and the mobility limitation of the SA chain due to Ca2+ cross-linking may lead to a decrease in the pH sensitivity of the hydrogels. Unfortunately, to date, there have been no reports on the semi-IPN hydrogels based on linear SA and cross-linked PNIPAAm.
In our previous study [20], we successfully prepared SA/PNIPAAm semi-IPN hydrogels with remarkable temperature and pH sensitivity based on linear SA and cross-linked PNIPAAm. In the present work, we focus on studying the deswelling kinetics of the semi-IPN hydrogels and explain how their deswelling rate was improved. The porous structure within the hydrogels observed by scanning electron microscopy (SEM) was formed in the presence of the ionized SA component during the polymerization process, which is advantageous to the migration of water molecules out of the gel network, resulting in rapid deswelling.
Experimental
Materials
NIPAAm (TCI, Japan) was purified by recrystallization from cyclohexane/toluene (60/40, v/v) before use to remove the inhibitor. Ammonium persulfate (APS) (Shanghai Chemical Reagent Co., China) as an initiator, N,N′-methylenebisacrylamide (BIS) (Fluka Chemical Co.) as a cross-linker, and N,N,N′,N′-tetramethylethylene diamine (TEMED) (Sigma Co.) as an accelerator were used as received. SA (Chemical Reagent Factory of Shanghai) has a β-d-mannuronic acid to α-l-guluronic acid ratio of 1.56 and a molecular weight of 2.9×105 g mol−1. All other reagents were of analytical grade and were used without further purification.
Preparation of SA/PNIPAAm semi-IPN hydrogels
Various ratios of SA to NIPAAm monomer and 2 wt % BIS based on the total monomers were dissolved in 13 ml deionized water and then 1 wt % APS and 1 wt % TEMED as redox initiators were added. Polymerization was carried out in ice–water bath for 24 h. After the polymerization was complete, the gel was cut into disks of 10-mm diameter and 1-3-mm thickness, and then immersed into an excessive amount of deionized water for 7 days to remove the residual unreacted monomers. Swollen polymeric gels were dried at room temperature for 24 h and then dried in a vacuum oven for 2 days at 40 °C prior to characterization. The feed compositions for the semi-IPN hydrogels are shown in Table 1.
VPTT determination
The VPTT of SA/PNIPAAm semi-IPN hydrogels were analyzed using a differential scanning calorimeter (DSC) (modulated DSC 2910, TA Co., USA). All hydrogels were immersed in deionized water at room temperature and allowed to swell for at least 24 h to reach the equilibrium state. The DSC analyses of the swollen hydrogels were performed from 25 to 45 °C at a heating rate of 3 °C min−1 under a nitrogen atmosphere with a flow rate of 40 ml min−1. Deionized water was used as the reference in the DSC analysis.
Scanning electron microscopy
The equilibrium-swollen samples of the SA/PNIPAAm semi-IPN hydrogels in deionized water at room temperature were quickly frozen in liquid nitrogen and then freeze-dried (−48 °C, 3.8×10−4 mbar) for at least 24 h until all water had sublimed. The freeze-dried hydrogels were fractured carefully and coated with gold on the surfaces of the hydrogels. Their surface morphology was observed using a scanning electron microscope (JSM-5600LV, JEOL, Japan).
Swelling ratio measurement
The swelling ratios of SA/PNIPAAm hydrogels were measured gravimetrically under a certain condition after the excess water on the hydrogel surface had been removed gently with tissue paper. The hydrogels were incubated in the medium for at least 24 h under each condition. The swelling ratio (Q) of the hydrogel is defined as
where Ws is the weight of water in the swollen hydrogel under a certain condition and Wd is the dry weight of the hydrogel.
Deswelling kinetics analysis
The deswelling kinetics of SA/PNIPAAm semi-IPN hydrogels was studied by recording the weight of water in the hydrogels. The water retention (WR) was calculated as
where Wt is the weight of the hydrogels at a given time interval during the course of deswelling after the swollen hydrogels at 25 °C had been quickly transferred into hot water of 45 °C or the swollen hydrogels in pH 9 medium had been transferred into the pH 1.2 medium. To analyze the deswelling process quantitatively, a semilogarithmic plot as a first-order rate analysis was applied to the time dependence of deswelling as follows:
where We and W0 are the weights of hydrogels in the equilibrium state under a certain condition and the weight of the hydrogel at 25 °C or pH 9, respectively, k is the rate constant, and t is the deswelling time.
Results and discussion
Preparation of SA/PNIPAAm semi-IPN hydrogels
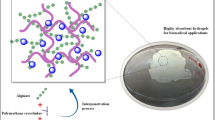
SA/PNIPAAm semi-IPN hydrogels were prepared by solution polymerization in an aqueous medium in the presence of SA, NIPAAm as the monomer, BIS as the crosslinker, and redox initiators, as shown in Fig. 1. In the present work, we employed a neutral solution as the polymerization solvent, and the pH value of the polymerization system measured by a pH meter was close to 7.0. Since the pH value is remarkably higher than the pKa of the β-d-mannuronic acid and α-l-guluronic acid units (4.0 and 3.2, respectively), SA is a negatively charged polyelectrolyte in the polymerization system. The strong electrostatic repulsions among SA carboxylate anions (–COO−) could have resulted in an expanded network of the hydrogel, which might have had an extremely high water uptake. Since the hydrogel network was reported to retain the memory of its formation history and molecular conformation [21, 22, 23], an expanded network structure with a special conformation would remain even after the hydrogel had been transferred to an acidic medium after the synthesis. The expanded porous structure was confirmed by SEM for the swollen hydrogel samples after being freeze-dried and fractured, as illustrated later.
VPTT of SA/PNIPAAm semi-IPN hydrogels
The DSC thermograms of conventional PNIPAAm and SA/PNIPAAm semi-IPN hydrogels are shown in Fig. 2. The temperature at the onset point of the DSC endotherm is referred to as the VPTT of the hydrogel as reported in previous studies [24]. At the VPTT, water in the hydrogels separated from the system, leading to a smaller heat capacity. It can be noted from Fig. 2 that all SA/PNIPAAm semi-IPN hydrogel samples exhibit a similar VPTT, around 33 °C, and there is no significant deviation from the VPTT of the conventional PNIPAAm hydrogel, indicating that, in the semi-IPN systems, the PNIPAAm network retains its own property because there is no chemical bond between SA and the PNIPAAm network.
Internal structure of SA/PNIPAAm semi-IPN hydrogels
The SEM micrographs of the internal structure of SA/PNIPAAm semi-IPN hydrogels are shown in Fig. 3. It is seen that the conventional PNIPAAm hydrogel has a relatively dense structure, while the semi-IPN hydrogels show a porous network structure. Their pore sizes increase with increasing SA content in the semi-IPNs. Therefore, it can be concluded from the results that a highly expanded network can be generated by electrostatic repulsions among SA carboxylate anions (–COO−) during the polymerization process. With increasing SA content, the expansion of the gel matrixes is enhanced, resulting in an increase in the pore size. When the temperature is above the hydrogel’s VPTT, the shrinking or deswelling occurs and thus the water molecules diffuse out easily as a result of numerous small pores in the hydrogel network. Therefore, the response rate is significantly enhanced by the incorporation of SA into the PNIPAAm hydrogel network during the deswelling process.
Response of SA/PNIPAAm semi-IPN hydrogels to environmental temperature
The effect of temperature on the equilibrium-swelling ratios of SA/PNIPAAm semi-IPN hydrogels and the conventional PNIPAAm hydrogel in deionized water are shown in Fig. 4. A VPTT of around 32 °C is observed for all the semi-IPN hydrogels and the conventional PNIPAAm hydrogel. It is well known that for the PNIPAAm hydrogel, the hydrogen bonds between the hydrophilic groups on its side chain and water molecules predominate over the hydrophobic interaction among the hydrophobic groups at temperatures below the VPTT, leading to the great water uptake of the PNIPAAm hydrogel. When SA was introduced into the gel network, the swelling ratios of the SA/PNIPAAm semi-IPN hydrogels were higher than those of the PNIPAAm hydrogel and the difference became more dramatic as the SA content in the semi-IPN hydrogel was increased, which is attributed to the expanded network structure formed during the polymerization process, as discussed earlier. Thus, the higher the SA content, the greater the network expansion, the more water was contained in the hydrogel. However, as the external temperature was increased to the VPTT, the swelling ratios for all hydrogels decreased sharply, at which point the hydrophobic interactions among the hydrophobic groups of the gels became dominant and thus the phase transition occurred. When the temperature was above the VPTT, the hydrophobic interactions became fully dominant in the systems, and the difference between the swelling ratios of the semi-IPN hydrogels and the PNIPAAm hydrogel tends to be less significant, as shown in Fig. 4.
Deswelling kinetics of SA/PNIPAAm semi-IPN hydrogels in response to temperature change
The deswelling kinetics of SA/PNIPAAm semi-IPN hydrogels after a temperature jump from the equilibrium-swollen state at 25 °C (below the VPTT) to deionized water at 45 °C (above the VPTT) is shown in Fig. 5, from which we can observe that the semi-IPN hydrogels have much higher response rates than the conventional PNIPAAm hydrogels. For instance, semi-IPN04, semi-IPN08 and semi-IPN13 lose about 80% water within 60 min, whereas the conventional PNIPAAm takes 2 h to lose only 5% water. The dramatic improvement in the response rate is attributed to the incorporation of SA into the PNIPAAm network. As observed by SEM, the porous structure was generated for SA/PNIPAAm semi-IPN hydrogels owing to the presence of ionized SA in the course of the preparation of the hydrogels. According to the mean-field theory, an expanded network with an increased osmotic pressure results in a rapidly discontinuous volume change during the phase transition [25, 26]. On the other hand, in the expanded state, the mobility of the polymer chains was much higher, so the semi-IPN hydrogels would deswell faster. Thus, a hydrogel with an expanded network has a great temperature response. Similar to other hydrophilic polymers such as poly(vinyl alcohol) and poly(ethylene glycol) reported in the literature [14, 17], the hydrophilic SA chains would also have acted as water-releasing channels in the semi-IPN hydrogels when the collapse occurred; thus, their deswelling rate was greatly improved. Clearly, more SA incorporated into the gel systems led to more water-releasing channels being formed, so the water molecules can be squeezed out of the gels more easily at temperatures above the VPTT.
To analyze the deswelling process quantitatively, ln[(Wt−We)/(W0−We)] against time t was plotted, as shown in Fig. 6. The plots were linearly fitted with a coefficient of more than 0.985, indicating the deswelling process can be apparently described by a first-order kinetics equation, and then we can obtain the deswelling rate constant from the slopes of the lines. The deswelling rate constants are 1.02×10−3, 5.56×10−3, 6.23×10−3 and 9.24×10−3 min−1 for the conventional PNIPAAm hydrogel, semi-IPN04, semi-IPN08, semi-IPN13, respectively. It is clear that k increases with increasing SA fraction. Moreover, the deswelling rate constants of semi-IPN hydrogels are significantly greater than that of the conventional PNIPAAm hydrogel owing to the incorporation of SA chains into the gel network. Accordingly, it is possible to design any hydrogel with the desired deswelling behavior by controlling the SA content.
Deswelling kinetics of SA/PNIPAAm semi-IPN hydrogels in response to pH change
When the equilibrium-swollen hydrogels in a pH 9.0 medium were transferred into an aqueous solution at pH 1.2, the deswelling kinetics of SA/PNIPAAm semi-IPN hydrogels is as shown in Fig. 7. Under basic conditions, the carboxylic acid groups in SA transform into the ionized form (COO−), so the electrostatic repulsion between the ionized groups caused the hydrogels to swell. As the semi-IPN hydrogels were transferred into an acidic medium at a pH below the pKa of SA, the COO− groups were protonated to form COOH groups. The hydrogen bonds between the carboxyl groups (–COOH) in SA and the amide groups (–CONH) in PNIPAAm kept the polymer chains of the SA/PNIPAAm hydrogels close to each other and restricted the expansion of the network at temperatures below their VPTTs [27]. With increasing SA content, the hydrogen bonds were enhanced; thus, the gel network had a more compact structure with increased SA content. From the plots of ln[(Wt−We)/(W0−We)] against time t (shown in Fig. 8), the deswelling rate constants for semi-IPN04, semi-IPN08 and semi-IPN13 are 0.94×10−3, 4.17×10−3 and 5.28×10−3 min−1, respectively. The deswelling rate constant increased significantly with the SA content in the semi-IPN hydrogels. This behavior may also be attributed to the porous structure within the gel network, which was advantageous to the migration of water molecules out of the gel when the gel volume change induced by pH stimuli occurred.
Conclusions
In the present study, SA/PNIPAAm semi-IPN hydrogels were prepared by the introduction of linear SA with pH sensitivity into the cross-linked PNIPAAm hydrogel network. These semi-IPN hydrogels exhibited rapid deswelling rates in response to both pH and temperature changes. The phenomenon was due to the formation of the porous structure within the hydrogels in the presence of ionized SA during the polymerization process, as observed by SEM. The deswelling behavior of the SA/PNIPAAm semi-IPN hydrogels could be well described with a first-order kinetics equation. The deswelling rate could be easily regulated by the SA content in the hydrogel network. Thus, it is expected that these types of semi-IPN hydrogels could be used in biomedical fields for stimuli-responsive drug delivery systems.
References
Kiler J, Scranton, AB, Peppas NA (1990) Macromolecules 23:4944
Chiu HC, Lin YF, Hung SH (2002) Macromolecules 35:5235
Kokufata E (1991) Nature 351:302
Kwon IC (1990) Mackomol Chem Macromol Symp 33:265
Serizawa T, Wakita K, Akashi M (2002) Macromolecules 35:10
Kim J, Lee S, Kim S, Lee Y (2002) Polymer 43:7549
Ju HK, Kim SY, Kim ST, Lee YM (2002) J Appl Polym Sci 83:1128
Tanaka Y, Kagamin Y, Matsuda A, Osada Y (1995) Macromolecules 28:2574
Zhang J, Peppas NA (2002) J Biomater Sci Polym Ed 5:511
Gutowska A, Bark JS, Kwon IC, Cha Y, Kim SW (1991) J Controlled Release 15:141
Fei H, Bae YH, Feijen T, Kim SW (1991) J Membr Sci 64:283
Liu F, Tao GL, Zhou RX (1993) Polym J 25:561
Bae YH, Okana T, Hsu R, Kim SW (1987) Macromol Chem Rapid Commun 8:481
Zhang JT, Cheng SX, Zhou RX (2003) Colloid Polym Sci 281:582
Yoshida R, Katsumi U, Kaneko Y, Sakai K, Sakurai Y, Okano T (1995) Nature 374:240
Kaneko Y, Nakamara S, Sakai K, Okano T (1998) Polym Gels Networks 6:333
Kim J, Lee CK, Lee YK, Kim SI (2003) J Appl Polym Sci 90:3032
Zhang J, Peppas NA (2000) Macromolecules 33:102
Stokke BT, Draget KI, Smidsrod O, Kajiwara (2000) Macromolecules 33:1853
Zhang GQ, Zha LS, Zhou MH, Ma JH, Liang BR J Polym Sci Part A Polym Chem (submitted)
Zhang XZ, Yang Y, Wang FJ, Chung TS (2002) Langmuir 18:2013
Nakamoto C, Motonaga T, Shibayama M (2001) Macromolecules 34:911
Alvarez LC, Guney O, Oya T, Salai Y, Tanaka K, Masamune G, Tanaka T (2000) Macromolecules 33:8693
Otake K, Inomata H, Konno M, Saito S (1990) Macromolecules 23:283
Hirokawa Y, Tanaka T (1984) J Chem Phys 81:6379
Tanaka T, Fillmore DJ, Sun ST, Nishio I, Swisiow G, Shah A (1980) Phys Rev Lett 45:1636
Kokufuta E, Wang BL,Yoshida R, Khokhlov AR, Hirata M (1998) Macromolecules 31:6878
Acknowledgement
This work was supported by the Doctorate Innovation Foundation of Dong Hua University, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, GQ., Zha, LS., Zhou, MH. et al. Rapid deswelling of sodium alginate/poly(N-isopropylacrylamide) semi-interpenetrating polymer network hydrogels in response to temperature and pH changes. Colloid Polym Sci 283, 431–438 (2005). https://doi.org/10.1007/s00396-004-1172-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1172-6