Abstract
Doxorubicin (DOX) is a widely used anti-tumor agent. The clinical application of the medication is limited by its side effect which can elicit myocardial apoptosis and cardiac dysfunction. However, the underlying mechanism by which DOX causes cardiomyocyte apoptosis is not clear. The aim of present study is to investigate the role of high-mobility group box 1 (HMGB1) in DOX-induced myocardial injury, and signal pathway involved in regulation of HMGB1 expression in cardiomyocytes with DOX. We found treatment of isolated cardiomyocytes and naive mice with the DOX resulted in an increased HMGB1 expression which was associated with increased myocardial cell apoptosis. Pharmacological (A-box) or genetic blockade (TLR4 deficiency, TLR4−/−) of HMGB1 attenuated the DOX-induced myocardial apoptosis and cardiac dysfunction. In addition, our study showed that DOX resulted in an increment in the generation of peroxynitrite (ONOO−) and an elevation in phosphorylation of c-Jun N terminal kinase (JNK). Pretreatment of myocytes with FeTPPS, a peroxynitrite decomposition catalyst, prevented DOX-induced JNK phosphorylation, HMGB1 expression, myocardial apoptosis and cardiac dysfunction. Genetic (JNK−/−) or pharmacological (SP600125) inhibition of JNK ameliorated the DOX-induced HMGB1 expression and diminished myocardial apoptosis and cardiac dysfunction. Taken together, our results indicate that HMGB1 mediates the myocardial injury induced by DOX and ONOO−/JNK is a key regulatory pathway of myocardial HMGB1 expression induced by DOX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin (DOX; Adriamycin) is a potent and widely used anti-tumor agent. It is particularly effective in treating breast and bladder cancer, Hodgkin’s lymphomas, and acute leukemia [22, 23]. However, clinic use of this medication is limited due to its toxicity to the heart which could result in irreversible myocardial injury and dysfunction [22, 23]. The current consensus held is that DOX induces myocardial cell oxidative stress, lipid peroxidation, inhibition of nucleic acid and protein synthesis [7, 32]. In addition, myocardial apoptosis is a key component of DOX-induced cardiotoxicity which could ultimately lead to cardiac failure [10]. However, the mechanism involved in the induction of the cardiomyocyte apoptosis remains unclear. Thus, the major aim of the present study is to investigate the underlying mechanism by which DOX induces myocardial apoptosis and establish optimal therapeutic approaches to prevent the injury induced by the medication.
Previous studies have demonstrated that induction of reactive oxygen species (ROS) including superoxide (O2 −) anion, hydroxyl radical and hydrogen peroxide, plays a key role in the pathogenesis of DOX-induced myocardial injury [10, 23]. Peroxynitrite (ONOO−) is a product of the reaction between nitric oxide (NO) and superoxide anion (O2 −), which can exert both oxidant and nitrosant activity [2, 16]. ONOO− has a high affinity to tyrosine residues, resulting in posttranslational modification of protein-bound tyrosine to 3-nitrotyrosine (3-NT). Recent studies have uncovered that peroxynitrite generation plays critical roles in ischemia-/reperfusion-induced myocardial injury and cardiac failure [9, 13, 15]. It has been demonstrated that myocardial nitrotyrosine formation was increased in DOX-treated rodents and believed to be contributing to the DOX-induced myocardial depression [16]. However, the downstream events of the peroxynitrite induction by the DOX remain unknown.
High-mobility group box 1(HMGB1) is an “alarmin” protein, which could trigger inflammatory responses in neighbor cells when the organisms are under stress or bacteria invasion [3, 6, 12]. In general, HMGB1 is passively released from necrotic cells [20]. Recent study indicated that it can be actively secreted by viable cells under certain pathological conditions [8]. The HMGB1 plays its biological roles by interaction with its receptor [e.g., Toll-like receptor-4 (TLR-4)] [18]. Activation of the receptor can lead to activation of NFκB, which will further increase expression of leukocyte adhesion molecules and the production of proinflammatory mediators by both hematopoietic and endothelial cells, thereby promoting inflammation and causing organ and cell damage [25, 27].
We and others have previously demonstrated that HMGB1 plays important roles in LPS-induced myocardial dysfunction as well as in ischemia-/reperfusion (I/R)-induced myocardial injury [1, 29, 30]. In mice with endotoxemia, LPS activates TLR4/PI3Kγ pathway which leads to an increased myocardial HMGB1 and contributes to decrease in myocardial contractility. While in I/R-induced myocardial apoptosis, HMGB1 contributes to myocardial apoptosis by potentiating the effect of TNFα. It seems that in different pathological situations, HMGB1 plays its roles by various mechanisms. The above findings promote us to further explore potential role(s) of HMGB1 in DOX-induced myocardial apoptosis and study the underlying cell signaling pathway by which the expression of HMGB1 is regulated. Using both in vitro and in vivo approaches, we provided evidences that (1) DOX could promote cardiomyocytes to increase HMGB1 expression, which contributes to DOX-induced myocytes apoptosis and cardiac dysfunction; (2) Activation of c-Jun N terminal kinase (JNK) by ONOO− after DOX treatment represents a key regulator of DOX-induced expression of HMGB1.
Materials and methods
Mice
C57BL/6 mice (wild type, 6 weeks old, 25 g) were obtained from Charles River Laboratories Canada (St. Constant, Quebec, Canada). TLR4−/− mice and JNK−/− mice on C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were housed in Vivarium Service at Victoria Research Labs with a 12 h light/dark cycle and free access to rodent chow and tap water. The mice were used for in vivo study. The breeding pairs of above mice were used for generating neonatal mice for isolation of cardiomyocytes for in vitro experiments. The procedure was performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 85-23, National Academy Press, Washington, DC, revised 1996). The study protocol was approved by Animal Care and Use Committee of Jiangsu University and University of Western Ontario.
Neonatal cardiomyocytes
Neonatal cardiomyocytes were isolated and cultured as described previously with some modifications [19]. Briefly, hearts were harvested from neonatal mice, minced and digested with Liberase (15 µg/ml, Roche) in Ca++ and Mg++ free Hank’s balance salt solution. After a washing step, the cells were suspended in Medium 199 (Sigma) with 10 % fetal bovine serum (FBS, Gibco). The cardiomyocytes were enriched by a pre-plating approach (to remove contaminating cells) before seeded into cell culture plates. After 48–72 h culture, the cells formed a confluent monolayer, consisting of 95 % cardiomyocytes, beating synchronically and could be used for in vitro experiments at this time.
Concentration of HMGB1 in plasma of circulation and supernatant of medium
Circulating HMGB1 in plasma and HMGB1 in cell culture medium were determined by ELISA as described previously with some modifications [29]. Briefly, the samples were collected and added into 96-well enzyme immunoassay plates coated with mouse anti-HMGB1 mAb (5 µg/ml, abcam). After incubation overnight at 4 °C, a biotin-conjugated rabbit anti-HMGB1 mAb (1:1,000, abcam) was added to the enzyme immunoassay plate. Subsequently, an Extravidin–HRP (1:2,000, Sigma) was added. Finally, TMB substrate (Cell Signaling) was added to amplify the immunity reaction. Color was developed, and optical density was determined by microplate reader (BIO-RAD) at 405 nm.
Knockdown HMGB1 with shRNA plasmid
HMGB1 small hairpin RNA plasmid (HMG-1 shRNA plasmid) and a negative control shRNA plasmid were purchased from Santa Cruz biotechnology, Inc. The transfection of shRNA plasmid was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Briefly, the neonatal cardiomyocytes were plated in 48-well plates in completed M199. Subsequently, the myocytes were transfected with HMG-1 shRNA plasmid or negative control. Forty eight hours after the shRNA transfection, the myocytes were ready for experiments.
Western blot analysis of HMGB1 expression, phosphorylated-JNK and myocardial nitrotyrosine
Expression of HMGB1 in myocardial tissue and in cardiomyocytes, phosphorylation of JNK and myocardial nitrotyrosine were evaluated by Western blot analysis. Cardiomyocytes and myocardial tissue lysates were analyzed using 12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Bio-Rad). Subsequently, the membranes were incubated with primary antibodies against following molecules, HMGB1 (1:2,000, abcam), JNK (1:1,000, Cell Signaling), phospho-JNK (1:1,000, Cell Signaling), nitrotyrosine (mono-antibody, 1:1,000, abcam) and β-actin (1:6,000, abcam) at 4 °C overnight, respectively. The membranes were then incubated with HRP-labeled secondary antibody at 37 °C for 2 h. Signals were detected by enhanced chemiluminescence (Amersham, USA). Densitometric analysis for the blots was performed with Multi-analyst image software.
Peroxynitrite production
Peroxynitrite within the cardiomyocytes was determined by measuring the oxidation of intracellular dihydrorhodamine 123 (DHR123; Molecular Probe), an fluorescence probe sensitive to peroxynitrite, as described previously [19]. Briefly, the cells were preloaded with DHR123 (10 µmol/L) for 1 h followed by DOX (0.5 µM) treatment. Subsequently, the cells were washed with cold PBS and lysed with CHAPS solution (0.1 % CHAPS, 50 mM K2HPO4, 0.1 mM EDTA, pH = 7.0) and DHR123 oxidation was measured with a Spectrofluorophotometer at excitation and emission wavelengths of 502 and 523 nm, respectively.
Apoptosis of cardiomyocytes
Apoptosis of cardiomyocytes was assessed by measuring the caspase-3 activity with an assay kit (Enzo) and detection of histone-associated DNA fragments with a Cell Death Detection ELISA assay kit (Roche) according to the manufacturer’s instructions. The caspase-3 activity was measured at 6 h after DOX treatment, while detection of histone-associated DNA fragments was done 24 h post DOX treatment.
Mouse model of DOX-induced cardiotoxicity
Male C57BL/6 wild type mice, TLR4−/−, and JNK−/− mice in C57BL/6 background were administrated intraperitoneally (i.p.) with a single dose of doxorubicin (Sigma) at 20 mg/kg. The mice given equal volume of vehicle were served as control. Myocardial function was assessed 5 days after the injection and mouse myocardium and blood sample were collected for detecting related parameters. For some experiments, DOX-treated mice were given either a selective peroxynitrite scavenger and decomposition catalyst 5,10,15,20-Tetrakis (4-sulfonatophenyl) porphyrinato Iron (III) Chloride (FeTPPS; 10 mg/kg, i.p.) started 1 h before DOX injection and continued in third and fifth day (10 mg/kg, i.p.) thereafter; or A-box (20 mg/kg, i.p.), a HMGB1 inhibitor, every other days starting 4 h after the DOX treatment.
Immunohistological analysis of nitrotyrosine
Myocardial tissue was fixed in 4 % formalin. The embedded tissue was cut into 5 µm slices. Tissue sections were incubated with a mouse anti-nitrotyrosine mAb (1:50, abcam) overnight at 4 °C. Subsequently, the sections were incubated with HRP conjugated goat anti-mouse secondary antibody (1:200, abcam) at room temperature for 1 h. Finally, color was developed with a peroxide-based substrate Vectastain DAB kit (BD Biosciences).
TUNEL assay
Paraffin sections were prepared, and in situ detection of apoptosis in the myocardial tissues was performed with an In Situ Cell Death Detection Kit, Fluorescein (Roche) following the instructions. After TUNEL labeling, sections were stained with mouse monoclonal Troponin T (1:100, abcam) for 1 h, followed by incubation with appropriate secondary antibody conjugated with Texas Red (1:200, Santa Cruz). Nucleus were labeled with DAPI, and the TUNEL positive cells were observed using microscope (Zeiss, using 40× objective). The percentage of apoptotic cells per total cells was determined in five randomly chosen fields.
Myocardial function
Myocardial function was measured as described previously [29]. In brief, mice were anesthetized with i.p. injection of ketamine (150 mg/kg) and xylazine (5 mg/kg), and mechanically ventilated. A Millar tip transducer catheter (Model SPR-893, 1.4 Fr.) was positioned in the left ventricle via the right carotid artery. Post recording the basic hemodynamic parameters, left-ventricular pressure–volume loops were generated by occlusion of the inferior caval vein using a PowerLab system (AD Instruments) connected to the Millar catheter. After recording heart rate (HR), end systolic volume, end diastolic volume, left-ventricular end systolic pressure (LVESP), left-ventricular end diastolic pressure (LVEDP), ±dP/dt, the inferior cava vein was occluded and pressure–volume loops were recorded. Subsequently, the end-systolic pressure–volume relation (ESPVR) was generated from pressure–volume loops and served as an index of myocardial contractility.
Statistical analysis
Data are expressed as mean ± SEM and analyzed with SPSS11.0 statistical software. Comparisons of data among groups were made using one-way analysis of variance (ANOVA) with Bonferroni’s post-test. A P value <0.05 was considered as a statistical significance.
Results
HMGB1 mediates DOX-induced myocardial apoptosis
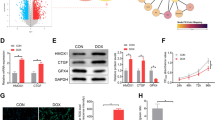
HMGB1 is a cytokine which has been demonstrated involved in ischemia-/reperfusion-induced myocardial injury and sepsis-induced myocardial depression [1, 29, 30]. To assess whether HMGB1 plays a role in DOX-induced myocardial apoptosis, we first evaluated HMGB1 expression and releasing by DOX-treated cardiomyocytes. As shown in Fig. 1a, treatment of cardiac myocytes with DOX resulted in increased HMGB1 expression and releasing. In addition, the DOX treatment induced myocyte apoptosis (Fig. 1b). TLR4 is a receptor that HMGB1 can interact with [24, 31]. To determine whether HMGB1 mediates the DOX-induced myocytes apoptosis, either pharmacological (A-box) or genetic inhibition of HMGB1 (TLR4 deletion and HMG1 shRNA plasmid transfection) was employed in the experiments. We found inhibition of HMGB1 with the A-box attenuated the DOX-induced apoptosis (Fig. 1b); as compared to cardiomyocytes derived from wild type mice, DOX induced much less apoptosis in cardiomyocytes derived from TLR4 deficient mice (Fig. 1c). To further confirm the role of HMGB1 in the DOX-induced myocardial apoptosis, HMGB1 knock down approach was used in our study. As shown in SFig. 1, the transfection of HMG-1 shRNA plasmid into cardiac myocytes resulted in a 70 % reduction in HMGB1 expression in naïve cells and prevented the increase in HMGB1 expression in DOX-treated cardiomyocytes. The knock down HMGB1 attenuated apoptosis in myocytes with DOX (Fig. 1d).
HMGB1 contributes to DOX-induced cardiomyocytes apoptosis. a Cardiac myocytes were treated with DOX (0.5 µM). At the time indicated, cardiomyocytes and cell culture medium were harvested. Intracellular and released HMGB1 of cardiomyocytes was analyzed by Western blot and ELISA, respectively. b Inhibition of HMGB1 prevented the myocyte apoptosis induced by DOX. Cardiac myocytes were challenged with DOX with or without A-box (10 µg/ml) for 6 or 24 h, myocyte apoptosis was determined by caspase 3 activity and cell death ELISA assays. c Cardiac myocytes derived from wild type and TLR4−/− mice were challenged with DOX for 6 or 24 h, and the control myocytes were treated with vehicle. The myocyte apoptosis was determined. n = 3. *P < 0.05 compared with time 0, or control group, # P < 0.05 compared with DOX group or wild type myocytes with DOX group. d Cardiac myocytes were transfected with HMG1 shRNA plasmid or shRNA negative control plasmid followed by DOX treatment for 6 or 24 h, respectively. The myocyte apoptosis was determined by caspase 3 activity and cell death ELISA. n = 3. *P < 0.05 compared with negative control group, # P < 0.05 compared with negative control + DOX group
JNK is involved in HMGB1 expression in cardiomyocytes with DOX
JNK is a kinase which has been reported involved in cell apoptosis and inflammation [11, 14, 21, 26]. To investigate whether JNK regulates DOX-induced HMGB1 expression, cardiomyocytes were treated with DOX and JNK phosphorylation was assessed. As shown in Fig. 2a, the treatment of cardiomyocytes with DOX resulted in activation of JNK as indicated by an increase in JNK phosphorylation which peaked at 10–15 min after the DOX treatment. To determine whether activation of JNK contributes to increase in HMGB1 expression by cardiomyocytes after the DOX treatment, both pharmacological and genetic inhibition of JNK were used in the study. As shown in Fig. 2b, the DOX-induced increase in myocytes HMGB1 protein expression was prevented by a JNK inhibitor, SP600125. Furthermore, DOX induced much less HMGB1 production in cardiomyocytes derived from JNK−/− mice as compared to those of myocytes from wild type mice (Fig. 2c). Collectively, these findings indicate that DOX-induced expression of HMGB1 is dependent on activation of JNK.
JNK is pivotal in DOX-induced HMGB1 expression in cardiomyocytes. a. Cardiac myocytes were treated with DOX. At the time indicated, the myocytes were harvested and JNK activation was assessed by Western blot for detection of JNK phosphorylation. b Inhibition of JNK prevents the DOX-induced HMGB1 expression. Cardiac myocytes were treated with DOX, with or without SP600125 (10 µM) for 4 h. Myocyte HMGB1 expression was assessed with Western blot. c Deficiency in JNK prevents the DOX-induced HMGB1 expression. Cardiac myocytes from wild type and JNK1 deficient mice were challenged with DOX for 4 h, and the control myocytes were challenged with vehicle. HMGB1 expression was assessed with Western blot. n = 3. *P < 0.05 compared with time 0 or control group, # P < 0.05 compared with DOX group or wild type myocytes with DOX
DOX increases intracellular ONOO−, activates JNK and regulates HMGB1 expression in cardiomyocytes which results in myocyte apoptosis
ONOO− is a reactive oxygen species, which is produced from reaction between nitric oxide (NO) and the superoxide anion (O2 −). It represents a key effector which mediates DOX-induced cardiomyocyte apoptosis [16]. In present study, we measured intracellular ONOO− levels using DHR123 as intracellular ONOO− probe. We found that challenge of cardiomyocytes with DOX resulted in an increased ONOO− generation, which is peaked at 10 min after DOX treatment (Fig. 3a). However, the production of ONOO− was significantly attenuated by FeTPPS, a selective decomposition catalyst of ONOO− (Fig. 3b). These results illustrated that DOX can increase ONOO− levels in cardiomyocytes, which was diminished by FeTPPS.
Peroxynitrite (ONOO−) is involved in HMGB1 expression in cardiomyocytes with DOX. a DOX treatment increased ONOO− production in cardiomyocytes. Cardiac myocytes were treated with DOX. At the time indicated, the ONOO− was detected with DHR123 (10 µM), a fluorescence probe for ONOO−. b FeTPPS, a cell permeable selective decomposition catalyst of ONOO−, prevented the increase in peroxynitrite induced by DOX. FeTPPS (25 µM) was added into the cell culture medium 1 h before DOX treatment. Intracellular ONOO− was determined 10 min after DOX treatment. c Cardiomyocytes were treated with DOX for 15 min, JNK phosphorylation was analyzed with Western blot. Inhibition of ONOO− with FeTPPS prevented DOX-induced increase in myocyte JNK phosphorylation. d Cardiomyocytes were treated with DOX for 4 h, HMGB1 expression was analyzed with Western blot. Inhibition of ONOO− with FeTPPS prevented DOX-induced increase in myocyte HMGB1 expression. n = 3. *P < 0.05 compared with time 0 or control group, # P < 0.05 compared with DOX group
Next, we further assessed the role of ONOO− in JNK activation and HMGB1 expression in cardiomyocytes with DOX. We found that DOX-induced JNK activation and HMGB1 expression in cardiomyocytes were prevented by FeTPPS (Fig. 3c, d). However, as compared to cardiomyocytes derived from wild type mice, myocytes from JNK deficient mice had similar levels of ONOO− after the DOX treatment (SFig. 2a). These results indicated that ONOO− is involved in regulation of DOX-induced HMGB1 expression in cardiomyocytes; and increase in that ONOO− is an upstream event of JNK activation.
Since HMGB1 contributes to the DOX-induced cardiomyocyte apoptosis and is regulated by ONOO−/JNK pathway, we further evaluated the role of ONOO−/JNK pathway in DOX-induced myocyte apoptosis. As shown in Fig. 4a, DOX-induced myocyte apoptosis was diminished by either FeTPPS or SP600125. In addition, DOX-induced apoptosis was attenuated significantly in JNK−/− cardiomyocytes as compared to those of cardiomyocytes derived from wild type mice (Fig. 4b).
Inhibition of ONOO−/JNK pathway attenuated DOX-induced cardiomyocyte apoptosis. a Cardiac myocytes were pretreated with FeTPPS or SP600125 followed by DOX treatment. Myocyte apoptosis were determined with caspase 3 activity and cell death ELISA assay. b Cardiomyocytes derived from wild type or JNK−/− mice were treated with DOX or vehicle, and myocyte apoptosis was assessed. n = 3. *P < 0.05 compared with control group, # P < 0.05 compared with wild type + DOX group or + P < 0.05 compared with DOX only group
ONOO−/JNK pathway regulates myocardial HMGB1 expression in mice with DOX
In order to confirm the silent features uncovered by our in vitro experiments, we designed in vivo experiments. As shown in Fig. 5a–c, increased ONOO− generation and JNK activation were detected in myocardium of mice with DOX. In addition, the DOX-induced ONOO− production and JNK activation were greatly attenuated when the DOX-treated mice were given FeTPPS. Furthermore, increased myocardial HMGB1 expression in mice with DOX was prevented by FeTPPS (Fig. 5d). To evaluate the role of JNK on DOX-induced myocardial HMGB1 expression, JNK1 deficient mice were administrated with DOX and myocardial HMGB1 expression and circulating HMGB1 were assessed. As compared to wild type mice, the myocardial levels of HMGB1 and circulating HMGB1 in JNK1 deficient mice were decreased (Fig. 5e). However, myocardium of JNK1 deficient mice had similar levels of peroxynitrite after DOX as compared to those of wild type counter part (SFig. 2b). The above results support our in vitro findings that ONOO−/JNK pathway regulates the myocardial HMGB1 expression.
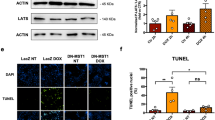
ONOO−/JNK pathway regulates myocardial and circulating HMGB1 in mice with DOX. Mice were injected (intraperitoneal, i.p.) with DOX (10 mg/kg), while control mice were injected with equal volume of vehicle. For DOX mice treated with FeTPPS, they were given FeTPPS (10 mg/kg, i.p.) every other day starting 1 h before the administration of DOX. On day 5 after DOX, cardiac tissue was harvested for myocardial level of ONOO− with nitrotyrosine staining and HMGB1 expression by Western blot. a Images are represents of nitrotyrosine staining of tissue section of 5 separate mouse hearts. b Western blot analysis was done to quantify the myocardial nitrotyrosine. Myocardial levels of ONOO− were increased in mice with DOX which were attenuated by FeTPPS treatment. c Myocardial JNK phosphorylation was increased in mice with DOX which was greatly attenuated by FeTPPS. d Myocardial levels of HMGB1 were increased in hearts of mice with DOX. FeTPPS treatment attenuated the increase in myocardial HMGB1 induced by DOX. n = 3, *P < 0.05 compared to control, # P < 0.05 compared with DOX group. e Deficiency in JNK1 prevented the DOX-induced increase in myocardial and circulating HMGB1. n = 3. *P < 0.05 compared with control, # P < 0.05 compared with WT mice with DOX
Inhibition of HMGB1 and ONOO−/JNK pathway prevents the DOX-induced myocardial apoptosis and dysfunction
In order to evaluate the role of HMGB1 on DOX-induced myocardial apoptosis and dysfunction, the DOX-treated mice were given A-box, a HMGB1 receptor competitive inhibitor. In addition, TLR4 deficient mice were administrated with DOX. The A-box treatment attenuated DOX-induced myocardial apoptosis (Fig. 6b), and prevented the DOX induced myocardial dysfunction (Fig. 7a, SFig. 3). Treatment of TLR4 deficient mice with DOX induced limited myocardial apoptosis and dysfunction as compared to their wild type counterpart (Figs. 6d, 7c and SFig. 3).
Inhibition of ONOO−/JNK/HMGB1 pathway attenuates myocardial apoptosis in mice with DOX. Wild type, TLR4−/− and JNK1−/− mice were i.p. injected with either vehicle or DOX. For inhibition of ONOO−, FeTPPS (10 mg/kg, i.p.) were given every other day starting 1 h before the administration of DOX. For pharmacological inhibition of HMGB1, A-box (20 mg/kg) was given to DOX mice every other days starting 4 h after the DOX treatment. Myocardial apoptosis was assessed 5 days after the DOX treatment. a Representative images of TUNEL staining. b–d Quantitative analysis of myocardial apoptosis. For each section, 10 high-power fields were counted for apoptotic cells. n = 5. *P < 0.05 compared with control, # P < 0.05 compared with WT mice with DOX
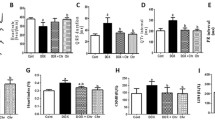
Inhibition of ONOO−/JNK/HMGB1 pathway improves myocardial function in mice with DOX. The mouse treatment groups were same as in Fig. 6. Myocardial function was assessed 5 days after the DOX treatment with pressure–volume loop analysis system. Left ventricle end systolic pressure–volume relation (ESPVR, mmHg/µl) was determined and was used as index of myocardial contractility. n = 5. *P < 0.05 compared with control, # P < 0.05 compared with WT mice with DOX
To further evaluate the role of ONOO−/JNK on DOX-induced myocardial apoptosis and dysfunction, mice with DOX were treated with FeTPPS and JNK1 deficient mice were administrated with DOX, myocardial apoptosis and cardiac function were assessed. As shown in Fig. 6b, administration of FeTPPS to mice with DOX attenuated myocardial apoptosis and improved the cardiac function (Fig. 7a, SFig. 3). Similarly, as compared to wild type mice, JNK1 deficient mice incurred less myocardial apoptosis and dysfunction after the administration of the DOX (Figs. 6c, 7b and SFig. 1). The results further confirm our in vitro findings which indicate that ONOO−/JNK pathway is involved in the DOX-induced myocardial apoptosis and cardiac dysfunction.
Discussion
Doxorubicin is an antitumor agent with side effect on myocardium which can cause myocardial injury [22, 23]. HMGB1 is a “danger signal”, whose release from cells serves to inform adjacent (or remote) cells of infection and/or injury, so that an appropriate defensive immune response can be generated [3, 6, 12]. Myocardial cells increase HMGB1 expression and release HMGB1 to extracellular milieu under stressful conditions; i.e., sepsis and ischemia-/reperfusion [1, 29, 30]. Extracellular HMGB1 exerts pro-inflammatory activity by binding to its receptors and contributes to myocardial apoptosis and dysfunction. In the present study, we addressed the role of HMGB1 in DOX-induced myocardial apoptosis. We demonstrate for the first time that (1) DOX can increase cardiomyocyte HMGB1 expression which contributes to DOX-induced myocardial apoptosis and cardiac dysfunction; (2) regulation of HMGB1 expression is dependent on ONOO−/JNK pathway.
One of major mechanism involved in cardiomyocytes apoptosis during DOX-induced cardiomyopathy is the induction of myocardial oxidative stress and nitrosative stress attributed to the generation of reactive oxygen species (ROS) and reactive nitrogen species, most notably peroxynitrite (ONOO−) derived from nitric oxide (NO) and superoxide (O2 −). Intracellular peroxynitrite further activates signaling pathway and causes injury to myocytes [16, 17]. One potential mechanism is that the peroxynitrite induces stress to myocardial cells and results in increase expression of HMGB1 due to the activation of the signaling pathway during DOX-induced cardiotoxicity. In our study, we have demonstrated that DOX treatment resulted in the induction of ONOO− which was evident by the increased DHR123 oxidation in cardiomyocytes. In myocardial tissue, the induction of ONOO− was marked by nitrotyrosine which was increased in the mice injected with DOX. As the selective decomposition catalyst of ONOO−, FeTPPS could reduce the formation of ONOO−, which has been demonstrated in our study and in the previous study [4]. Meanwhile, FeTPPS can prevent the increase in HMGB1 expression and attenuate cardiomyocyte apoptosis induced by DOX. These results indicate that ONOO− induced by DOX is involved in the regulation of myocardial HMGB1 expression and contributes to cardiomyocyte apoptosis.
c-Jun N terminal kinase (JNK) is an important member of the mitogen-activated protein kinase (MAPK) superfamily, which is readily to be activated by many environmental stimuli [5, 14]. As a pro-apoptotic kinase, JNK is believed to play pivotal roles in cardiomyocyte apoptosis in various pathologies [14, 28]. The main mechanism of JNK activation is the cell stress including the ROS, such as ONOO− [11, 14, 21]. In our study, we found that DOX increased myocyte ONOO− generation, which in turn caused the activation of JNK. Inhibition of ONOO− with FeTPPS can prevent the activation of JNK and attenuate the DOX-induced myocyte apoptosis. Further, genetical (JNK−/−) and pharmacological (SP600125) inhibition of JNK offered significant protection against apoptosis induced by DOX. Those results support that ONOO−/JNK pathway is a pivotal mechanism in the DOX-induced myocyte apoptosis.
The regulatory pathways involved in the HMGB1 expression differ in different conditions. We have previously reported that TLR4/PI3Kγ pathway regulates myocardial HMGB1 expression in sepsis [29]. However, others have demonstrated that the peroxynitrite contributes to the HMGB1 up regulation in infracted myocardium [13]. In the present study, our results indicate that DOX-induced HMGB1 expression is regulated by JNK activation based on following evidences, (1) DOX induced activation of JNK at 10–15 min, and increased expression of HMGB1 at about 4 h, which implied that HMGB1 might be the downstream event to the activation of JNK in DOX-induced apoptosis; (2) suppression of JNK led to reduced HMGB1 expression in both in vitro and in vivo settings.
As shown in our study, neither genetic methods (JNK and TLR4 deficiency) nor pharmacological inhibitors (A-box, FeTPPS and SP600125) to inhibit HMGB1 could completely rescue the myocytes from DOX-induced injury. It implies that in addition to the release of HMGB1 in cardiac tissue, there are other mechanisms that contribute to DOX-induced myocardial injury. As reported by others [28], such mechanisms include inhibition of nucleic acids and protein synthesis, lipid peroxidation, and release of vasoactive amines. Previous studies have discovered that HMGB1 is a ligand of several receptors which include TLR4, TLR2 and RAGE [18, 25]. In our study, deletion of TLR4 can only partially abrogate the DOX-induced myocardial apoptosis and dysfunction. This implies HMGB1 may mediate the DOX-induced myocardial injury through other receptors (i.e., TLR2, and RAGE).
In summary, the present study showed that HMGB1 was involved in DOX-induced apoptosis of cardiomyocytes and cardiac dysfunction. In addition, ONOO−/JNK pathway has been identified as a regulator for expression of HMGB1 after DOX treatment. These results suggested that inhibition of HMGB1 could provide a means of reducing DOX-induced cardiomyopathy.
References
Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A (2008) High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117:3216–3226. doi:10.1161/CIRCULATIONAHA.108.769331
Baker CS, Dutka DP, Pagano D, Rimoldi O, Pitt M, Hall RJ, Polak JM, Bonser RS, Camici PG (2002) Immunocytochemical evidence for inducible nitric oxide synthase and cyclooxygenase-2 expression with nitrotyrosine formation in human hibernating myocardium. Basic Res Cardiol 97:409–415. doi:10.1007/s003950200050
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5. doi:10.1189/jlb.0306164
Bolton C, Scott GS, Smith T, Flower RJ (2008) The acute and chronic phases of chronic relapsing experimental autoimmune encephalomyelitis (CR EAE) are ameliorated by the peroxynitrite decomposition catalyst, 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron (III) chloride, (FeTPPS). Eur J Pharmacol 601:88–93. doi:10.1016/j.ejphar/2008.10.029
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252. doi:10.1016/s0092-8674(00)00116-1
Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P (2005) HMGB1: guiding immunity from within. Trends Immunol 26:381–387. doi:10.1016/j.it.2005.04.009
Ewer MS, Ewer SM (2010) Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol 7:564–575. doi:10.1038/nrcardio.2010.121
Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3:995–1001. doi:10.1093/embo-reports/kvf198
Jiao XY, Gao E, Yuan Y, Wang Y, Lau WB, Koch W, Ma XL, Tao L (2009) INO-4885 [5,10,15,20-tetra[N-(benzyl-4′-carboxylate)-2-pyridinium]-21H,23H-porphine iron(III) chloride], a peroxynitrite decomposition catalyst, protects the heart against reperfusion injury in mice. J Pharmacol Exp Ther 328:777–784. doi:10.1124/jpet.108.144352
Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S (2002) Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem 234–235:119–124. doi:10.1023/A:1015976430790
Li Y, Arita Y, Koo HC, Davis JM, Kazzaz JA (2003) Inhibition of c-Jun N-terminal kinase pathway improves cell viability in response to oxidant injury. Am J Respir Cell Mol Biol 29:779–783. doi:10.1165/rcmb.2003-0087RC
Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5:331–342. doi:10.1038/nri1594
Loukili N, Rosenblatt-Velin N, Li J, Clerc S, Pacher P, Feihl F, Waeber B, Liaudet L (2011) Peroxynitrite induces HMGB1 release by cardiac cells in vitro and HMGB1 upregulation in the infarcted myocardium in vivo. Cardiovasc Res 89:586–594. doi:10.1093/cvr/cvq373
Michel MC, Li Y, Heusch G (2001) Mitogen-activated protein kinases in the heart. Naunyn Schmiedebergs Arch Pharmacol 363:245–266. doi:10.1007/s002100000363
Mihm MJ, Coyle CM, Schanbacher BL, Weinstein DM, Bauer JA (2001) Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc Res 49:798–807. doi:10.1016/S0008-6363(00)00307-2
Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P (2009) Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol 296:H1466–H1483. doi:10.1152/ajpheart.00795.2008
Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C (2003) Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation 107:896–904. doi:10.1161/01.CIR.0000048192.52098.DD
Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279:7370–7377. doi:10.1074/jbc.M306793200
Rui T, Cepinskas G, Feng Q, Kvietys PR (2003) Delayed preconditioning in cardiac myocytes with respect to development of a proinflammatory phenotype: role of SOD and NOS. Cardiovasc Res 59:901–911. doi:10.1016/S0008-6363(03)00502-9
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195. doi:10.1038/nature00858
Shen HM, Liu ZG (2006) JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med 40:928–939. doi:10.1016/j.freeradbiomed.2005.10.056
Singal PK, Siveski-Iliskovic N, Kaul N, Sahai M (1992) Significance of adaptation mechanisms in adriamycin induced congestive heart failure. Basic Res Cardiol 87:512–518. doi:10.1007/BF00788661
Takemura G, Fujiwara H (2007) Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49:330–352. doi:10.1016/j.pcad.2006.10.002
Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR (2005) The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 201:1135–1143. doi:10.1084/jem.20042614
van Beijnum JR, Buurman WA, Griffioen AW (2008) Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 11:91–99. doi:10.1007/s10456-008-9093-5
Volz HC, Laohachewin D, Seidel C, Lasitschka F, Keilbach K, Wienbrandt AR, Andrassy J, Bierhaus A, Kaya Z, Katus HA, Andrassy M (2012) S100A8/A9 aggravates post-ischemic heart failure through activation of RAGE-dependent NF-kappaB signaling. Basic Res Cardiol 107:1–16. doi:10.1007/s00395-012-0250-z
Volz HC, Seidel C, Laohachewin D, Kaya Z, Muller OJ, Pleger ST, Lasitschka F, Bianchi ME, Remppis A, Bierhaus A, Katus HA, Andrassy M (2010) HMGB1: the missing link between diabetes mellitus and heart failure. Basic Res Cardiol 105:805–820. doi:10.1007/s00395-010-0114-3
Xiao J, Moon M, Yan L, Nian M, Zhang Y, Liu C, Lu J, Guan H, Chen M, Jiang D, Jiang H, Liu PP, Li H (2012) Cellular FLICE-inhibitory protein protects against cardiac remodelling after myocardial infarction. Basic Res Cardiol 107:1–21. doi:10.1007/s00395-011-0239-z
Xu H, Su Z, Wu J, Yang M, Penninger JM, Martin CM, Kvietys PR, Rui T (2010) The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipopolysaccharide-induced myocardial dysfunction via a TLR4/phosphatidylinositol 3-kinase gamma pathway. J Immunol 184:1492–1498. doi:10.4049/jimmunol.0902660
Xu H, Yao Y, Su Z, Yang Y, Kao R, Martin CM, Rui T (2011) Endogenous HMGB1 contributes to ischemia-reperfusion-induced myocardial apoptosis by potentiating the effect of TNF-α/JNK. Am J Physiol Heart Circ Physiol 300:H913–H921. doi:10.1152/ajpheart.00703.2010
Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107:11942–11947. doi:10.1073/pnas.1003893107
Zhu W, Soonpaa MH, Chen H, Shen W, Payne RM, Liechty EA, Caldwell RL, Shou W, Field LJ (2009) Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation 119:99–106. doi:10.1161/CIRCULATIONAHA.108.799700
Acknowledgments
The study was supported by Grants from heart and stroke foundation of Ontario (NA-6316) and the Jiangsu Provincial Foundation for Creative Talents.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2012_267_MOESM1_ESM.tif
Supplementary Fig. 1 Transfection of cardiomyocytes with HMG1 shRNA plasmid resulted in a decrease in cardiomyocyte HMGB1 and prevented the DOX-indued increase in myocyte HMGB1. Cardiomyocytes were transfected with HMG1 shRNA plasmid or shRNA negative control plasmid. Subsequently, the myocytes were incubated with medium with or without doxorubicin (DOX, 0.5 µM). The HMGB1 levels in cardiomyocytes were evaluated with Western blot. Transfection cardiomyocytes with the HMG1 shRNA plasmid inhibited HMGB1 expression in cardiomyocytes. n=3. *P < 0.05 compared with negative control without DOX group, # P < 0.05 compared with negative control+DOX group. (TIFF 44693 kb)
395_2012_267_MOESM2_ESM.jpg
Supplementary Fig.2 Increase in peroxynitrite has been detected in myocytes deficient in JNK1 and myocardium of JNK deficient mice. a. Cardiomyocytes derived from wild type or JNK1-/- mice were treated with DOX (0.5 μM), the control myocytes were treated with vehicle. Myocyte peroxynitrite production was measures with DHR123. The peroxynitrite production was no statistical difference between wild type myocytes and JNK1-/- myocytes after DOX treatment. n=3, *P<0.05 compared to respective control; b. Wild type and JNK1-/- mice were administrated with DOX (10 mg/kg) or vehicle. Mouse hearts were harvested 5 days after the DOX and myocardial peroxynitrite was determined with Western blot by antibody againsts nitrotyrosine. The myocardial nitrotyrosine was no statistical difference between myocardium of wild type mice and those of JNK1-/- mice after DOX treatment. n=5, *P <0.05 compared to respective control. (JPEG 38 kb)
395_2012_267_MOESM3_ESM.jpg
Supplementary Fig.3Wild type, TLR4-/- and JNK1-/- mice were administrated (i.p.) with either vehicle or DOX. FeTPPS (10mg/kg, i.p.) were given to DOX mice every other day starting 1 hour before the administration of DOX for inhibition of ONOO-. A-box (20mg/kg) was given to DOX mice every other days starting 4 hrs after the DOX treatment for inhibition of HMGB1. The heart rate (HR), left ventricle end systolic volume, end diastolic volume, left-ventricular end systolic pressure (LVESP), left-ventricular end diastolic pressure (LVEDP) and ±dP/dt were measured 5 days after the administration of DOX with a pressure-volume loop analysis system. n=5. *P <0.05 compared with control, #P <0.05 compared with wild type mice with DOX. (JPEG 190 kb)
Rights and permissions
About this article
Cite this article
Yao, Y., Xu, X., Zhang, G. et al. Role of HMGB1 in doxorubicin-induced myocardial apoptosis and its regulation pathway. Basic Res Cardiol 107, 267 (2012). https://doi.org/10.1007/s00395-012-0267-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0267-3