Abstract
Remote ischemic preconditioning (rIPC) induced by transient limb ischemia (li-rIPC) leads to neurally dependent release of blood-borne factors that provide potent cardioprotection. We hypothesized that transcutaneous electrical nerve stimulation (TENS) is a clinically relevant stimulus of rIPC. Study 1: seven rabbits were subjected to lower limb TENS; six to li-rIPC, and six to sham intervention. Blood was drawn and used to prepare a dialysate for subsequent analysis of cardioprotection in rabbit Langendorff preparation. Study 2: 14 healthy adults underwent upper limb TENS stimulation on one study day, 10 of whom also underwent li-rIPC on another study day. Blood was drawn before and after each stimulus, dialysate prepared, and cardioprotective activity assessed in mouse Langendorff preparation. The infarct size and myocardial recovery were measured after 30 min of global ischemia and 60 or 120 min of reperfusion. Animal validation: compared to control, TENS induced marked cardioprotection with significantly reduced infarct size (TENS vs. sham p < 0.01, rIPC vs. sham p < 0.01, TENS vs. rIPC p = ns) and improved functional recovery during reperfusion. Human study: compared to baseline, dialysate after rIPC (pre-rIPC vs. post-rIPC, p < 0.001) and TENS provided potent cardioprotection (pre-TENS vs. post-TENS p < 0.001) and improved myocardial recovery during reperfusion. The cardioprotective effects of TENS dialysates were blocked by pretreatment of the receptor heart with the opioid antagonist naloxone. TENS is a novel method for inducing cardioprotection and may provide an alternative to the limb ischemia stimulus for induction of rIPC clinically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic preconditioning (IPC) is a potent innate mechanism of protection that can reduce myocardial infarction size by over 50 % in experimental models [30]. Preconditioning can result from both local and remote (rIPC) ischemic stimuli [33], the most clinically relevant of which is transient limb ischemia (li-rIPC). In the original study by Kharbanda and colleagues, transient ischemia of the hindlimb using a tourniquet was shown to reduce subsequent myocardial infarction (MI) size in pigs, and in the same study rIPC induced by transient ischemia of the arm (using a standard blood pressure cuff) protected against endothelial dysfunction after prolonged ischemia of the contralateral arm in human volunteers [19]. Subsequently, there have been many experimental studies using the technique [3, 19, 35], and translation to randomized clinical trials [5, 20, 50] has been rapid. While the exact nature of the signal transduction within the limb and between the limb and the heart remains to be fully elucidated, it is known that li-rIPC leads to the release of blood-borne cardioprotective factors. We have shown previously that these cardioprotective factors are small (dialyze across a 15 kDa membrane) and hydrophobic (elute from a C18 column), and when plasma dialysate from preconditioned animals or humans is used to treat naïve hearts in Langendorff preparation, of fresh isolated cardiomyocytes, provide potent cardioprotection [42]. Furthermore, in animal models, we and others have shown that the release of these factors can be induced by intra-arterial adenosine [10, 45], or by local adenosine release [9, 25], direct femoral nerve stimulation [35, 45], and nociceptive stimulation via topical capsaicin [18, 35]. Conversely, these effects can be abrogated by adenosine receptor blockade [32, 49], femoral nerve section [10, 45], pretreatment with intra-arterial infusion of the NO-donor SNAP [45] which is neuro-inhibitory to small sensory nerve fibers, and topical DMSO (a sensory fiber-blocking agent) prior to capsaicin treatment [12]. Interestingly, in a recent clinical study, li-rIPC in diabetic patients led to release of blood-borne cardioprotective factors in those without, but not those with, peripheral neuropathy [17], further emphasizing the importance of the peripheral nerves in the mechanism of release of blood-borne cardioprotective factors.
We hypothesized that other forms of peripheral nerve stimulation may provide a clinically relevant method to cause release of the cardioprotective factor(s). Transcutaneous electrical nerve stimulation (TENS) is simple to apply, is used widely clinically [4, 43], and has an excellent safety profile. Given our prior observations showing induction of RIPC by direct nerve stimulation [35], we studied the use TENS as a possible peripheral nerve stimulus of rIPC in animal studies, and subsequently in human volunteers, using our previously described method of preparing blood dialysate, which we test in Langendorff heart preparation [42]. Although the exact nature of the circulating cardioprotective factor(s) remains to be identified, this system provides a facile bioassay for the release of such factors that can be tested in naïve hearts, not subjected directly to preconditioning stimuli.
Materials and methods
All animal protocols were approved by the Animal Care and Use Committee of the Hospital for Sick Children in Toronto and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). The human studies were approved by the institutional clinical research ethics board, and written informed consent was obtained from each subject.
Experimental design
Study 1: Transcutaneous electrical nerve stimulation (TENS) as a novel method of remote preconditioning in a rabbit model
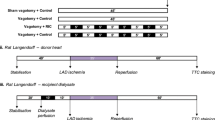
The donor rabbits were premedicated with 0.25 mg/kg akmezine (containing ketamine, acepromazine and atropine). After 10 min, pentobarbital sodium (30 mg/kg) was injected via a cannula placed in a marginal ear vein and the animal was intubated and ventilated. All rabbits were anticoagulated with heparin (100 IU/kg) via a marginal ear vein. The left carotid artery of sham and treated rabbits was exposed and cannulated with a 5-Fr catheter to collect blood for dialysate preparation.
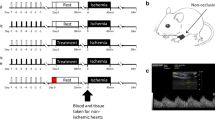
The 31 New Zealand white rabbits (male: weight 3–3.5 kg) were randomly allocated to five groups: group 1 (sham, n = 6), animals were anesthetized for 50 min; group 2 (li-rIPC, n = 6), remote ischemic preconditioning was induced through four cycles of 5 min of hindlimb ischemia (via a tourniquet), followed by 5 min of reperfusion as previously described [36]; group 3 (TENS, n = 7), transcutaneous electrical nerve stimulation was delivered using the Nicolet Endeavour TENS machine (Nicolet Endeavor CR, CareFusion, Madison, WI, USA) via applied electrode pads directly onto the medial side of the hindlimb, with a stimulus of 500 μs pulse width, 3.1 Hz, 2–3 mA, four cycles of 5 min of stimulation followed by 5 min of rest. Blood was taken at the end of the intervention protocol (see below).
Study 2: TENS as a novel method of remote preconditioning in human study
Fifteen healthy human subject volunteers (7 males, 8 females, all Caucasian) between the ages of 25–32 years were recruited. Potential subjects were excluded if affected by recent (within 1 month) inter-current or chronic illnesses (e.g., diabetes mellitus, hypertension). Informed consent was obtained before enrollment in the study. The subjects were required to abstain from vigorous exercise, coffee and alcohol consumption for 24 h prior to each study; all were non smokers. Nine subjects underwent rIPC and TENS with the order of the stimulus randomly assigned, and with at least 1 week separating each study intervention (see below). Five subjects underwent only TENS. One subject underwent only rIPC due to scheduling difficulties. Samples of 30 ml of whole blood were stored in lithium-heparin tubes before and after each intervention to prepare dialysate for study in mouse Langendorff preparation.
Induction of li-rIPC
Li-rIPC was performed on another study day separated by at least 1 week from the TENS study. Decision as to which intervention was performed first was made by a coin toss. Li-rIPC was administered in the usual protocol, whereby four cycles of 5 min of ischemia, induced by blood pressure cuff to the upper arm inflated to 200 mmHg, was followed by 5 min of reperfusion [19]. Blood samples for preparation of dialysate were collected at baseline and following li-rIPC.
Transcutaneous electrical nerve stimulation (TENS) preconditioning
Using the same TENS machine as in rabbit experiments, subjects received a therapeutic stimulus via an upstream and downstream electrode pair placed on the skin of the upper arm coinciding with the C5 dermatome. The stimulus was set to the same pulse width and frequency as in rabbits (500 μs pulse width, 3.1 Hz), with amplitude titrated to tolerance of discomfort for each subject (range of 8–20 mA). In all cases, this was associated with mild muscle twitch. Four cycles of 5 min of stimulation followed by 5 min of rest was performed.
Dialysate preparation
Approximately, 80 ml of rabbit blood was obtained from the donor rabbit at the end of treatment. Bleeding was limited to <2 min to minimize secondary hemodynamic effects. Blood gases and electrolyte measurements were taken at the end of the blood draw to confirm that each rabbit remained well oxygenated and neither acidotic nor hyperkalemic.
Human samples of 30 ml of whole blood were obtained before and after (within 15 min) each intervention.
Laboratory staff were blinded to treatment allocation and timing of sampling. Both human and rabbit blood plasma were obtained by centrifugation (3,000 rpm for 20 min) of the whole blood. To prepare the dialysate, 50 ml rabbit plasma or 15 ml human plasma was placed in dialysis tubing with a 12–14 kDa cutoff membrane (Spectra/Por; Spectrum Laboratories, Inc.; Rancho Dominguez, CA, USA) and dialyzed against a 20-fold volume of Krebs–Henseleit buffer while stirring overnight at 4 °C and then stored at −80 °C. Before perfusion of the rabbit donor hearts (Study 1), d-glucose and NaHCO3 were added to the final concentration of 11 and 10 mmol/l, respectively, to the dialysate and then filtered through a 0.2 μm filter.
Prior to perfusion of the mouse hearts (Study 2), d-glucose, NaHCO3 and EDTA were added to a final concentration of 15, 25 and 0.5, respectively, to the human dialysate. All perfusates were equilibrated with 95 % oxygen–5 % CO2 and adjusted to a pH of 7.35–7.4.
Langendorff preparation
Rabbit dialysate was studied using a rabbit-heart Langendorff preparation (one heart studied per dialysate sample). Human blood samples were smaller in volume due to the practicality of taking large amounts of blood from volunteers and thus human dialysate from human plasma was studied using a mouse-heart Langendorff. A total of 45 mice (male, C57BL6, 9–11 weeks old) were used for li-rIPC pre- and post-groups (10 human dialysates in each group); 61 mice were used for TENS pre- and post-groups (14 human dialysates in each group), each dialysate was used to perfuse two to three mouse hearts and the infarct size data averaged as one data point (see Tables 1 and 2).
Rabbit Langendorff
The thirty-one New Zealand white rabbits (male: weight 3–3.5 kg) were used for rabbit Langendorff. A heart from an untreated rabbit was quickly excised (anesthesia procedures same as above), mounted on a Langendorff perfusion apparatus, and perfused under non-recirculating conditions at constant pressure (80 mmHg) at 37 °C. Krebs–Henseleit buffer contained (in mM) 119 NaCl, 4.7 KCl, 1.2 MgSO4, 1.8 CaCl2, 1.2 KH2PO4, 25 NaHCO3, and 11 glucose, gassed with 95 % oxygen–5 % CO2. The solution was continuously oxygenated with 95 % oxygen–5 % CO2 to maintain a final pH of 7.4. The buffer was vacuum filtered with a 0.2-μm nitrocellulose filter to remove particulates. Once placed onto the perfusion apparatus, each heart was submerged in 37 °C Krebs–Henseleit buffer for the duration of the experiment. A water-filled balloon, custom made with thin plastic Saran Wrap on PE-160 polyethylene tubing, was inserted into the left ventricle (LV) through the mitral valve and connected to a pressure transducer (ML844; AD Instruments; Colorado Springs, CO, USA). The balloon was inflated with water to adjust LV end-diastolic pressure (LVEDP) to 6–8 mmHg at the beginning of the experiment, and the volume was kept constant for the duration of the study.Each heart was allowed to stabilize for 20 min and then perfused with 1,000 ml dialysate for 15–22 min, depending on the coronary flow. Then, standard buffer was added for the remaining 8–15 min of a total of 30 min of pretreatment after stabilization. The hearts were then subjected to 30 min of global ischemia (37 °C) and 120 min of reperfusion.
Mouse Langendorff
One hundred and six C57BL6 mice (male, 10–11 weeks old) were used. Mice received heparin (200 IU, i.p. Sigma) and were anesthetized with pentobarbital (60 mg/kg, i.p. Ceva Sante animale) and then intubated and ventilated. Hearts were rapidly excised by bilateral thoracotomy, placed in ice-cold buffer and the aorta cannulated with a 20-gauge metal cannula. Isolated hearts were mounted on the Langendorff perfusion apparatus (Radnoti Technologies Inc., Monrovia, CA, USA) and perfused with dialysate for 30 min under non-recirculating conditions at a constant pressure of 80 mm Hg (~2 ml/min) with 37 °C Krebs–Henseleit buffer (KHB) (consisting of the following in mmol/L: NaCl 120.0, NaHCO3 25.0, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.5, EDTA 0.5 and glucose 15). The left auricle was removed and a balloon, made with saran wrap and PE60 polyethylene tubing, was inserted into the left ventricle (LV) through the mitral valve and connected to a pressure transducer. The balloon was inflated with water to adjust left ventricular end-diastolic pressure (LVEDP) to 7–10 mmHg at the beginning of the experiment and the volume was kept constant for the duration of the study.
After a 20-min stabilization period, isolated hearts were perfused for 30 min using dialysate, then washed out for 5 min, and then subjected to 30 min of no-flow global ischemia followed by 60 min of reperfusion.
Hemodynamic measurements, including heart rate (HR), peak left ventricular pressure (LVP), maximum rate of contraction (+dP/dt max), maximum rate of relaxation (−dP/dt min), and LVEDP, were recorded on a data acquisition system (PowerLab, ADInstruments) throughout the procedure. The LV developed pressure (LVDP) was calculated as the difference between the systolic and end-diastolic LV pressures.
Opioid antagonist naloxone treatment
Twenty-six C57BL/6 mice were divided into five groups for mouse Langendorff. Group 1 (pre-TENS, n = 3) used stored frozen Pre-TENS dialysate to perfuse mouse heart for 30 min before global ischemia; procedure was the same as described above. Group 2 (post-TENS, n = 3) used frozen post-TENS dialysate to perfuse mouse heart for 30 min before global ischemia. In Group 3 (naloxone alone, n = 8), after a 20-min stabilization period, hearts were perfused with opioid antagonist naloxone (10 μm/l) for 10 min, changed to Krebs–Henseleit buffer for 35 min, then subjected to 30 min of no-flow global ischemia followed by 60 min of reperfusion. Naloxone infusion resumed at the onset of reperfusion and continued throughout the reperfusion period. Group 4 (naloxone + pre-TENS, n = 6) and Group 5 (naloxone + post-TENS, n = 6) hearts were perfused with opioid antagonist naloxone (10 μm/l) for 10 min before perfusing pre- or post-TENS human dialysate, then hearts were perfused for 30 min using human TENS dialysate, washed out for 5 min and then subjected to 30 min of no-flow global ischemia followed by 60 min of reperfusion. Naloxone infusion resumed at the onset of reperfusion and continued throughout the reperfusion period. The naloxone dose regime was used based on an established protocol previously reported by Gross et al. [31].
Measurement of infarct size
At the end of the reperfusion period, hearts were weighed and frozen at −80 °C. The frozen rabbit heart was cut transversely into 2-mm-thick slices using a rabbit Heart Slicer Matrix. The mouse hearts were cut into 1-mm-thick slices using a Mouse Heart Slicer Matrix (Zivic Instruments; Pittsburgh, PA, USA) and stained with 1.25 % TTC in 200 mm Tris/HCl (pH 7.4) for 15 min in a 37 °C water bath. After fixation for 2 h in 10 % neutral-buffered formaldehyde, each slice was photographed by electronic scanning (CanoScan 4400F). The viable myocardium stained brick red, and infarct tissues appeared pale. The infarct and LV areas were measured via automated planimetry using Adobe Photoshop CS2 software, with infarct size expressed as a percentage of the total LV area.
Statistical analysis
Statistical significance was determined for most comparisons by using one-way ANOVA, followed by post hoc testing (Newman–Keuls) where appropriate. Values of p ≤ 0.05 were considered to be statistically significant. Data are shown as mean ± S.E.M. (standard error).
Results
There were no statistically significant differences in baseline hemodynamic functional parameters between any of the groups. No rabbit hearts were excluded from the study. 16 mice heart were excluded from the study after the initial 20-min stabilization period if they met one of the following functional criteria: (1) coronary flow >5 ml/min, (2) heart rate lower than 300 beats/min, (3) left ventricular systolic pressure below 80 mmHg, or significant cardiac arrhythmias.
Study 1: Transcutaneous electrical nerve stimulation (TENS) is a novel method of remote preconditioning in a rabbit model
As shown in Fig. 1, perfusion with li-rIPC dialysate significantly decreased the infarct size in comparison to the sham dialysate (28.1 ± 5.3 % in rIPC vs. 45.4 ± 3.4 % in sham, p < 0.01). Comparison of TENS dialysate with sham also showed significantly decreased infarct size (27.2 ± 2.9 % in TENS vs. 45.4 ± 3.4 % in sham, p < 0.01).
As shown in Fig. 2, perfusion with li-rIPC and TENS dialysate improved post-ischemic cardiac performance in isolated perfused hearts. Recovery of post-ischemic contractile function (assessed by LVDP) was greater in li-rIPC and TENS dialysate-perfused hearts than in sham hearts. By the end of the 120-min reperfusion period, a significantly greater functional recovery was observed in li-rIPC and TENS hearts (44.3 ± 2.4 % in rIPC and 50.6 ± 3.0 % in TENS, of pre-ischemic value) compared with sham (32.0 ± 3.5 % of pre-ischemic value, p < 0.05 and p < 0.01 respectively, Fig. 2a). Maximal rates of contraction (dP/dt max) and maximal rates of relaxation (dP/dtmin) were also significantly increased (improved) in li-rIPC and TENS compared to sham hearts (dP/dt max 49.7 ± 4.9 % in li-rIPC and 57.25 ± 6.3 % in TENS vs. 35.0 ± 4.6 % in sham, p < 0.05; dP/dt min 45.9 ± 2.2 % in rIPC and 48.6 ± 5.3 % in TENS vs. 36.5 ± 4.7 %, Fig. 2b, c); these results indicated that post-ischemic contractile function was improved by li-rIPC and TENS. Diastolic recovery was also improved by li-rIPC and TENS (Fig. 2d), In rIPC and TENS groups, LVEDP was significantly lower than that of sham at the beginning of reperfusion and decreased steadily throughout the reperfusion period. At the end of the reperfusion period, LVEDP was lower in li-rIPC and TENS dialysate-perfused hearts (24.9 ± 4.8 mmHg in rIPC and 21.7 ± 3.8 mmHg in TENS) than in sham dialysate-perfused hearts (37.4 ± 6.8 mmHg, Fig. 2b).
Transcutaneous nerve stimulation improves post-ischemic cardiac performance in isolated rabbit perfused hearts. The time course of LVDP (a), dP/dt max (b), dP/dt min (c) and LVEDP (d) for all groups are shown. Haemodynamic parameters in a–c are expressed as a percentage of the pre-ischemic values, whereas LVEDP values (d) are shown in mmHg. Values are mean ± SEM, n = 6–7 per group. Hearts perfused with dialysate after li-RIPC and TENS showed improved functional recovery compared to sham dialysate (e.g., for LVDP p < 0.05, p < 0.01 respectively) (see text for details). *p < 0.01 vs. sham; # p < 0.05 vs. sham
Study 2: Human transcutaneous electrical nerve stimulation dialysate reduces infarct size and improves post-ischemic cardiac performance in perfused mouse hearts
In humans, perfusion with li-rIPC dialysate significantly decreased the infarct size when compared with baseline blood dialysate (26.6 ± 1.5 % post-rIPC vs. 41.7 ± 1.8 % pre-rIPC p < 0.001). Comparison of TENS dialysate with baseline dialysate decreased the infarct size to a similar degree (29.5 ± 2.2 % post-TENS vs. 39.8 ± 2.5 % pre-TENS, p < 0.001) (Fig. 3).
Transcutaneous nerve stimulation in humans invokes cardioprotection in dialysate-perfused mouse hearts. Dialysate obtained after either li-rIPC (n = 10) or TENS (n = 14) reduced infarct size expressed as percentage of LV area, compared to dialysate obtained prior to either stimulus. Values are mean ± SEM. *p < 0.01
As shown in Fig. 4, perfusion with either the rIPC or TENS dialysates significantly improved the post-ischemic cardiac performance in isolated perfused mouse hearts. The recovery of LVDP was significantly greater in hearts perfused with post-li-rIPC (87.6 ± 3.8 % of preischemic value) and post-TENS dialysate (86.8 ± 2.3 %) than in hearts perfused with the pre-rIPC (72.7 ± 2.8 %, p < 0.05) and pre-TENS dialysate (73.9 ± 3.6 %, p < 0.01; Fig. 4a). Both dP/dt max and dP/dt min were significantly improved with post-rIPC dialysate compared to pre-rIPC (p < 0.01, and p < 0.05 respectively). The improvement in dP/dt max was also statistically significant after TENS (p < 0.01) (Fig. 4b, c). Diastolic recovery was also improved by li-rIPC and TENS (Fig. 4d). The LVEDP was lower in the post-li-rIPC and TENS compared to the pre-rIPC group (18.9 ± 1.9 % in post-rIPC vs. 26.3 ± 2.1 % in pre-rIPC, p < 0.05) and TENS groups (17.6 ± 1.6 % in post-TENS vs. 24.2 ± 2.9 % in pre-TENS, p = 0.059) (Fig. 4d).
Human transcutaneous nerve stimulation dialysate improves post-ischemic cardiac performance in mouse perfused hearts The time course of LVDP (a), dP/dt max (b), dP/dt min (c) and LVEDP (d) for all groups are shown. Hemodynamic parameters in a–c are expressed as a percentage of the pre-ischemic values, whereas LVEDP values d are shown in mmHg. Values are mean ± SEM, n = 10 in li-rIPC group and n = 14 in TENS group. Compared to baseline (untreated dialysate) hearts perfused with dialysate after li-RIPC and TENS showed improved functional recovery (see text for details). *p < 0.01; # p < 0.05 vs. baseline (untreated dialysate)
Opioid antagonist naloxone blocked the cardioprotection induced by human TENS dialysate in perfused mouse hearts
Administration of the opioid antagonist naloxone abrogated the cardioprotective effect of TENS dialysate (infarct size 58.6 ± 4.4 % naloxone + post-TENS vs. 56.4 ± 4.0 % naloxone + pre-TENS) (Fig. 5a). Naloxone also abrogated the improvement in post-ischemic cardiac performance by either the TENS dialysates. The recovery of LVDP was same in hearts perfused with pre- and post-TENS (60.6 ± 3.9 % of preischemic value in naloxone + post- vs. 64.8 ± 4.0 % in naloxone + pre-TENS) (Fig. 5b). Similarly, LVEDP was unchanged in the TENS dialysate-perfused hearts compared to the pre-TENS groups (37.9 ± 1.8 % in naloxone + post-TENS vs. 32.4 ± 3.3 % in naloxone + pre-TENS) (Fig. 5c). To confirm the effectiveness of human dialysate after being stored for a prolonged period, stored frozen pre- and post-TENS dialysates were used in additional studies. Perfusion with TENS dialysate significantly decreased the infarct size when compared with baseline blood dialysate (29.0 ± 1.8 % post-TENS vs. 42.47 ± 1.8 % pre-TENS p < 0.01). While there was an apparent trend toward increased infarct size in the naloxone-treated hearts, there was no statistically significant difference between pre-TENS dialysate (no naloxone) and naloxone + pre-TENS groups (Fig. 5a). As shown in Fig. 5b, c, perfusion with the stored untreated TENS dialysates significantly improved the post-ischemic cardiac performance in isolated perfused mouse hearts.
The opioid antagonist naloxone blocks the cardioprotection induced by human TENS dialysate in perfused mouse hearts. Stored dialysate obtained after TENS reduced infarct size compared to dialysate obtained prior to stimulus, but administration of the opioid antagonist naloxone abrogated the cardioprotective effect of TENS dialysate (a). Naloxone also abrogated the improvement in post-ischemic cardiac performance (b and c) by TENS dialysates. The end of points LVDP (b) and LVEDP (c) are shown. n = 3 in TENS groups, n = 8 in naloxone alone group, n = 6 in naloxone + TENS groups. The right hand panels (pre-/post-TENS data, without naloxone, show data on similarly stored dialysate analyzed to exclude an adverse effect of storage on cardioprotective capacity of the dialysate. Values are mean ± SEM. *p < 0.01, # p < 0.05 vs. baseline (untreated dialysate)
Subjective experience of discomfort in TENS and rIPC
Subjects were asked to grade the discomfort during the stimulus on a 1–10 scale (1 no discomfort, 10 extreme discomfort). There was no statistically significant difference between the TENS (median 6/10, IQR 4–6) and rIPC (median 4.5, IQR 2–6) (p = 0.42) for the entire cohort or when the analysis was restricted to subjects who were subjected to both methods (p = 0.44).
Discussion
We have shown for the first time that peripheral nerve stimulation using transcutaneous electrical nerve stimulation evokes the release of dialyzable factors into the bloodstream in both rabbits and healthy human volunteers that result in potent cardioprotection in isolated hearts. The importance of these findings lay not only in the potential utility of TENS as a novel preconditioning stimulus, but also in revealing that TENS may share some characteristics to the signal transduction previously demonstrated for rIPC induced through transient limb ischemia.
The exact nature of the mechanisms by which a peripheral stimulus can lead to cardioprotection remains somewhat controversial. It is clearly a complex phenomenon, with recent data showing that its effects in humans, at least in terms of intracellular signaling, may be different to those described in smaller animals [15]. Similarly, clinical studies in patients have yielded, perhaps unexpected, but mechanistically relevant observations that potentially cast light on the mechanisms of the remote preconditioning response, for example the observation that RIPC may be abrogated by the use of propofol [23, 24]. Several studies have suggested that a direct neural connection is required between the peripheral organ and the heart [9, 11, 13, 25, 27, 32, 38, 49, 52]. We [42, 45] and others [7, 8, 22, 39, 51] have shown that cardioprotection is induced, at least in part, via the release of blood-borne factors resulting from the remote stimulus. Although it remains to be fully elucidated, not least in terms of their exact identification and characterization, several recent studies have improved our understanding of the signal transduction within the limb that leads to the release of these blood-borne cardioprotective factor(s). We have previously shown that li-rIPC results in release of a dialyzable (<15 kDa), hydrophobic factor(s) that provides cross-species (human dialysate–rabbit heart, human dialysate–mouse heart) cardioprotection [42]. Our model, whereby blood sampled from an animal or subject before and after a rIPC stimulus is used to prepare a dialysate that can be tested in a naïve heart in Langendorff preparation, allows dissection of direct neural from indirect humoral effects of the rIPC stimulus that may confound in vivo assessments. Subsequent studies using this model have shown that other stimuli within the limb can lead to release of blood-borne cardioprotective factors, with similar physical characteristics and effectiveness as li-rIPC. These studies have not only obviated the dependence of cardioprotection upon a direct neural connection between limb and heart, but have also provided strong evidence for dependence on intact neural pathways within the limb itself. Most relevant to the current study are our prior observations that direct femoral nerve stimulation and topical capsaicin invoke blood-borne cardioprotection, and that femoral nerve transection prior to li-rIPC [42] and pretreatment of the skin with DMSO (to block non-myelinated peripheral nerve transmission) prior to topical capsaicin abrogate cardioprotection [35].
While our proof-of-principle study was not designed to study the exact nature of the neural signal transduction by which TENS might induce release of blood-borne cardioprotective factors, our data clearly show that TENS is equally effective to li-rIPC, both in terms of infarct size reduction and post-ischemic myocardial recovery after reperfusion, and is similarly blocked by pretreatment with naloxone. This suggests that both li-rIPC and TENS release endogenous opioids, and its downstream effect involves stimulation of opiate receptors within the myocardium. We have previously shown that naloxone abrogates the cardioprotective effect of dialysate obtained from human subjects after li-rIPC in an isolated cardiomyocyte model [41], and it appears from the current observations that TENS may liberate substances with similar properties. Figure 5 shows that co-administration of naloxone abrogated the effect of dialysate from TENS-treated animals. These experiments were performed post hoc, on stored dialysate, and for that reason we repeated experiments with stored dialysate, without naloxone, to ensure that cardioprotection was not lost by storage alone. Nonetheless, there does appear to be a trend toward increased infarct size with naloxone alone and, while not statistically significant, we cannot completely exclude a type 2 error on the basis of the study numbers used. However, this would not alter our findings that naloxone does reverse the cardioprotective effect of post-TENS dialysate. The previous observation that 30 min of TENS stimulation to the hand or leg in patients differentially increases either Met-enkephalin (MEAP) or dynorphin A (depending on stimulation frequency, 2 vs. 100 Hz respectively) in CSF is interesting in this regard [14].
Although the li-rIPC stimulus is a mature, safe, and facile technique that has been used in multiple clinical trials, TENS represents an alternative stimulus of rIPC that may have some clinical advantages (e.g., avoiding the need to render a limb ischemic, maintain access to the limb for infusions and monitoring). However, more studies are required to establish the clinical utility of TENS under these circumstances, not least because the li-rIPC is a complex stimulus that induces other effects such as modification of ischemia-induced platelet reactivity [26], alteration of protein phosphorylation [2, 44, 48], altered protein expression [28, 46, 53] and modification of gene expression, [6, 16, 21, 34, 40, 46, 47] and functional activity of circulating neutrophils [37, 41] that may be important contributors to the clinical benefits of the technique and may or may not be invoked by TENS.
Study limitations
This was a proof of principle study, and while the observations are unique, the TENS protocol we used was idiosyncratic. We chose settings commonly used clinically in our practice and used the same pulse width and frequency, albeit at lower amplitude in rabbits. Future experiments should be designed to address the mode of stimulation, optimal TENS settings, and biology of the stimulus. In terms of the latter, we are unable to exclude the possibility that the TENS stimulus was not, at least in part, effective via direct muscular stimulation and possible ‘exercise-induced’ ischemia. We have previously shown that vigorous exercise also releases blood-borne cardioprotective agents [29], and in both our animal and human studies TENS stimulation was associated with underlying muscle stimulation manifest as twitch. Furthermore, in the first study examining the limb as a possible site for inducing rIPC, Birnbaum and colleagues showed in rabbits that direct electrical gastrocnemius muscle stimulation in association with partial limb ischemia, but neither muscle stimulation nor partial ischemia alone, was cardioprotective [1]. Further studies are clearly required to establish the mechanisms underlying the release of cardioprotective factors by TENS, the optimal stimulus, and the time course of the portfolio of downstream effects that may occur in target tissues.
We have already mentioned significant caveats regarding the relative clinical utility regarding rIPC stimulated by limb ischemia or TENS. For example, at a practical level TENS stimulation should not be applied, for example, in those with pacemakers. From a mechanistic viewpoint, while our model does allow demonstration of stimulation of blood-borne cardioprotection and allows us to report that TENS works, at least in part, by release of humoral factors, we do not have data showing the effectiveness in an entirely in vivo system. This is particularly important in terms of the ability of TENS to confer cardiac and other organ protection in humans, as our current data only provide indirect evidence in an in vitro ‘bio-assay’. Clearly, future studies should address the potential for TENS to provide protection in vivo and compare directly the effects of the TENS stimulus with the increasing data, showing a broad range of effects of li-rIPC. Furthermore, a more sophisticated analysis of pain and discomfort, using appropriate visual analog tools, would be required in larger numbers of subjects before superiority of either technique could be established definitively, in this regard.
In summary, we have shown that TENS is a novel stimulus of remote ischemic preconditioning that is equally effective to that induced by transient limb ischemia in isolated heart preparation. Further studies will be required to establish the optimal delivery and clinical utility of this novel method of cardioprotection.
References
Birnbaum Y, Hale SL, Kloner RA (1997) Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation 96:1641–1646. doi:10.1161/01.CIR.96.5.1641
Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R (2008) Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 102:131–135. doi:10.1161/CIRCRESAHA.107.164699
Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT (2010) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375:727–734. doi:10.1016/S0140-6736(09)62001-8
Celander O, Folkow B (1953) The nature and the distribution of afferent fibres provided with the axon reflex arrangement. Acta Physiol Scand 29:359–370. doi:10.1111/j.1748-1716.1953.tb01031.x
Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN (2006) Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 47:2277–2282. doi:10.1016/j.jacc.2006.01.066
Conti A, Scala S, D’Agostino P, Alimenti E, Morelli D, Andria B, Tammaro A, Attanasio C, Della Ragione F, Scuderi V, Fabbrini F, D’Esposito M, Di Florio E, Nitsch L, Calise F, Faiella A (2007) Wide gene expression profiling of ischemia–reperfusion injury in human liver transplantation. Liver Transplant 13:99–113. doi:10.1002/lt.20960
Dickson EW, Blehar DJ, Carraway RE, Heard SO, Steinberg G, Przyklenk K (2001) Naloxone blocks transferred preconditioning in isolated rabbit hearts. J Mol Cell Cardiol 33:1751–1756. doi:10.1006/jmcc.2001.1436
Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, Przyklenk K (1999) Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol 277:H2451–H2457
Ding YF, Zhang MM, He RR (2001) Role of renal nerve in cardioprotection provided by renal ischemic preconditioning in anesthetized rabbits. Sheng Li Xue Bao 53:7–12
Dong JH, Liu YX, Ji ES, He RR (2004) Limb ischemic preconditioning reduces infarct size following myocardial ischemia–reperfusion in rats. Sheng Li Xue Bao 56:41–46
Dong JH, Liu YX, Zhao J, Ma HJ, Guo SM, He RR (2004) High-frequency electrical stimulation of femoral nerve reduces infarct size following myocardial ischemia–reperfusion in rats. Sheng Li Xue Bao 56:620–624
Evans MS, Reid KH, Sharp JB Jr (1993) Dimethylsulfoxide (DMSO) blocks conduction in peripheral nerve C fibers: a possible mechanism of analgesia. Neurosci Lett 150:145–148. doi:10.1016/0304-3940(93)90522-m
Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD (1996) Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94:2193–2200. doi:10.1161/01.CIR.94.9.2193
Han JS, Chen XH, Sun SL, Xu XJ, Yuan Y, Yan SC, Hao JX, Terenius L (1991) Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 47:295–298
Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M (2012) STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res 110:111–115. doi:10.1161/CIRCRESAHA.111.259556
Huda R, Chung DH, Mathru M (2005) Ischemic preconditioning at a distance: altered gene expression in mouse heart and other organs following brief occlusion of the mesenteric artery. Heart Lung Circ 14:36–43. doi:10.1016/j.hlc.2004.11.006
Jensen RV, Stottrup NB, Kristiansen SB, Botker HE (2012) Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol 107:285. doi:10.1007/s00395-012-0285-1
Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X (2009) Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation 120:S1–S9. doi:10.1161/CIRCULATIONAHA.108.843938
Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106:2881–2883. doi:10.1161/01.CIR.0000043806.51912.9B
Kharbanda RK, Nielsen TT, Redington AN (2009) Translation of remote ischaemic preconditioning into clinical practice. Lancet 374:1557–1565. doi:10.1016/S0140-6736(09)61421-5
Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, Redington AN (2004) The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 19:143–150. doi:10.1152/physiolgenomics.0 0046.2004
Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN (2005) Remote ischemic preconditioning of the recipient reduces myocardial ischemia–reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation 79:1691–1695
Kottenberg E, Musiolik J, Thielmann M, Jakob H, Peters J, Heusch G (2014) Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J Thorac Cardiovasc Surg 147(1):376–382. doi: 10.1016/j.jtcvs.2013.01.005
Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J (2012) Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand 56:30–38. doi:10.1111/j.1399-6576.2011.02585.x
Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker DJ (2002) Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol 283:H29–H37. doi:10.1152/ajpheart.0 1031.2001
Linden MD, Whittaker P, Frelinger AL 3rd, Barnard MR, Michelson AD, Przyklenk K (2006) Preconditioning ischemia attenuates molecular indices of platelet activation-aggregation. J Thromb Haemost 4:2670–2677. doi:10.1111/j.1538-7836.2006.02228.x
Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ (2005) Remote ischemic preconditioning provides early and late protection against endothelial ischemia–reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 46:450–456. doi:10.1016/j.jacc.2005.04.044
Lu Y, Zhou J, Xu C, Lin H, Xiao J, Wang Z, Yang B (2008) JAK/STAT and PI3 K/AKT pathways form a mutual transactivation loop and afford resistance to oxidative stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem 21:305–314. doi:10.1159/000129389
Michelsen MM, Stottrup NB, Schmidt MR, Lofgren B, Jensen RV, Tropak M, St-Michel EJ, Redington AN, Botker HE (2012) Exercise-induced cardioprotection is mediated by a bloodborne, transferable factor. Basic Res Cardiol 107:260. doi:10.1007/s00395-012-0260-x
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136. doi:10.1161/01.CIR.74.5.1124
Peart JN, Gross GJ (2006) Cardioprotective effects of acute and chronic opioid treatment are mediated via different signaling pathways. Am J Physiol Heart Circ Physiol 291:H1746–H1753. doi:10.1152/ajpheart.0 0233.2006
Pell TJ, Baxter GF, Yellon DM, Drew GM (1998) Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol 275:H1542–H1547
Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P (1993) Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899. doi:10.1161/01.CIR.87.3.893
Raza A, Dikdan G, Desai KK, Shareef A, Fernandes H, Aris V, de la Torre AN, Wilson D, Fisher A, Soteropoulos P, Koneru B (2010) Global gene expression profiles of ischemic preconditioning in deceased donor liver transplantation. Liver Transplant 16:588–599. doi:10.1002/lt.22049
Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, Dai X, Manlhiot C, Li J, Redington AN (2012) Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol 107:241. doi:10.1007/s00395-011-0241-5
Saade NE, Massaad CA, Ochoa-Chaar CI, Jabbur SJ, Safieh-Garabedian B, Atweh SF (2002) Upregulation of proinflammatory cytokines and nerve growth factor by intraplantar injection of capsaicin in rats. J Physiol 545:241–253. doi:10.1113/jphysiol.2002.028233
Saxena P, Shaw OM, Misso NL, Naran A, Shehatha J, Newman MA, d’Udekem Y, Thompson PJ, Konstantinov IE (2011) Remote ischemic preconditioning stimulus decreases the expression of kinin receptors in human neutrophils. J Surg Res 171:311–316. doi:10.1016/j.jss.2009.11.011
Schoemaker RG, van Heijningen CL (2000) Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol 278:H1571–H1576
Serejo FC, Rodrigues LF Jr, da Silva Tavares KC, de Carvalho AC, Nascimento JH (2007) Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol 49:214–220. doi:10.1097/FJC.0b013e3180325ad9
Sergeev P, da Silva R, Lucchinetti E, Zaugg K, Pasch T, Schaub MC, Zaugg M (2004) Trigger-dependent gene expression profiles in cardiac preconditioning: evidence for distinct genetic programs in ischemic and anesthetic preconditioning. Anesthesiology 100:474–488
Shimizu M, Saxena P, Konstantinov IE, Cherepanov V, Cheung MM, Wearden P, Zhangdong H, Schmidt M, Downey GP, Redington AN (2010) Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res 158:155–161. doi:10.1016/j.jss.2008.08.010
Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN (2009) Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 117:191–200. doi:10.1042/CS20080523
Sjolund BH (1988) Peripheral nerve stimulation suppression of C-fiber-evoked flexion reflex in rats. Part 2: parameters of low-rate train stimulation of skin and muscle afferent nerves. J Neurosurg 68:279–283
Soond SM, Townsend PA, Barry SP, Knight RA, Latchman DS, Stephanou A (2008) ERK and the F-box protein betaTRCP target STAT1 for degradation. J Biol Chem 283:16077–16083. doi:10.1074/jbc.M800384200
Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A (2010) Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol 299:H1598–H1603. doi:10.1152/ajpheart.0 0396.2010
Steinbeck JA, Methner A (2005) Translational downregulation of the noncatalytic growth factor receptor TrkB.T1 by ischemic preconditioning of primary neurons. Gene Expr 12:99–106
Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP (2003) Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet 362:1028–1037. doi:10.1016/S0140-6736(03)14412-1
Stephanou A, Scarabelli TM, Brar BK, Nakanishi Y, Matsumura M, Knight RA, Latchman DS (2001) Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J Biol Chem 276:28340–28347. doi:10.1074/jbc.M101177200
Takaoka A, Nakae I, Mitsunami K, Yabe T, Morikawa S, Inubushi T, Kinoshita M (1999) Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effects of “remote preconditioning”. J Am Coll Cardiol 33:556–564. doi:10.1016/S0735-1097(98)00559-2
Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G (2010) Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 105:657–664. doi:10.1007/s00395-010-0104-5
Weinbrenner C, Nelles M, Herzog N, Sarvary L, Strasser RH (2002) Remote preconditioning by infrarenal occlusion of the aorta protects the heart from infarction: a newly identified non-neuronal but PKC-dependent pathway. Cardiovasc Res 55:590–601. doi:10.1016/S0008-6363(02)00446-7
Wolfrum S, Schneider K, Heidbreder M, Nienstedt J, Dominiak P, Dendorfer A (2002) Remote preconditioning protects the heart by activating myocardial PKCepsilon-isoform. Cardiovasc Res 55:583–589. doi:10.1016/S0008-6363(02)00408-X
Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, Qiu Y, Li JJ, Bolli R (1999) Nuclear factor-kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res 84:1095–1109. doi:10.1161/01.RES.84.9.1095
Acknowledgments
This study was supported by grants from the Leducq Foundation and Canadian Institutes of Health Research.
Conflicts of interest
ANR has submitted a patent application regarding the use of TENS as a preconditioning stimulus.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. L. Redington is joint first author.
Rights and permissions
About this article
Cite this article
Merlocco, A.C., Redington, K.L., Disenhouse, T. et al. Transcutaneous electrical nerve stimulation as a novel method of remote preconditioning: in vitro validation in an animal model and first human observations. Basic Res Cardiol 109, 406 (2014). https://doi.org/10.1007/s00395-014-0406-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-014-0406-0