Abstract
The chemical modification of Plantago psyllium mucilage (Psy), an anionic polysaccharide, was done by grafting polyacrylamide (PAM) chains to prepare a graft-copolymer (Psy-g-PAM). It was synthesized in the presence of nitrogen using ceric ammonium nitrate–nitric acid redox initiator and characterized by IR spectroscopy, scanning electron microscopy and viscosity measurements. This grafted copolymer was tested for its flocculation efficiency in textile wastewater by the standard Jar test method. The effects of polymer concentration, pH and contact time on the percentage removal of solid wastes [total dissolved solids (TDS) and suspended solids (SS)] and color from textile effluent are reported. The optimum dose was found to be 1.6 mg l−1, at which maximum solid removal (SS and TDS) was seen. The most suitable pH for TDS and color removal was neutral (7.0) and for SS removal alkaline pH (9.2) was found to be most suitable. The optimum treatment duration for solid waste removal was 5 h. The X-ray diffraction analysis of Psy-g-PAM and solid waste before and after treatment suggests the interaction of the solid waste and Psy-g-PAM copolymer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indian textile industry is broadly classified into two groups: cotton industry and woolen industry. The industries based on cotton and woolen fibers also include synthetic fiber blends. These industries in general consume large volumes of water of high purity. Consequently, these units discharge large quantities of effluent that normally exhibit polluting characteristics. The discharge of the effluents to the environment without proper treatment may have long-lasting consequences. In the last decade, industry and government have become increasingly aware of the need to clean up textile effluents and reduce river pollution.
Several new and advanced materials and methods have continuously been investigated and developed. One such method is flocculation. The new materials include acrylic monomers grafted natural polysaccharides [1]. These new flocculants have started gaining importance for their shear stability, greater efficiency and environmentally-friendly nature. Natural polymers such as starch and its chemically modified forms [2, 3, 4], polyacrylamide (PAM)-grafted sodium alginate, amylopectin, guargum, xanthan gum [1] and polyacrylonitrile (PAN)-grafted polysaccharides [5, 6, 7] have been reported in the literature for domestic and industrial wastewater treatment. We have recently reported the use of some food-grade polysaccharides like fenugreek [8], Okra mucilage [9, 10], sodium alginate [11], PAM-grafted psyllium [12], PAM-grafted sodium alginate [13] and PAN-grafted Plantago psyllium mucilage (Psy) [14] for wastewater treatment. In the present communication, the synthesis, characterization and use of Psy-g-PAM as a flocculant for suspended solids (SS), total dissolved solids (TDS) and color removal from textile effluent are reported.
Experimental
Psy was obtained from its husk (Sidhpur Sat-Isabgol Factory, Gujrat, India) and was used after purification. It was purified by precipitation from aqueous solution with alcohol and finally washed with acetone. Acrylamide, ceric ammonium nitrate (extra pure, Merck chemical Co.) and nitric acid (AnalaR grade, BDH, India) were used as received. The structure of the graft copolymer was confirmed by its Fourier transform (FT) IR spectrum (Brucker vector-22 spectrometer). The intrinsic viscosities of the sample prepared and pure Psy were measured using an Ostwald viscometer. The scanning electron micrographs of Psy were obtained using a JEOL JSM-840 scanning electron microscope.
Textile wastewater samples were obtained from Saroj Textile Mills, Panki Industrial Area, Kanpur, India. Samples were collected from the main stream of wastewater coming out from the processing unit on three consecutive days. The samples were characterized within the next 24 h and were stored under refrigeration. Flocculation studies were conducted within the next 24 h. The pH values of the wastewater samples and mucilage solution in water were measured using a CP 931microprocessor pH meter. The conductivity of the wastewater samples was measured using a CC 631 conductivity meter, turbidity was measured using a HACH model 2100A turbidity meter and the chemical oxygen demand (COD) was measured by the usual standard method [15]. The buffer solutions prepared by using readymade buffer tablets (E. Merck Chemicals) were used for maintaining the pH of the wastewater samples. The concentration of the black color of the textile effluent was measured spectrophotometrically using a Systronics model 106 visible spectrophotometer.
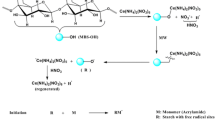
The Psy-g-PAM was synthesized by grafting acrylamide onto purified Psy by a radical polymerization method in aqueous system using ceric ion and nitric acid redox initiator. The detailed method is given in our previous publications [13, 16]. Psy (1 g), acrylamide monomer (0.21 mol) and ceric ammonium nitrate (0.05×10−3 mol) in 300 ml distilled water were used for the synthesis [16]. There is always a possibility of the formation of the homopolymer in grafting; however, Owen and Shen [17] showed that if the monomer (here PAM) concentration is less than 2.0 mol and the ceric ammonium nitrate concentration is less than 0.1 mol the chances of forming the homopolymer is much less. In the present study their concentration was kept within these critical values. It was thus assumed that no homopolymerization took place. The total monomer conversion was calculated by as follows [18]:
Flocculation studies were conducted by Jar test method. The method is described in our previous publications [13, 19]. The solid contents were calculated by the standard equation [20].
To determine the TDS, known volumes of filtered samples were evaporated and the solids thus obtained were dried and weighed. The SS in the wastewater were determined by subtracting the TDS from the total solids.
Flocculation studies were carried out at three pH values: 4.0, 7.0 and 9.2. The X-ray diffraction (XRD) patterns of powder samples of the grafted copolymer, the solid waste and the flocs were obtained at ambient conditions using an Iso-Debyflux-2002 X-ray diffractometer (Rich and Scifert) with a Cu Kα radiation source.
Results and discussion
Characterization
The FTIR spectrum of purified Psy shows characteristic peaks of –OH between 3,609 and 3,288 cm−1, of −C=O of −COOH between 1,635 and 1,617 cm−1 and of the ether linkage at 1,419 cm−1. The most prominent peak is the −C=O peak of the −COOH group present in Psy (Fig. 1, curve a), whereas the spectrum of the Psy-g-PAM sample (Fig. 1, curve b) has characteristic peaks of −OH and −NH overlapping between 3,609 and 3,288 cm−1, of the ether linkage between 1,543 and 1,438 cm−1, of −C= O of the amide at 1,636 cm−1, of −CH2 at 2,924 cm−1, of C–N stretching at 1,261 cm−1 and of the out-of-plane NH band at 800–600 cm−1. Further, a comparative study of the scanning electron micrographs of purified Psy and Psy-g-PAM indicated that grafting had taken place [21] (Fig. 2). The intrinsic viscosity values of pure mucilage and copolymer were found to be 1.29 and 5.73 dl g−1, respectively.
The pH values, conductivity values and COD of the wastewater samples ranged between 4.0 and 4.2, between 5.6 and 6.0 mS and between 815 and 910 mg l−1, respectively. Total solid contents and SS ranged between 5,590 and 5,625 mg l−1 and between 95 and 120 mg l−1, respectively, whereas the turbidity ranged between 18.0 and 18.28 NTU. The pH values of 100 ml aqueous solutions having different concentrations of Psy-g-PAM were slightly alkaline (between 7.64 and 7.87). The pH of the textile effluent after addition of different doses of Psy-g-PAM was found to vary between 5 and 6.
Flocculation test
Effect of polymer dose
Plots of percentage removal of SS and TDS versus polymer dose are shown in Fig. 3. It is apparent that with an increase in polymer dose, the percentage removal of solid waste (SS and TDS) increases, but after a certain dose of polymer, i.e. 1.6 mg l−1, a decreasing trend in solid removal is seen with an increase in polymer concentration. This behavior could be explained by the fact that the optimal dose of flocculant in suspension causes larger amounts of solid to aggregate and settle. However, Chan and Chiang [2] suggest that an overoptimal amount of flocculant in suspension would cause the aggregated particles to redisperse in the suspension and would also disturb particle settling.
Effect of contact time
The effect of contact time on the percentage removal of SS and TDS from the effluent at various polymer concentrations is shown in Fig. 4. The optimum polymer dose (1.6 mg l−1) causes the maximum SS removal, i.e. around 87%, after 5 h, whereas the maximum TDS removal, i.e. around 27%, was achieved after only 1 h. After this duration the reverse trend was seen. The most plausible reason for this trend may be the change in the surface chemistry of the effluent with time and may be due to the destabilization of the aggregated particles after the optimal time duration [22].
Percentage removal of suspended solids versus contact time: copolymer dose 0.4 mg l−1 (closed circles), 0.8 mg l−1 (closed triangles), 1.2 mg l−1 (closed squares), 1.6 mg l−1 (closed diamonds). Percentage removal of total dissolved solids versus contact time: copolymer dose 0.4 mg l−1 (open circles), 0.8 mg l−1 (open triangles), 1.2 mg l−1 (open squares), 1.6 mg l−1 (open diamonds)
Effect of pH
The flocculation efficiency of the grafted copolymer was found to be a maximum at alkaline pH (9.2) for SS removal and neutral pH (7.0) was best suited for TDS removal using the optimum concentration. The percentage removal of SS and dissolved solids with various contact times at different pH is shown in Fig. 5. The maximum SS removal at pH 4.0, 7.0 and 9.2 was around 89, around 27 and around 93%, respectively, after 5 h. In the case of TDS, the removal was around 19, around 72 and around 15% at pH 4.0, 7.0 and 9.2, respectively, after 3 h. From the plots it could be inferred that though the maximum SS removal (93%) is seen after 5 h of contact time at pH 9.2 the acidic pH (4.0) is also favorable. Heavy metals, like Zn, Mn, Ni, Cu, Cd, Al and Fe, are usually present in the textile effluent, either in the form of their hydroxide at alkaline pH [22] or as oxides at acidic pH [23]. The increase of TDS removal at neutral pH may be due to such substances, which favor neutral pH for substantial precipitation. From the results, it is clear that the solid removal by using this copolymer would require a two-step process. In the first step, TDS removal might be achieved by maintaining the neutral pH and then by increasing the pH to alkaline, SS removal might be achieved. This whole process would require around 6 h to get the maximum total solid removal.
Percentage removal of suspended solids versus contact time: copolymer dose 1.6 mg l−1, pH 4.0 (closed circles), 7.0 (closed triangles), 9.2 (closed squares). Percentage removal of total dissolved solids versus contact time: copolymer dose 1.6 mg l−1, pH 4.0 (open circles), 7.0 (open triangles), 9.2 (open squares)
Adsorption of the color by the copolymer
The wastewater sample was blackish. The concentration of the color content in the textile effluent was measured before and after treatment using a visible spectrophotometer. The adsorption of color by the optimal copolymer dose, 1.6 mg l−1 with various contact times and pH is shown in Fig. 6. The absorbance of the color was measured at λ max=520 nm. It is apparent from the figure that the maximum color removal was seen at neutral pH (7.0). At this pH, the maximum percentage removal was found to be 15.24%, at acidic pH, 4.0, the percentage color removal was found to be7.14, whereas at alkaline pH, 9.2, the color removal was only 3.75% after 4 h of contact time.
Although the XRD patterns do not give any specific evidence for the mechanism of flocculation, they may be used as supportive evidence. Figure 7 presents the comparison of XRD patterns observed for the solid waste, the grafted copolymer and flocs at room temperature from 2θ=10–90″ (error range of 2θ is 0.01–0.31). The diffraction pattern in Fig. 7a shows the crystalline nature of the solid waste, whereas that in Fig. 7b shows the completely amorphous nature of the copolymer. The flocs showed a diffraction pattern quite different from the diffractograms of the solid waste and the grafted copolymer. The 2θ (diffraction angle) and the d values (diffracting intensities) observed in Fig. 7a are changed altogether in Fig. 7c). This constitutes primary evidence that a different crystal type was formed [24]. The changes in the 2θ angle and d values indicate the change in the nature of the crystalline waste material in the wastewater during the flocculation process. This may be due to interactions between free hydroxyls groups of polysaccharide and the contents of the textile waste [9].
Anionic polymers are known to make larger flocs by a bridging mechanism. In this experiment, however, the extent of the change observed in Figs. 7a and c suggests that apart from secondary bonding between the flocculant and the solid waste, there may also be the involvement of primary bonding, like chelation [25] between crystalline matter of the waste and the grafted copolymer.
Conclusions
Water-soluble Psy-g-PAM was found to be a very effective flocculant, capable of removing more than 93% of SS, 72% of TDS and 15.24% of color from the wastewater sample. The efficacy of Psy-g-PAM in flocculating textile wastewater is dependent on the pH of the medium under the test conditions. The most suitable pH for TDS and color removal was found to be neutral and the optimal time was 3–4 h, whereas alkaline pH was well suited for SS removal and the time requirement was 5 h. A very low flocculant concentration of 1.6 mg l−1 is capable of removing an appreciable amount of solids and color. The flocculation results show that the overall treatment procedure would be a two-step process. XRD patterns were used as supportive evidence for suggesting the possible mechanism of flocculation.
References
Singh RP, Karmakar GP, Rath SK, Pandey SR, Tripathy T, Panda J, Kanan K, Jain S K, Lan NT (2000) Polymer Eng Sci 40:46
Chan WC, Chiang C Y (1995) Appl Polym Sci 58:1721
Khalil MI, Farag S (1998) J Appl Polym Sci 69:45
Triphati T, Pandey SR,. Bhagat RP, Singh RP (2001) Eur Polym J 37:125
Min BG, Kim C, Lee S (2000) Annu Tech Conf Soc Plast Eng 58:2396
Kang DW, Choi RC, Kweon DK (1999) J Appl Polym Sci 73:469
Eromosele IC, Bayero SS (2000) Bio Resour Technol 71:279
Mishra A, Agarwal M, Yadav A (2003) Colloid Polym Sci 281:164
Agarwal M, Rajani S, Mishra A (2001) Macromol Mater Eng 286:560
Agarwal M, Rajani S, Rai JSP, Mishra A (2002) Int J Polym Mater (in press)
Rajani S, Vankar PS, Mishra A (2001) Colourage 48:29
Agarwal M, Rajani S, Mishra A (2002). J Polym Res 9:69
Rajani S, Agarwal M, Vankar PS, Mishra A (2002) Asian Textile J 11: 48
Rajani S, Agarwal M, Mishra A (2002) Water Qual Res J Can 37:371
Eaton AD, Clesceri LS, Greenberg AE (1995) Standard methods for examination of water and effluent, 19th edn. APHA, Washington, DC, pp 5–12
Mishra A, Rajani S, Agarwal M, Dubey R (2002) Polym Bull 48:439
Owen DR, Shen TC (1977) In: Harris FW, Seymour RB (eds) Structure–solubility relationship in polymers. Academic, New York, pp
Athawale VD, Rathi SC (1999) J Macromol Sci Rev Macromol Chem Phys C 39:445
Jha A, Agarwal S., Mishra A, Rai JSP (2001) Iran Polym J 10:85
Eaton AD, Clesceri LS, Greenberg AE (1995) Standard methods for examination of water and effluent, 19th edn. APHA, Washington, DC, pp 2–53
Shukla GS, Tiwari SC, Dixit SK (1990) J Appl Polym Sci 40:1425
Hocking HB, Klichuk KA, Lowen S(1999) J Macromol Sci Rev Macromol Chem Phys C 39:177
Ayumi U, Teruyuki T, Toshiyoki T, Koji M (2000) Water Res 34:751
Huang L, Allen E, Tonelli AE (1999) Polymer 40:3211
Kang DW, Choi RC, Kweon DK (1999) J Appl Polym Sci 73:469
Acknowledgements
The authors are grateful to the University Grants Commission and the Council of Scientific and Industrial Research, New Delhi, India, for providing financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, A., Srinivasan, R., Bajpai, M. et al. Use of polyacrylamide-grafted Plantago psyllium mucilage as a flocculant for treatment of textile wastewater. Colloid Polym Sci 282, 722–727 (2004). https://doi.org/10.1007/s00396-003-1003-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-003-1003-1