Abstract
Autophagy is a reparative, life-sustaining process by which cytoplasmic components are sequestered in double membrane vesicles and degraded upon fusion with lysosomal compartments. Mice with a macrophage-specific deletion of the essential autophagy gene Atg5 develop plaques with increased apoptosis and oxidative stress as well as enhanced plaque necrosis. This finding indicates that basal autophagy in macrophages is anti-apoptotic and present in atherosclerotic plaques to protect macrophages against various atherogenic stressors. However, autophagy is impaired in advanced stages of atherosclerosis and its deficiency promotes atherosclerosis in part through activation of the inflammasome. Because basal autophagy can be intensified selectively in macrophages by specific drugs such as mammalian target of rapamycin (mTOR) inhibitors or Toll-like receptor 7 (TLR7) ligands, these drugs were recently tested as potential plaque stabilizing compounds. Stent-based delivery of the mTOR inhibitor everolimus promotes a stable plaque phenotype, whereas local administration of the TLR7 ligand imiquimod stimulates inflammation and plaque progression. Therefore, more drugs capable of inducing autophagy should be tested in plaque macrophages to evaluate the feasibility of this approach. Given that drug-induced macrophage autophagy is associated with pro-inflammatory responses due to cytokine release, induction of postautophagic necrosis or activation of phagocytes after clearance of the autophagic corpse, cotreatment with anti-inflammatory compounds may be required. Overall, this review highlights the pros and cons of macrophage autophagy as a drug target for plaque stabilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autophagy is an evolutionarily conserved subcellular process for bulk destruction of proteins and entire organelles [54, 80]. Different autophagic pathways have been described, all sharing the import of cytoplasmic components into lysosomes. For the most prevalent form of autophagy (macroautophagy, further referred to as autophagy), the process starts with a largely undefined membranous structure, known as a phagophore or isolation membrane (Fig. 1). After elongation and maturation, the phagophore forms a double-membrane vacuole or autophagosome that engulfs small portions of the cytosol including protein aggregates, lipid droplets, and complete organelles (Fig. 1). Autophagosomes eventually fuse with a lysosome, thereby generating an autolysosome. During this final step, incorporation of the outer autophagosomal membrane within the lysosomal membrane allows degradation of the remaining inner single-membrane and the cytoplasmic content of the autophagosome by lysosomal hydrolases (Fig. 1).
Schematic overview of macroautophagy. The process is initiated by formation of nascent membranous structures (isolation membranes or phagophores), which sequestrate small portions of the cytoplasm and organelles for degradation. Expansion of the isolation membrane and enclosure of the cytoplasmic cargo lead to the formation of autophagosomes. These autophagic vacuoles dock and fuse with lysosomes to form autolysosomes in which the cargo is degraded
The autophagic process is classically considered to be a pathway contributing to cellular homeostasis and adaptation to stress by removing damaged or unwanted intracellular material. In this way, it may act as a pro-survival mechanism in an adverse and stressful environment. However, excessive stimulation of the autophagic machinery is deleterious and may lead to caspase-independent cell death [8, 15], although most evidence linking autophagy to cell death is circumstantial and not always convincing [33]. Because recent findings raise doubts as to the very existence of autophagic cell death [72], it has been postulated that cells undergoing continuous autophagic stimulation may die with autophagy, but not by autophagy. Accordingly, we prefer to use the terms “autophagy-mediated death” or “autophagy-associated death”, rather than “autophagic death”, as recently recommended [29].
Autophagy in atherosclerosis
Autophagy has been associated with a plethora of different pathological conditions including heart disease, cancer, neurodegeneration, and infectious diseases [40, 55, 74]. In vivo evidence for autophagy in atherosclerosis is limited [41, 42, 69], probably due to the technical limitations associated with immunohistochemical detection of autophagy-related proteins [43, 44]. Nonetheless, transmission electron microscopy images of atherosclerotic plaques reveal structures consistent with autophagosomes and certain marker proteins have been detected in crude lysates of atherosclerotic plaques by immunoblot analysis [42]. Moreover, a growing body of in vitro evidence indicates that autophagy could be stimulated in atherosclerosis by oxidized lipids (e.g., oxLDL, 7-ketocholesterol) [20, 46, 57, 83], reactive oxygen species (ROS) [22], endoplasmic reticulum stress [81], certain pro-inflammatory cytokines such as IFNγ [53], and conditions of hypoxia or metabolic stress [26]. Despite the protective actions of autophagy in several human pathologies, the role of autophagy in atherosclerosis is poorly understood [41, 42, 69]. Recently, however, it has been demonstrated that LDL receptor knockout mice with a macrophage-specific deletion of the essential autophagy gene Atg5 develop plaques with increased apoptosis and oxidative stress as well as enhanced plaque necrosis [35], which confirms the overall hypothesis that basal levels of autophagy are anti-apoptotic and present in atherosclerotic plaques to protect macrophages against various atherogenic stressors. Given that foam cell formation is a major event in atherosclerosis, it is also important to note that lipid droplets in macrophage foam cells are delivered to lysosomes via autophagy, where lysosomal acid lipase acts to hydrolyze lipid droplet-derived cholesteryl esters to generate free cholesterol mainly for ABCA1-dependent efflux [59]. This process is specifically induced upon macrophage cholesterol loading and may contribute to macrophage survival [48, 59]. However, recent evidence suggests that basal autophagy in macrophages is only atheroprotective during early atherosclerosis. Basal autophagy becomes dysfunctional in the more advanced stages of atherosclerosis and its deficiency promotes atherosclerosis in part through activation of the inflammasome [62]. This finding highlights the need for compounds that stimulate the pro-survival effects of autophagy in vivo.
Drug-induced autophagy
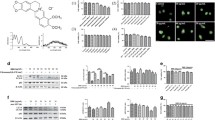
Basal autophagy can be intensified by several drugs (Fig. 2). The most commonly used inducer of autophagy is rapamycin (also known as sirolimus), which acts through inhibition of mTORC1. Although this protein has been known as a key regulator of autophagy for more than a decade, the underlying regulatory mechanism remains unclear [78]. Recent evidence suggests that mTORC1 phosphorylates ULK1, a protein kinase that interacts with several other proteins including Atg13 and FIP200, which are required for the induction of autophagy [24]. Because rapamycin has poor aqueous solubility and chemical stability, several rapamycin analogs such as everolimus (RAD-001), temsirolimus (CCI-779), deforolimus (AP23573) and zotarolimus (ABT-578) have been developed. These agents have activities that are similar to those of rapamycin and are clinically used to treat in-stent restenosis [19] or several types of cancer [5].
Examples of drug-induced macrophage autophagy. The autophagic process was stimulated in mouse macrophages through inhibition of mTORC1 (treatment with 10-μM everolimus, 8 h) or via initiation of an mTOR-independent pathway (treatment with 30-μg/ml imiquimod, 8 h). Formation of autophagic vacuoles was analyzed by transmission electron microscopy. Everolimus- or imiquimod-treated cells showed many autophagic vacuoles as compared to untreated control cells. The boxed area (showing autophagic vacuoles in an imiquimod-treated cell) is also presented at higher magnification. N nucleus. Scale bar 2 μm
Apart from rapamycin or its derivatives, autophagy could be regulated by the release of calcium from the endoplasmic reticulum under stress conditions [13]. Indeed, treatment of cells with the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitors thapsigargin [21] or alisol B [32] leads to elevated cytosolic Ca2+ concentrations, which eventually results in increased autophagy. Similar effects can be obtained with Ca2+-mobilizing agents such as the Ca2+ ionophore ionomycin [21], cadmium [77], or resveratrol [76]. High levels of cytosolic calcium activate a variety of autophagy-stimulating proteins, most notably calmodulin, which activates calmodulin-dependent kinase kinase β (CaMKKβ), followed by AMPK-dependent inhibition of mTOR [13].
mTORC1-independent regulation of autophagy can be obtained through lithium, sodium valproate, and carbamazepine, which are compounds that lower the myo-inositol-1,4,5-triphosphate levels [64]. Moreover, using chemical high-throughput screens, various small molecule enhancers of rapamycin (SMERs) have been identified that enhance the clearance of aggregate-prone proteins independent of rapamycin [65]. Other mTOR-independent autophagy inducers are the disaccharide trehalose [63], the polyamine spermidine [16], or the Toll-like receptor 7 (TLR7) ligand imiquimod [14].
Drug-induced autophagy in atherosclerosis
Reports that describe drug-induced autophagy in atherosclerosis are highly limited. Recently, however, everolimus and imiquimod have been tested on experimental plaques to induce an mTOR-dependent and -independent type of autophagy, respectively, albeit with different outcome as described in more detail below.
Everolimus-induced macrophage autophagy promotes a stable plaque phenotype
Local stent-based delivery of the rapamycin derivative everolimus in atherosclerotic plaques from cholesterol-fed rabbits leads to a marked reduction in macrophage content without altering the amount of smooth muscle cells (SMCs) [49, 75]. Consistent with this finding, a large body of evidence indicates that everolimus has pleiotropic anti-atherosclerotic effects that may prevent or delay the pathogenesis of atherosclerosis. These effects include inhibition of SMC migration and proliferation [31], loss of monocyte chemotaxis [4], and the prevention of lipid accumulation in both macrophages and SMCs [37, 38, 52]. Accordingly, everolimus may have therapeutic potential not only to prevent macrophage accumulation, but also to enhance the macrophage cholesterol efflux and to promote reverse cholesterol transport. Indeed, ApoE−/− mice fed a Western-type diet and treated with the related compound sirolimus (4 mg/kg/day) for 12 weeks showed a 36 % reduction in cholesterol content of the aortic arch as compared to untreated control mice fed the Western-type diet only [6].
Given that everolimus has a strong inhibitory effect on protein synthesis [51, 75] and that inhibition of protein synthesis leads to selective macrophage death in atherosclerotic plaques [9], it has been suggested that everolimus-induced macrophage depletion is a consequence of autophagy-mediated cell death. Along these lines, in vitro studies showed that everolimus promotes several features of autophagy in macrophages including bulk degradation of long-lived proteins, processing of microtubule-associated protein light chain 3 (LC3), and cytoplasmic vacuolization (Fig. 2), followed by cellular death [49, 75] (Fig. 3). However, dephosphorylation of the downstream mTOR target p70 S6 kinase after everolimus treatment, most notably dephosphorylation at Thr389, occurs at very low concentrations (0.1–1 nM) whereas induction of macrophage death only occurs after treatment with high everolimus concentrations (>3 μM) [49] (Fig. 3). As a consequence, it could be assumed that inhibition of other intracellular proteins, but not of mTOR, is responsible for the everolimus-induced macrophage death. Because the viability of macrophages is not affected using the same concentration of tacrolimus [75], an mTOR-independent everolimus-analog, this theory is unlikely. Moreover, mTOR gene silencing after transfection of mTOR-specific siRNA is associated with selective induction of macrophage cell death [75]. Possibly, other mTOR-mediated pathways, but not dephosphorylation of p70 S6 kinase, are responsible for the induction of everolimus-induced macrophage death. Still, several in vitro findings suggest that inhibition of protein translation rather than differential expression of cell death proteins drives the selective induction of macrophage death by everolimus. Measurements of oxygen consumption as well as immunodetection of markers for DNA synthesis/repair indicate that plaque macrophages are metabolically highly active and thus more sensitive to protein synthesis inhibitors as compared to SMCs [7, 36, 47]. Moreover, inhibition of translation in SMCs by rapamycin induces a modulation towards a differentiated, quiescent, contractile phenotype through upregulation of smooth muscle α-actin, calponin, and myosin heavy chain, which may render SMCs relatively insensitive to cell death mediated by inhibition of protein translation [39]. Interestingly, macrophages are more sensitive to everolimus-induced cell death after foam cell formation (Fig. 3). Everolimus is highly lipophilic and may easily accumulate in lipid-laden macrophages to cytotoxic levels or levels that evoke autophagy-mediated cell death.
Viability of macrophages and macrophage-derived foam cells after treatment with the mTOR inhibitor everolimus. Mouse macrophages were grown in the presence or absence of acetylated LDL (acLDL, 100 μg/ml) for 24 h to induce foam cell formation. AcLDL was vortexed for 2 min to obtain aggregated LDL to facilitate acLDL uptake. Next, both untreated (control) and acLDL-laden macrophages were exposed to 1–10 μM everolimus for 24 h. **P < 0.01, ***P < 0.001 versus untreated (0 μM everolimus) (1-way ANOVA, followed by Dunnett test, n = 3); # P < 0.05, ## P < 0.01 versus control (cells without acLDL) (unpaired Student’s t test, n = 3)
Also noteworthy is the finding that bioresorbable scaffolds eluting everolimus trigger a healing process in the vessel wall, both in pigs [58] and humans (ABSORB trial) [70], which results in late lumen enlargement (up to 10 mm2 at 24-month follow up) as well as critical plaque and media regression (nearly 13 %). A significant reduction in plaque growth (up to 85 % in the brachiocephalic artery and ~60 % at the aortic root) was also observed when osmotic minipumps eluting everolimus were implanted subcutaneously in LDL receptor deficient mice for a period of 12 weeks [56]. At present, this phenomenon of atheroregression is poorly understood. Given that everolimus triggers autophagy-mediated macrophage death and that everolimus inhibits cell proliferation, we hypothesize that everolimus is capable of inhibiting (or reversing) the basic mechanisms that control plaque growth and destabilization, as recently suggested [23].
Imiquimod-induced macrophage autophagy is associated with inflammation and plaque progression
Ligands of Toll-like receptor 7 (TLR7), in particular the imidazoquinoline imiquimod, are able to induce autophagy through myeloid differentiation primary response gene 88 (MyD88) which is an adaptor protein that targets Beclin 1 [14, 73]. TLR7 signaling enhances the interaction of MyD88 with Beclin 1 and reduces the binding of Beclin 1 to Bcl-2, which finally leads to autophagy [14, 73]. Immunohistochemical evidence and immunoblot analysis indicate that TLR7 is expressed in macrophages but not in SMCs, i.e., only macrophages are sensitive to imiquimod-induced autophagy [12]. Surprisingly, local delivery of imiquimod in atherosclerotic plaques of cholesterol-fed rabbits via osmotic minipumps does not deplete macrophages, but rather stimulates macrophage accumulation [12]. Other imiquimod effects are upregulation of vascular adhesion molecule-1 in endothelial cells, infiltration of T-lymphocytes, and enlargement of the plaque area [12]. Moreover, treatment of macrophages with imiquimod in vitro enhances the expression of adipocyte differentiation-related protein (ADRP, also known as ADFP or adipophilin) [17], which plays an important role in cholesterol ester accumulation. Chronic activation of this protein may facilitate foam cell formation and plaque progression [30].
The contradictory in vivo effects of everolimus and imiquimod might be misleading because the concentration of the drug and the level of autophagy induced by both compounds in the plaque could be entirely different. Stents eluting everolimus may yield high local concentrations of the eluted drug which could rapidly stimulate autophagy-mediated macrophage death. In contrast, imiquimod delivered to the vessel wall via osmotic minipumps may be diluted in the surrounding tissue. In vitro experiments showed that high concentrations of imiquimod (>10 μg/ml) are needed to induce autophagy-mediated death in macrophages [12]. Moreover, imiquimod-induced cell death requires pronounced expression of TLR7. When using a high concentration of imiquimod (10 μg/ml) in vitro, autophagy-mediated cell death is much more pronounced in macrophage cell lines such as J774A.1, which abundantly express TLR7, whereas bone marrow-derived macrophages showing moderate TLR7 expression levels reveal moderate levels of autophagy without the induction of cell death [12]. Even in response to 30 μg/ml imiquimod, approximately 50 % of bone marrow-derived macrophages survives [12], suggesting that TLR7 expression in macrophages of atherosclerotic plaques might be insufficient for imiquimod-induced autophagy-mediated death.
Both imiquimod and everolimus trigger cytokine release by macrophages independent of autophagy
If imiquimod does not easily trigger macrophage death, the question arises as to the underlying mechanism of imiquimod-induced plaque progression. As already mentioned above, imiquimod exerts its biological activity in macrophages through direct activation of TLR7 signaling, which in turn activates the transcription factor NF-κB to stimulate the release of proinflammatory cytokines and chemoattractant proteins such as IL-6, MCP-1, Rantes, and TNFα [12]. These proteins may enhance the expression of adhesion molecules, including VCAM-1, and trigger plaque inflammation by recruiting both monocytes and T-lymphocytes [12]. Interestingly, also mTOR inhibitors such as everolimus and rapamycin stimulate secretion of pro-inflammatory cytokines (e.g., IL-6, TNFα) and chemokines (e.g., MCP1, Rantes) by macrophages prior to induction of autophagy mediated death, albeit not via NF-κB but through activation of p38 MAP kinase [51]. Cytokine production is similar in macrophages lacking the essential autophagy gene Atg7, suggesting that NF-κB or p38 activation is an off-target effect of imiquimod and everolimus, respectively, and not a consequence of autophagy induction [12, 51]. Of note, cytokines are not released by macrophages undergoing starvation, an autophagic condition known to inhibit mTOR similar to everolimus [51], indicating that mTOR inhibition is not sufficient to induce cytokine release. In addition, because sustained starvation leads to macrophage death [51], a decrease in macrophage viability is not a prerequisite for cytokine synthesis. It has been demonstrated previously that mTOR inhibition via starvation is associated with enhanced de novo protein synthesis, rather than with inhibition of protein synthesis [51]. Given that protein synthesis inhibitors have the potential to induce macrophage death via p38 [10], it is likely that inhibition of protein translation by everolimus is a stressful condition for macrophages that could mediate p38 activation and cytokine synthesis. Fortunately, imiquimod-induced cytokine release and recruitment of leukocytes can be inhibited by coincubation with dexamethasone [12]. Another option is the use of clobetasol, one of the most powerful corticosteroids with anti-inflammatory activity. Combined stent-based delivery of clobetasol and everolimus in rabbit plaques downregulates TNFα expression as compared to everolimus-treated plaques, yet does not affect the ability of everolimus to induce macrophage clearance [51].
Everolimus-induced macrophage depletion: a matter of necrotic death?
Autophagy-mediated cell death leads to secondary necrosis when dead cells are not properly phagocytized, which is similar to apoptotic death. Because it is plausible to believe that autophagic cells literally digest themselves to death, at least when the autophagic stimulus is extensive and long-lasting, it could be expected that the cytoplasmic content progressively decreases, resulting in minimal inflammation after post-autophagic necrosis. Based on this theory, it has been proposed that induction of autophagy-mediated death is the preferred type of death to deplete macrophages in atherosclerotic plaques [50]. However, in vitro experiments showed that the intracellular protein content of autophagic macrophages prior to cell lysis does not dramatically change (Fig. 4). If autophagic cells are not efficiently phagocytized, as shown for apoptotic cells in advanced plaques [68], not only cytokines but also many other intracellular proteins with pro-inflammatory potential could be released in the plaque (Fig. 4a). p43, for example, is one of the pro-inflammatory proteins released by macrophages not only after in vitro treatment with everolimus but also after starvation. Recently, p43 was found to be abundantly expressed in macrophages of advanced human atherosclerotic plaques [45]. The protein is synthesized as a 34-kDa precursor molecule and acts as a pro-inflammatory molecule by stimulating synthesis of cytokines and adhesion molecules in macrophages and endothelial cells, respectively [45]. Experiments with monocytic THP1 or U937 cells revealed that p43 is cleaved during cell death into an inactive isoform p43(ARF) by calpains [45, 71]. However, bone marrow-derived macrophages undergoing autophagy-mediated cell death secrete mainly full-length p43, most likely due to low calpain expression. Macrophages treated with recombinant full-length p43 activate both p38 and NF-κB, and secrete various cytokines (IL-6, MCP1, Rantes TNFα) (Fig. 4b), an effect that could be blocked by clobetasol. Besides p43, high mobility group box 1 (HMGB1) is released after starvation or everolimus-induced macrophage death. HMGB1 is a well-known danger signal passively released from necrotic or damaged cells [67]. It stimulates monocytes and macrophages to release pro-inflammatory cytokines [3, 79], enhances the expression of the vascular adhesion molecules ICAM-1 and VCAM-1 and various pro-inflammatory molecules (e.g. TNFα, IL-8, MCP-1) in endothelial cells [18, 79], and acts as a strong chemotactic agent for SMCs. HMGB1 is abundantly expressed in macrophages, SMCs, and endothelial cells of carotid and coronary atheromatous plaques, but not in SMCs of fibrous plaques or normal media [25], suggesting that the release of HMGB1 in plaques may form a serious adverse effect in terms of plaque inflammation. From a theoretical point of view, post-autophagic necrosis could be prevented by enhancing phagocytosis of autophagic cells. However, even when autophagic macrophages are efficiently removed by phagocytes, inflammation may still occur. Indeed, recent evidence suggests that phagocytic uptake of cells dying through autophagy leads to a pro-inflammatory response characterized by the induction and secretion of IL-6, TNFα, IL-8, and IL-10 [61]. Moreover, phagocytosis of autophagic cells (but not apoptotic cells) induces inflammasome activation and IL-1β secretion [60]. In this light, autophagy is not immunologically silent, as originally thought, unless the pro-inflammatory responses are inhibited by an anti-inflammatory agent.
Prolonged stimulation of autophagy induces macrophage necrosis and the release of pro-inflammatory proteins. a Mouse macrophages were starved in Earle’s Balanced Salt Solution or treated with 10-μM everolimus in RPMI medium for up to 72 h to induce autophagy. Proteins from crude cell lysates or extracellular medium were separated on polyacrylamide gels and stained with coomassie blue. Release of p43 and HMGB1 by autophagic macrophages was examined via western blotting. b Mouse macrophages were treated with 100-nM recombinant full-length p43 for 0–120 min. Phosphorylation of p38 MAPK (Thr180/Tyr182), NF-κB p65 (Ser536), and IkBα (Ser32/36) was analyzed by western blotting. In another series of experiments, mouse macrophages were incubated in RPMI medium (control) or RPMI supplemented with 100 nM p43 for 24 h. The culture medium was then analyzed using a cytokine antibody array (for full array layout, see [51])
Concluding remarks
Two types of autophagy can be induced in mammalian cells, namely mTOR-dependent and mTOR-independent autophagy. Stimulation of the mTOR-dependent type of autophagy in macrophages (via everolimus or analogs) leads to inhibition of protein synthesis and triggers autophagy-mediated cell death, macrophage depletion, and the formation of a stable plaque phenotype (Fig. 5). Initiation of the mTOR-independent type of autophagy in macrophages via imiquimod seems detrimental because it is associated with inflammation and plaque progression (Fig. 5). At this moment, it is unclear whether these adverse effects are specific to imiquimod or inherent to induction of mTOR-independent autophagy pathways. Therefore, alternative drugs capable of inducing mTOR-independent autophagy should be tested in plaque macrophages. However, a major concern with pharmacological induction of autophagy is the occurrence of pleiotropic effects of the compounds being used. Trehalose, for example, is not only an autophagy inducer but also a chemical chaperone because of its ability to influence protein folding through direct protein-trehalose interactions [66]. Moreover, some well-known autophagy inducers (e.g., lithium chloride) do not induce autophagy in macrophages, but apoptosis [11]. It is unclear whether other mTOR-independent autophagy inducers trigger autophagy selectively in macrophages and not in other cell types of the plaque. The sphingosine-1-phosphate analog FTY720 reveals several potentially atheroprotective effects, including the ability to induce autophagy [34, 82], and reduces atherosclerosis in ApoE−/− mice [27, 28]. However, recent findings identify FTY720 as a new autophagy-blocking agent that promotes both apoptotic and nonapoptotic cell death, at least in certain cell types [1, 2, 34, 82]. Overall, mTOR inhibitors seem to be the first choice for autophagy induction in macrophages, even though they have a strong inhibitory effect on protein synthesis and thus may affect many metabolic pathways independent of autophagy. Also of note, systemic administration of mTOR inhibitors at levels suitable for autophagy induction in macrophages leads to systemic immunosuppression, which is not favorable, leaving only local treatment as a justified approach. In addition, drug-induced macrophage autophagy could be associated with pro-inflammatory responses due to off-target effects of the compound being used (e.g., activation of p38 MAPK or NF-κB). These adverse effects may lead to cytokine release, induction of postautophagic necrosis, or inflammasome activation after phagocytosis of the autophagic corpse, so that cotreatment with anti-inflammatory compounds would be a wise precaution.
Both everolimus and imiquimod stimulate macrophage autophagy in atherosclerosis, albeit with a different outcome. Everolimus is a potent inhibitor of mTOR which leads to not only initiation of autophagy but also inhibition of protein translation. At high everolimus concentrations (μM range), impaired protein translation combined with mTOR-dependent induction of autophagy-specific genes triggers autophagy-mediated macrophage death, followed by macrophage depletion and formation of a more stable plaque phenotype. Interestingly, treatment of macrophages in culture with everolimus promotes cytokine production in an autophagy-independent way through activation of p38 MAPK. Because everolimus-eluting stents have beneficial effects in patients, it remains to be determined whether everolimus-induced cytokine secretion by autophagic macrophages after stenting has any clinical significance. Imiquimod is a moderate inducer of macrophage autophagy and does not evoke macrophage death. Local treatment of plaques with imiquimod causes activation of NF-κB and the release of pro-inflammatory cytokines, resulting in enhanced VCAM-1 expression on endothelial cells, infiltration of T-cells, macrophage accumulation, and plaque enlargement. Treatment with corticosteroids, such as dexamethasone (DEX), suppresses these pro-inflammatory effects
References
Alinari L, Baiocchi RA, Praetorius-Ibba M (2012) FTY720-induced blockage of autophagy enhances anticancer efficacy of milatuzumab in mantle cell lymphoma: is FTY720 the next autophagy-blocking agent in lymphoma treatment? Autophagy 8:416–417. doi:10.4161/auto.19050
Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, Mani R, Mao Y, Yu B, Quinion C, Towns WH, Chen CS, Goldenberg DM, Blum KA, Byrd JC, Muthusamy N, Praetorius-Ibba M, Baiocchi RA (2011) FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood 118:6893–6903. doi:10.1182/blood-2011-06-363879
Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192:565–570. doi:10.1084/jem.192.4.565
Baetta R, Granata A, Canavesi M, Ferri N, Arnaboldi L, Bellosta S, Pfister P, Corsini A (2009) Everolimus inhibits monocyte/macrophage migration in vitro and their accumulation in carotid lesions of cholesterol-fed rabbits. J Pharmacol Exp Ther 328:419–425. doi:10.1124/jpet.108.144147
Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S (2008) mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr Cancer Drug Targets 8:647–665
Basso MD, Nambi P, Adelman SJ (2003) Effect of sirolimus on the cholesterol content of aortic arch in ApoE knockout mice. Transplant Proc 35:3136–3138. doi:10.1016/j.transproceed.2003.10.050
Bjornheden T, Bondjers G (1987) Oxygen consumption in aortic tissue from rabbits with diet-induced atherosclerosis. Arteriosclerosis 7:238–247
Clarke PG, Puyal J (2012) Autophagic cell death exists. Autophagy 8:867–869. doi:10.4161/auto.20380
Croons V, Martinet W, Herman AG, Timmermans JP, De Meyer GRY (2007) Selective clearance of macrophages in atherosclerotic plaques by the protein synthesis inhibitor cycloheximide. J Pharmacol Exp Ther 320:986–993. doi:10.1124/jpet.106.113944
Croons V, Martinet W, Herman AG, Timmermans JP, De Meyer GRY (2009) The protein synthesis inhibitor anisomycin induces macrophage apoptosis in rabbit atherosclerotic plaques through p38 mitogen-activated protein kinase. J Pharmacol Exp Ther 329:856–864. doi:10.1124/jpet.108.149948
De Meyer I, Martinet W, Van Hove CE, Schrijvers DM, Hoymans VY, Van VL, Fransen P, Bult H, De Meyer GRY (2011) Inhibition of inositol monophosphatase by lithium chloride induces selective macrophage apoptosis in atherosclerotic plaques. Br J Pharmacol 162:1410–1423. doi:10.1111/j.1476-5381.2010.01152.x
De Meyer I, Martinet W, Schrijvers DM, Timmermans J-P, Bult H, De Meyer GRY (2012) Toll-like receptor 7 stimulation by imiquimod induces macrophage autophagy and inflammation in atherosclerotic plaques. Basic Res Cardiol 107:269. doi:10.1007/s00395-012-0269-1
Decuypere JP, Bultynck G, Parys JB (2011) A dual role for Ca(2+) in autophagy regulation. Cell Calcium 50:242–250. doi:10.1016/j.ceca.2011.04.001
Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V (2008) Toll-like receptors control autophagy. EMBO J 27:1110–1121. doi:10.1038/emboj.2008.31
Denton D, Nicolson S, Kumar S (2012) Cell death by autophagy: facts and apparent artefacts. Cell Death Differ 19:87–95. doi:10.1038/cdd.2011.146
Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F (2009) Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 11:1305–1314. doi:10.1038/ncb1975
Feingold KR, Kazemi MR, Magra AL, McDonald CM, Chui LG, Shigenaga JK, Patzek SM, Chan ZW, Londos C, Grunfeld C (2010) ADRP/ADFP and Mal1 expression are increased in macrophages treated with TLR agonists. Atherosclerosis 209:81–88. doi:10.1016/j.atherosclerosis.2009.08.042
Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF (2003) Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 101:2652–2660. doi:10.1182/blood-2002-05-1300
Giordano A, Romano A (2011) Inhibition of human in-stent restenosis: a molecular view. Curr Opin Pharmacol 11:372–377. doi:10.1016/j.coph.2011.03.006
Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A (2008) Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J 410:525–534
Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 25:193–205. doi:10.1016/j.molcel.2006.12.009
Huang J, Lam GY, Brumell JH (2011) Autophagy signaling through reactive oxygen species. Antioxid Redox Signal 14:2215–2231. doi:10.1089/ars.2010.3554
Jia L, Hui RT (2009) Everolimus, a promising medical therapy for coronary heart disease? Med Hypotheses 73:153–155. doi:10.1016/j.mehy.2009.03.011
Jung CH, Ro SH, Cao J, Otto NM, Kim DH (2010) mTOR regulation of autophagy. FEBS Lett 584:1287–1295. doi:10.1016/j.febslet.2010.01.017
Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A (2004) Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol 24:2320–2325. doi:10.1161/01.ATV.0000145573.36113.8a
Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E (2007) Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev 21:1621–1635. doi:10.1101/quad.1565707
Keul P, Lucke S, von Wnuck LK, Bode C, Graler M, Heusch G, Levkau B (2011) Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res 108:314–323. doi:10.1161/CIRCRESAHA.110.235028
Keul P, Tolle M, Lucke S, von Wnuck LK, Heusch G, Schuchardt M, van der Giet M, Levkau B (2007) The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 27:607–613. doi:10.1161/01.ATV.0000254679.42583.88
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, Ahn HJ, Ait-Mohamed O, Ait-Si-Ali S, Akematsu T, Akira S, Al-Younes HM, Al-Zeer MA, Albert ML, Albin RL, Alegre-Abarrategui J, Aleo MF, Alirezaei M, Almasan A, Almonte-Becerril M, Amano A, Amaravadi R, Amarnath S, Amer AO, Andrieu-Abadie N, Anantharam V, Ann DK, Anoopkumar-Dukie S, Aoki H, Apostolova N, Arancia G, Aris JP, Asanuma K, Asare NY, Ashida H, Askanas V, Askew DS, Auberger P, Baba M, Backues SK, Baehrecke EH, Bahr BA, Bai XY, Bailly Y, Baiocchi R, Baldini G, Balduini W, Ballabio A, Bamber BA, Bampton ET, Banhegyi G, Bartholomew CR, Bassham DC, Bast RC, Jr., Batoko H, Bay BH, Beau I, Bechet DM, Begley TJ, Behl C, Behrends C, Bekri S, Bellaire B, Bendall LJ, Benetti L, Berliocchi L, Bernardi H, Bernassola F, Besteiro S, Bhatia-Kissova I, Bi X, Biard-Piechaczyk M, Blum JS, Boise LH, Bonaldo P, Boone DL, Bornhauser BC, Bortoluci KR, Bossis I, Bost F, Bourquin JP, Boya P, Boyer-Guittaut M, Bozhkov PV, Brady NR, Brancolini C, Brech A, Brenman JE, Brennand A, Bresnick EH, Brest P, Bridges D, Bristol ML, Brookes PS, Brown EJ, Brumell JH, Brunetti-Pierri N, Brunk UT, Bulman DE, Bultman SJ, Bultynck G, Burbulla LF, Bursch W, Butchar JP, Buzgariu W, Bydlowski SP, Cadwell K, Cahova M, Cai D, Cai J, Cai Q, Calabretta B, Calvo-Garrido J, Camougrand N, Campanella M, Campos-Salinas J, Candi E, Cao L, Caplan AB, Carding SR, Cardoso SM, Carew JS, Carlin CR, Carmignac V, Carneiro LA, Carra S, Caruso RA, Casari G, Casas C, Castino R, Cebollero E, Cecconi F, Celli J, Chaachouay H, Chae HJ, Chai CY, Chan DC, Chan EY, Chang RC, Che CM, Chen CC, Chen GC, Chen GQ, Chen M, Chen Q, Chen SS, Chen W, Chen X, Chen X, Chen X, Chen YG, Chen Y, Chen Y, Chen YJ, Chen Z, Cheng A, Cheng CH, Cheng Y, Cheong H, Cheong JH, Cherry S, Chess-Williams R, Cheung ZH, Chevet E, Chiang HL, Chiarelli R, Chiba T, Chin LS, Chiou SH, Chisari FV, Cho CH, Cho DH, Choi AM, Choi D, Choi KS, Choi ME, Chouaib S, Choubey D, Choubey V, Chu CT, Chuang TH, Chueh SH, Chun T, Chwae YJ, Chye ML, Ciarcia R, Ciriolo MR, Clague MJ, Clark RS, Clarke PG, Clarke R, Codogno P, Coller HA, Colombo MI, Comincini S, Condello M, Condorelli F, Cookson MR, Coombs GH, Coppens I, Corbalan R, Cossart P, Costelli P, Costes S, Coto-Montes A, Couve E, Coxon FP, Cregg JM, Crespo JL, Cronje MJ, Cuervo AM, Cullen JJ, Czaja MJ, D’Amelio M, Darfeuille-Michaud A, Davids LM, Davies FE, De FM, de Groot JF, de Haan CA, De ML, De MA, De T, V, Debnath J, Degterev A, Dehay B, Delbridge LM, Demarchi F, Deng YZ, Dengjel J, Dent P, Denton D, Deretic V, Desai SD, Devenish RJ, Di GM, Di PG, Di PC, Diaz-Araya G, Diaz-Laviada I, Diaz-Meco MT, Diaz-Nido J, Dikic I, Dinesh-Kumar SP, Ding WX, Distelhorst CW, Diwan A, Djavaheri-Mergny M, Dokudovskaya S, Dong Z, Dorsey FC et al. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544. doi:10.4161/auto.19496
Larigauderie G, Furman C, Jaye M, Lasselin C, Copin C, Fruchart JC, Castro G, Rouis M (2004) Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler Thromb Vasc Biol 24:504–510. doi:10.1161/01.ATV.0000115638.27381.97
Lavigne MC, Grimsby JL, Eppihimer MJ (2012) Antirestenotic mechanisms of everolimus on human coronary artery smooth muscle cells: inhibition of human coronary artery smooth muscle cell proliferation, but not migration. J Cardiovasc Pharmacol 59:165–174. doi:10.1097/FJC.0b013e31823a39c7
Law BY, Wang M, Ma DL, Al-Mousa F, Michelangeli F, Cheng SH, Ng MH, To KF, Mok AY, Ko RY, Lam SK, Chen F, Che CM, Chiu P, Ko BC (2010) Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol Cancer Ther 9:718–730. doi:10.1158/1535-7163.MCT-09-0700
Levine B, Yuan J (2005) Autophagy in cell death: an innocent convict? J Clin Invest 115:2679–2688. doi:10.1172/JCI26390
Liao A, Hu R, Zhao Q, Li J, Li Y, Yao K, Zhang R, Wang H, Yang W, Liu Z (2012) Autophagy induced by FTY720 promotes apoptosis in U266 cells. Eur J Pharm Sci 45:600–605. doi:10.1016/j.ejps.2011.12.014
Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I (2012) Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab 15:545–553. doi:10.1016/j.cmet.2012.01.022
Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ (1999) Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res 41:473–479
Ma KL, Ruan XZ, Powis SH, Moorhead JF, Varghese Z (2007) Anti-atherosclerotic effects of sirolimus on human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 292:H2721–H2728. doi:10.1152/ajpheart.01174.2006
Ma KL, Varghese Z, Ku Y, Powis SH, Chen Y, Moorhead JF, Ruan XZ (2010) Sirolimus inhibits endogenous cholesterol synthesis induced by inflammatory stress in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 298:H1646–H1651. doi:10.1152/ajpheart.00492.2009
Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ (2004) The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol 286:C507–C517. doi:10.1152/ajpcell.00201.2003
Martinet W, Agostinis P, Vanhoecke B, Dewaele M, De Meyer GRY (2009) Autophagy in disease: a double-edged sword with therapeutic potential. Clin Sci (Lond) 116:697–712. doi:10.1042/CS20080508
Martinet W, De Meyer GR (2008) Autophagy in atherosclerosis. Curr Atheroscler Rep 10:216–223
Martinet W, De Meyer GRY (2009) Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res 104:304–317. doi:10.1161/CIRCRESAHA.108.188318
Martinet W, De Meyer GRY, Andries L, Herman AG, Kockx MM (2006) Detection of autophagy in tissue by standard immunohistochemistry: possibilities and limitations. Autophagy 2:55–57
Martinet W, De Meyer GRY, Andries L, Herman AG, Kockx MM (2006) In situ detection of starvation-induced autophagy. J Histochem Cytochem 54:85–96. doi:10.1369/jhc.5A6743.2005
Martinet W, De Meyer I, Cools N, Timmerman V, Bult H, Bosmans J, De Meyer GRY (2010) Cell death-mediated cleavage of the attraction signal p43 in human atherosclerosis: implications for plaque destabilization. Arterioscler Thromb Vasc Biol 30:1415–1422. doi:10.1161/ATVBAHA.110.206029
Martinet W, De BM, Schrijvers DM, De Meyer GR, Herman AG, Kockx MM (2004) 7-ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24:2296–2301. doi:10.1161/01.ATV.0000146266.65820.a1
Martinet W, Knaapen MW, De Meyer GRY, Herman AG, Kockx MM (2002) Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 106:927–932. doi:10.1161/01.CIR.0000026393.47805.21
Martinet W, Schrijvers DM, De Meyer GRY (2012) Molecular and cellular mechanisms of macrophage survival in atherosclerosis. Basic Res Cardiol 107:297. doi:10.1007/s00395-012-0297-x
Martinet W, Verheye S, De Meyer GRY (2007) Everolimus-induced mTOR inhibition selectively depletes macrophages in atherosclerotic plaques by autophagy. Autophagy 3:241–244
Martinet W, Verheye S, De Meyer GRY (2007) Selective depletion of macrophages in atherosclerotic plaques via macrophage-specific initiation of cell death. Trends Cardiovasc Med 17:69–75. doi:10.1016/j.tcm.2006.12.004
Martinet W, Verheye S, De Meyer I, Timmermans JP, Schrijvers DM, Van Brussel I, Bult H, De Meyer GRY (2012) Everolimus triggers cytokine release by macrophages: rationale for stents eluting everolimus and a glucocorticoid. Arterioscler Thromb Vasc Biol 32:1228–1235. doi:10.1161/ATVBAHA.112.245381
Mathis AS, Jin S, Friedman GS, Peng F, Carl SM, Knipp GT (2007) The pharmacodynamic effects of sirolimus and sirolimus-calcineurin inhibitor combinations on macrophage scavenger and nuclear hormone receptors. J Pharm Sci 96:209–222. doi:10.1002/jps.20751
Matsuzawa T, Kim BH, Shenoy AR, Kamitani S, Miyake M, Macmicking JD (2012) IFN-gamma elicits macrophage autophagy via the p38 MAPK signaling pathway. J Immunol 189:813–818. doi:10.4049/jimmunol.1102041
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741. doi:10.1016/j.cell.2011.10.026
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075. doi:10.1038/nature06639
Mueller MA, Beutner F, Teupser D, Ceglarek U, Thiery J (2008) Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR−/− mice despite severe hypercholesterolemia. Atherosclerosis 198:39–48. doi:10.1016/j.atherosclerosis.2007.09.019
Muller C, Salvayre R, Negre-Salvayre A, Vindis C (2011) HDLs inhibit endoplasmic reticulum stress and autophagic response induced by oxidized LDLs. Cell Death Differ 18:817–828. doi:10.1038/cdd.2010.149
Onuma Y, Serruys PW, Perkins LE, Okamura T, Gonzalo N, Garcia-Garcia HM, Regar E, Kamberi M, Powers JC, Rapoza R, van BH, van der Giessen W, Virmani R (2010) Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation 122:2288–2300. doi: 10.1161/CIRCULATIONAHA.109.921528
Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL (2011) Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab 13:655–667. doi:10.1016/j.cmet.2011.03.023
Petrovski G, Ayna G, Majai G, Hodrea J, Benko S, Madi A, Fesus L (2011) Phagocytosis of cells dying through autophagy induces inflammasome activation and IL-1beta release in human macrophages. Autophagy 7:321–330. doi:10.4161/auto.7.3.14583
Petrovski G, Zahuczky G, Majai G, Fesus L (2007) Phagocytosis of cells dying through autophagy evokes a pro-inflammatory response in macrophages. Autophagy 3:509–511
Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF (2012) Autophagy links inflammasomes to atherosclerotic progression. Cell Metab 15:534–544. doi:10.1016/j.cmet.2012.02.011
Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282:5641–5652. doi:10.1074/jbc.M609532200
Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170:1101–1111. doi:10.1083/jcb.200504035
Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, Webster JA, Lewis TA, O’Kane CJ, Schreiber SL, Rubinsztein DC (2007) Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol 3:331–338. doi:10.1038/nchembio883
Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC (2009) Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ 16:46–56. doi:10.1038/cdd.2008.110
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195. doi:10.1038/nature00858
Schrijvers DM, De Meyer GRY, Kockx MM, Herman AG, Martinet W (2005) Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol 25:1256–1261. doi:10.1161/01.ATV.0000166517.18801.a7
Schrijvers DM, De Meyer GRY, Martinet W (2011) Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol 31:2787–2791. doi:10.1161/ATVBAHA.111.224899
Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, Veldhof S, Webster M, Thuesen L, Dudek D (2009) A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373:897–910. doi:10.1016/S0140-6736(09)60325-1
Shalak V, Guigou L, Kaminska M, Wautier MP, Wautier JL, Mirande M (2007) Characterization of p43(ARF), a derivative of the p43 component of multiaminoacyl-tRNA synthetase complex released during apoptosis. J Biol Chem 282:10935–10943. doi:10.1074/jbc.M611737200
Shen S, Kepp O, Kroemer G (2012) The end of autophagic cell death? Autophagy 8:1–3. doi:10.4161/auto.8.1.16618
Shi CS, Kehrl JH (2008) MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem 283:33175–33182. doi:10.1074/jbc.M804478200
Sridhar S, Botbol Y, Macian F, Cuervo AM (2012) Autophagy and disease: always two sides to a problem. J Pathol 226:255–273. doi:10.1002/path.3025
Verheye S, Martinet W, Kockx MM, Knaapen MW, Salu K, Timmermans JP, Ellis JT, Kilpatrick DL, De Meyer GRY (2007) Selective clearance of macrophages in atherosclerotic plaques by autophagy. J Am Coll Cardiol 49:706–715. doi:10.1016/j.jacc.2006.09.047
Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P (2010) AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem 285:9100–9113. doi:10.1074/jbc.M109.060061
Wang SH, Shih YL, Ko WC, Wei YH, Shih CM (2008) Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell Mol Life Sci 65:3640–3652. doi:10.1007/s00018-008-8383-9
Wang X, Proud CG (2011) mTORC1 signaling: what we still don’t know. J Mol Cell Biol 3:206–220. doi:10.1093/jmcb/mjq038
Yang H, Wang H, Czura CJ, Tracey KJ (2005) The cytokine activity of HMGB1. J Leukoc Biol 78:1–8. doi:10.1189/jlb.1104648
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12:814–822. doi:10.1038/ncb0910-814
Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281:30299–30304. doi:10.1074/jbc.M607007200
Zhang N, Qi Y, Wadham C, Wang L, Warren A, Di W, Xia P (2010) FTY720 induces necrotic cell death and autophagy in ovarian cancer cells: a protective role of autophagy. Autophagy 6:1157–1167. doi:10.4161/auto.6.8.13614
Zhang YL, Cao YJ, Zhang X, Liu HH, Tong T, Xiao GD, Yang YP, Liu CF (2010) The autophagy-lysosome pathway: a novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells. Biochem Biophys Res Commun 394:377–382. doi:10.1016/j.bbrc.2010.03.026
Acknowledgments
This work was supported by the Fund for Scientific Research (FWO)-Flanders (projects G.0431.11N, G.0448.11N, G.0443.12N and G.0074.12N) and the University of Antwerp. The authors are indebted to Lieve Svensson, Francis Terloo and Dominique De Rijck for excellent technical assistance during transmission electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinet, W., De Meyer, I., Verheye, S. et al. Drug-induced macrophage autophagy in atherosclerosis: for better or worse?. Basic Res Cardiol 108, 321 (2013). https://doi.org/10.1007/s00395-012-0321-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0321-1