Abstract
Receptor for advanced glycation end products (RAGE) and connective tissue growth factor (CTGF) play a key role in diabetic myocardial fibrosis, and peroxisome proliferator-activated receptor-γ (PPAR-γ) activation has been reported to reduce RAGE and CTGF expression. This study investigated the effects of the PPAR-γ agonist, rosiglitazone, on myocardial expression of RAGE and CTGF, extent of cardiac fibrosis, and left ventricular (LV) diastolic function in type 2 diabetic (T2D) rats. Twenty-week-old T2D rats were randomized to treatment with either 20 weeks of rosiglitazone (20 mg/kg) or saline (n = 10 in each group). Serial echocardiographic examinations were performed just before randomization (20 weeks) and at study completion (40 weeks). Fibrosis extent and RAGE and CTGF expression were assessed in previously imaged hearts by picrosirius red staining, and by real-time reverse transcriptase-coupled polymerase chain reaction (RT-PCR) and immunoblotting, respectively. Results of the latter assessments were further validated by immunohistochemical staining. Rosiglitazone treatment significantly improved E/A ratio in serial echocardiography assessment, and reduced LV collagen volume fraction as demonstrated by picrosirius red staining. Real-time RT-PCR and immunoblots of myocardial tissue from rosiglitazone-treated rats revealed reduced RAGE and CTGF mRNA and protein signals compared to those of saline-treated T2D rats, which were consistent with reduced proportions of myocardial RAGE and CTGF staining in the hearts of T2D rats. PPAR-γ agonist therapy reduces cardiac fibrosis and improves LV diastolic dysfunction as assessed by serial echocardiographic imaging. Suppression of RAGE and CTGF expression in the diabetic myocardium appears to contribute to the antifibrotic effect of rosiglitazone. These results support the potential of PPAR-γ agonists as antifibrotic agents in diabetic cardiomyopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial fibrosis, involving interstitial fibrosis and perivascular fibrosis, is a major component of pathological changes in diabetic cardiomyopathy [1, 3, 22, 23, 25]; it initially contributes to left ventricular (LV) diastolic dysfunction, subsequently to systolic dysfunction, and finally to congestive heart failure [2, 10, 22, 32]. A short period of hyperglycemia induces definitive remodeling, mainly involving the cellular compartment, of the LV myocardium [26], and sustained hyperglycemia inhibits Sonic hedgehog-induced monocyte chemotaxis [9], may reduce metalloproteinase activity (collagen degradation) and increase accumulation of collagen, which results in myocardial stiffness and impaired contractility in the diabetic heart [1, 30].
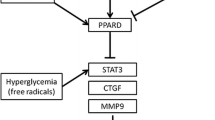
Connective tissue growth factor (CTGF) and the receptor for advanced glycation end products (RAGE) are expressed at significantly higher levels in association with the fibrotic process in the diabetic heart [6]. CTGF is a profibrotic cytokine of the CCN family of immediate early genes, and it is one of the earliest growth factors transcriptionally induced in cardiac myocytes by hypertrophic stimuli [4, 13]. A recent study demonstrated that CTGF secreted by cardiomyocytes induced collagen production in cardiac fibroblasts in vitro, and cardiomyocyte CTGF expression correlated with excessive interstitial fibrosis and diastolic heart failure in vivo, which suggests that CTGF plays a key role in the process of cardiac fibrosis [16]. RAGE upregulates CTGF through interaction with AGE in cultured human dermal fibroblasts and mesangial cells [28, 29], and recent studies have shown that RAGE is involved through its activity on CTGF expression in myocardial remodeling and fibrosis [6, 8, 24].
Many cytokines including CTGF and RAGE contribute to myocardial fibrosis in various animal models, and although many researchers have tried to find a way to reduce myocardial fibrosis by direct antagonism and inhibition of cytokines [5, 11, 19, 25], findings could not be translated into clinical applications and did not provide further insight into the mechanisms for myocardial fibrosis suppression. Peroxisome proliferator-activated receptor (PPAR)-γ agonists inhibit CTGF expression in cardiac fibroblast [17] and attenuate angiotensin II-induced cardiac fibrosis by inhibiting myocardial macrophage infiltration [5]. PPAR-γ agonists have also been shown to inhibit RAGE expression in vascular endothelial cells and vascular smooth muscle cells [18]. Moreover, PPAR-γ agonists have been tested as potential therapeutic agents in the suppression of collagen synthesis in the lung and liver, both in vitro and in vivo [12, 20]. However, the effects of PPAR-γ agonists on myocardial CTGF and RAGE expression have not been defined, and few studies have investigated whether PPAR-γ activation can attenuate cardiac fibrosis in overt diabetes in vivo. We posited that PPAR-γ agonists may inhibit the expression of RAGE and CTGF in association with attenuation of myocardial fibrosis in the diabetic heart, and utilized serial echocardiography to examine LV diastolic function alterations induced by rosiglitazone, as a pure PPAR-γ agonist, while analyzing myocardial RAGE and CTGF expression to address the effects of PPAR-γ activation in the hearts of T2D rats.
Methods and materials
Experimental animals
Twenty male Otsuka Long–Evans Tokushima Fatty (OLETF) rats were used for the T2D rat model, while ten male Long–Evans Tokushima Otsuka (LETO) rats were used for the matched nondiabetic controls. The starting age was 5 weeks. The Tokushima Research Institute, Otsuka Pharmaceuticals (Tokushima, Japan), kindly provided all of the OLETF and LETO rats. The rats were caged individually and received normal rat chow and tap water in a temperature-controlled environment under a 12-h artificial light and dark cycle. The animals were cared for in accordance with our institution’s animal facility guidelines, and all the protocols were approved by the Animal Care and Use Committee of the College of Medicine, the Catholic University of Korea.
Experimental protocol
At the age of 20 weeks, the 20 OLETF rats were equally divided into the treatment group (RT-OLETF, n = 10) and the non-treatment group (UT-OLETF, n = 10), and LETO rats (LETO, n = 10) were used only as nondiabetic controls. The OLETF rats in the treatment group received rosiglitazone (20 mg/kg per day, GlaxoSmithKline, Worthing, West Sussex, UK) through a gavage tube for 20 weeks from the age of 20 to 40 weeks. The same volume of saline was administered, using a gavage tube, to the OLETF rats in the non-treatment group and to the LETO rats. Every week, the dose of rosiglitazone was recalculated based on weights measured after 8 h of fasting.
Weight, hemodynamic data and intraperitoneal glucose tolerance test (IP-GTT)
In all 20-week-old rats, at rest immediately prior to drug administration, weight, blood pressure and heart rate were measured using a tail-cuff plethysmography (BP-2000, Visitech system, Apex, NC, USA) method after 12 h of fasting. At the age of 40 weeks, weight, blood pressure and heart rate were measured using the same method. IP-GTT was performed around 8 o’clock in the morning after a 12-h fast. Twenty-five percent glucose solution, at a dose of 2 g/kg, was administered by intraperitoneal injection. During fasting states, and at 30, 60, 90, and 120 min after glucose loading, blood was drawn from the tail vein, and serum glucose level was measured using the Beckman Glucose Analyzer (Beckman instrument Co., Palo Alto, CA, USA).
Echocardiogram
At 20 and 40 weeks of age, transthoracic Doppler echocardiography was performed with the Sequoia 256 equipment and a 15 MHz phased-array transducer (Acuson, Sequoia 256, Mountain View, CA, USA). After the rats were anesthetized, the left side of the chest was shaved to gain a clear image. All rats had their serial echocardiography done in the left lateral decubitus position, including measurements for LV end-systolic dimension (LVESD), LV end-diastolic dimension (LVEDD) and LV Fractional Shortening [FS (%)]. Using a pulsed wave Doppler, early diastolic peak velocity (E velocity) of the LV inflow, late diastolic peak velocity (A velocity), deceleration time (DT) and LV isovolumic relaxation time (IVRT) were measured as indices of LV diastolic function. Mean values of three measurements were used in analyses.
Histopathology
After killing at 40 weeks of age, rats were perfused with saline via the left ventricle, after which the LV was isolated and cut into three pieces perpendicular to the long axis of the heart. The middle part of heart including the papillary muscle was fixed with 10% formalin for 24 h, embedded in paraffin and cut into 5-μm sections for collagen-specific picrosirius red staining and immunohistochemical staining. The remaining heart tissue segments were snap-frozen for subsequent RT-PCR and Western blot analyses.
Immunohistochemistry was performed for CTGF (rabbit polyclonal antibody to CTGF; Abcam Inc., Cambridge, MA, USA) and RAGE (rabbit polyclonal antibody to RAGE; Santa Cruz Biotechnology, Santa Cruz, CA, USA), using the avidin–biotin peroxidase method. Briefly, tissue slides treated with 0.3% hydrogen peroxide were incubated for 60 min with a primary antibody, followed by the respective biotinylated secondary antibody. The reaction was visualized with a 3-amino-9-ethyl-carbazol substrate (AEC, Sigma), and counterstained with hematoxylin solution. Adjacent sections treated with non-immune IgG provided controls for antibody specificity.
Quantitative histological measurement of stained myocardium
Stained tissue sections were viewed with a microscope (Olympus AX70 TR62A02, Tokyo, Japan), and images were digitally captured with an Olympus DP70 digital camera (Olympus, Tokyo, Japan). All slides were examined under ×100 and ×200 magnification, and measurements were obtained using the Image pro plus imaging software (version 4.5; Media Cybernetics, Silver Spring, MD, USA). The collagen volume fraction (CVF) [7] was determined as the percentage of positive staining area per total myocardium stained with collagen-specific picrosirius red. CTGF and RAGE stained areas were measured as the percentage of positive staining area per total myocardium in five different randomly selected positions of each section.
Real-time reverse transcriptase-coupled polymerase chain reaction (RT-PCR)
Total RNA was extracted from each LV using the TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA was subjected to reverse transcription using the Onestep RT kit (Invitrogen). Expression of CTGF, RAGE and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was evaluated by real-time PCR using the SYBR Green dye. The primers used were as follows: rat CTGF (sense, 5′-GGCTGGAGAAGCAGAGTCGT-3′ and antisense, 5′-GATGCACTTTTTGCCCTTCTTAA-3′), rat RAGE (sense, 5′-GAATCCTCCCCAATGGTTCA-3′ and antisense 5′-GCCCGACACCGGAAAGT-3′), and rat GAPDH (sense, 5′-GTATGACTCTACCCACGGCAAGT and antisense, 5′-TTCCCGTTGATGACCAGCTT-3′). Results were expressed as an mRNA relative value, which was corrected for GAPDH and obtained by real-time PCR.
Western blot analysis
Cardiac tissue was lysed in RIPA buffer [0.1% SDS, 1% deoxycholate, 1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 10 mM Tris pH7.4, 10% protease inhibitor cocktails (Sigma–Aldrich)] for 30 min at 4°C and then centrifuged at 13,000 rpm at 4°C for 30 min. The supernatant fluid was collected and protein content was quantified using the Bio-Rad Protein assay reagent (Bio-Rad, Regents Park, NSW, Australia). Equal amounts of protein for each sample were separated on 8% sodium dodecylsulfate-polyacrylamide gels and transferred to Immobilon-PVDF membranes (Millipore, Billerica, MA, USA) for 2 h at 4°C, blocked in 5% skim milk (Difco, Sparks, MD, USA) for 30 min at room temperature. Membranes were incubated with mouse anti-RAGE monoclonal antibody (Santa Cruz Biotechnology, CA, USA) overnight at 4°C and washed in Tris-buffered saline with 0.1% Tween-20 (TBST) and incubated for 1 h with the appropriate HRP-conjugated secondary anti-mouse IgG (Santa Cruz Biotechnology, CA, USA). After washing with TBST, antibody binding was detected using an enhanced chemiluminescence (ECL) reagent (Amersham Biosciences/GE Healthcare, Rydalmere, NSW, Australia). Western blotting against β-actin (Santa Cruz Biotechnology) was used as a control to assess for equal protein loading.
Statistics
Statistical analysis was performed using the SPSS software program (Version 11, SPSS, Inc., Chicago, IL, USA), and all measured values were reported as a mean ± SD. Differences in measured values among the multiple groups were analyzed by the analysis of variance with Bonferroni’s multiple comparison tests. For all statistical tests, a P value less than 0.05 was considered statistically significant.
Results
Weight, hemodynamic data and glucose tolerance test
Body weight was greater in both of the OLETF groups compared to the LETO group. In addition, both OLETF groups showed higher baseline and 2-h glucose levels on the IP-GTT than did the LETO group at 20 weeks of age. Body weight in both OLETF groups was significantly increased compared to the LETO group from 20 to 40 weeks of age, regardless of rosiglitazone treatment (p < 0.05). After intraperitoneal glucose loading, 2-h glucose levels were significantly higher in both OLETF groups compared to the LETO group at the age of 40 weeks (p < 0.05), while 2-h glucose levels in rosiglitazone-treated OLETF rats (RT-OLETF) were significantly lower than in the untreated OLETF rats (UT-OLETF) (p < 0.05; Table 1). As shown in Table 2, there were no significant differences in systolic BP, diastolic BP, mean BP or heart rate among the three groups at the age of 20 weeks. However, at 40 weeks of age, systolic BP, in both OLETF groups, was significantly higher than in the LETO group (LETO) (p < 0.05). However, systolic blood pressures in the RT-OLETF and UT-OLETF groups showed no significant differences.
Doppler echocardiography
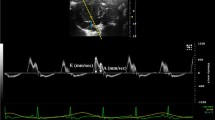
At the age of 20 weeks, all echocardiographic parameters compared among the three comparison groups (Table 3). Echocardiography performed at the age of 40 weeks, after the completion of treatments, revealed that while LVEDS, LVEDD and LV FS (%), as indices of LV systolic function, showed no significant differences among the three comparison groups, Doppler indices of mitral valve flow, reflecting LV diastolic function, showed that the E and A velocities of the UT-OLETF groups were significantly lower than those of the LETO and RT-OLETF groups (p < 0.05). The E/A ratio was 1.7 ± 0.1, 1.5 ± 0.1 and 1.7 ± 0.1 in the LETO, UT-OLEFT and RT-OLETF groups, respectively. The E/A ratio of the RT-OLETF group was significantly higher than that of the UT-OLETF (p < 0.05). In addition, deceleration time (DT) and LV isovolumic relaxation time (IVRT) were significantly decreased in the RT-OLETF group compared to the UT-OLETF group (Table 4; Fig. 1). Therefore, while improvement in LV diastolic dysfunction was clearly observed in rosiglitazone -treated T2D rats compared to untreated T2D rats, there was no improvement in LV systolic function.
Comparison of left ventricular mitral inflow patterns at the age of 40 weeks. The early to late diastolic peak velocity (E/A) ratio was reduced and the deceleration time (DT) of early diastolic inflow was increased in the OLETF rats (middle). These parameters were normalized in the rosiglitazone-treated OLETF rats (right). E early diastolic peak velocity, A late diastolic peak velocity
Myocardial fibrosis
The CVF (%) was calculated quantitatively to assess myocardial fibrosis in each specimen using collagen-specific picrosirius red staining. The extent of myocardial fibrosis in the RT-OLETF group was decreased compared to that of the UT-OLETF group, and was almost the same as that of the LETO group (Fig. 2a). CVF was 2.6 ± 0.8% in the LETO group, 5.7 ± 2.0% in the UT-OLETF group, and 3.2 ± 1.3% in the RT-OLETF group. CVF in the RT-OLETF group was significantly lower than that in the UT-OLETF group (p < 0.001), whereas there was no significant difference with that in the LETO group (Fig. 2b).
a Representative micrographs of myocardium with picrosirius red stain at 40 weeks. Fibrosis was detected by picrosirius red stain that produces a red color. Myocardial fibrosis was greater in the OLETF rat (middle) than in the LETO rat (left) and the rosiglitazone-treated OLETF rat (right). Magnification ×100. b LV collagen volume fraction (CVF) in LETO, untreated OLETF and treated OLETF rats. All values are expressed as mean ± SD. *p < 0.01 versus LETO rats and †p < 0.01 versus OLETF rats
CTGF and RAGE expression
To compare RAGE and CTGF expression in the myocardium among the three groups, expression at both the mRNA and protein levels was examined. Compared with the LETO group, mRNA levels of RAGE and CTGF were significantly increased in the UT-OLETF group, while rosiglitazone significantly reduced mRNA expression of CTGF and RAGE in the myocardium of T2D rats. Consistent with the latter mRNA findings, increased CTGF and RAGE protein expression was detected in the LV of T2D rats (OLETF) compared with controls. In the LV of untreated T2D rats, CTGF immunostaining localized predominantly to interstitial spaces and endothelial cells, while RAGE immunostaining localized predominantly to the interstitial spaces of the LV, endothelial cells and media areas of blood vessels. Therefore, treatment with rosiglitazone in diabetic rats was associated with a significant reduction in CTGF and RAGE expression assessed by RT-PCR, Western blotting and immunohistochemical staining (Figs. 3, 4, 5, 6).
a Immunohistochemical staining for CTGF in LV sections (1–4) and perivascular structure (5–8) from the negative control (1, 5), LETO (2, 6), OLETF (3, 7), and rosiglitazone-treated OLEFT (4, 8) rats. Antibodies were detected by the diaminobenzidine method that produces a brown color (arrow). Counterstaining of nuclei with hematoxylin (blue). Magnification, ×200. b LV CTGF staining (%) in LETO, untreated OLETF and treated OLETF rats. All values are expressed as mean ± SD. *p < 0.01 versus LETO rats and †p < 0.01 versus OLETF rats
a Immunohistochemical staining for RAGE in LV sections (1–4) and perivascular structure (5–8) from the negative control (1, 5), LETO (2, 6), OLETF (3, 7) and rosiglitazone-treated OLETF (4, 8) rats. Antibodies were detected by the diaminobenzidine method that produces a brown color (arrow). Counterstaining of nuclei with hematoxylin (blue). Magnification, ×200. b. LV RAGE staining (%) in LETO, untreated OLETF and treated OLETF rats. All values are expressed as mean ± SD. *p < 0.01 versus LETO rats and †p < 0.01 versus OLETF rats
Real-time RT-PCR for CTGF (a) and RAGE mRNA (b). Rosiglitazone also significantly suppressed the expression of CTGF and RAGE mRNA. The mRNA values are shown as a relative value, corrected for GAPDH obtained by real-time polymerase chain reaction. All values are expressed as mean ± SD. *p < 0.001 versus LETO rats and †p < 0.001 versus OLETF rats
Western blot for CTGF (a) and RAGE (b). The upper band represents CTGF (a) and RAGE (b), while the lower bands represent the loading control (β-actin). Rosiglitazone also significantly suppressed the expression of CTGF and RAGE. All values are expressed as mean ± SD. *p < 0.001 versus LETO rats and †p < 0.001 versus OLETF rats
Discussion
This study demonstrates that PPAR-γ activation in the myocardium of T2D rats reduces myocardial fibrosis in association with lower RAGE and CTGF expression, and that the PPAR-γ agonist rosiglitazone improves LV diastolic dysfunction in experimental diabetic cardiomyopathy. Serial echocardiographic imaging clearly showed the development of age-related diastolic dysfunction in diabetic rats and the improvement of such dysfunction after PPAR-γ agonist therapy.
Forty-week-old diabetic rats showed significantly increased myocardial fibrosis and expression of RAGE and CTGF in their hearts, which results in impaired LV diastolic function. In contrast to the study by Mizushige et al. [21], in which OLETF rats already showed signs of myocardial fibrosis and LV diastolic dysfunction in the prediabetic period, in our study, while diabetes and mild obesity developed in most OLETF rats, diastolic function was not significantly different among three groups at the age of 20 weeks. However, at the age of 40 weeks, all indices of LV diastolic function were different among three groups, and rosiglitazone significantly improved LV diastolic function in OLETF rats. These findings are consistent with the results previously reported by Kim et al. [14].
CTGF is a novel and potent profibrotic factor. CTGF expression is increased in the hypertrophied and failing heart, and CTGF production from cardiac myocytes induces collagen production in cardiac fibroblasts [16]. CTGF has been shown to induce extracellular matrix synthesis and accumulation of collagen by direct stimulation of fibroblasts in response to TGF-β1 [23]. CTGF also mediates cardiac fibrosis and dysfunction independently from TGF-β1 activation in transgenic mice over-expressing protein kinase C-2 [31]. Diabetic hearts have been shown to express significantly higher levels of AGEs, RAGE and CTGF [6]. In addition, AGEs have been reported to upregulate CTGF expression through interaction with RAGE in cultured human dermal fibroblasts and mesangial cells [28, 29]. Our study also showed that CTGF and RAGE were expressed at significantly higher levels in the myocardial interstitium and endothelial cells in association with significantly higher extents of myocardial fibrosis in 40-week-old OLETF rats. Taken together, the increased expression and robust interactions of AGE, RAGE and CTGF are thought to play an important role in the development of diabetes-related cardiac fibrosis.
Our study revealed that PPAR-γ activation in diabetic rats could suppress both myocardial RAGE and CTGF expression and fibrosis. In untreated OLETF rats, RAGE and CTGF proteins were expressed at significantly higher levels in the myocardial interstitium and endothelial cells. Rosiglitazone treatment significantly suppressed myocardial expression of RAGE and CTGF. RAGE stimulates the release of profibrotic growth factors including CTGF in the diabetic environment [26]. Recently, PPAR-γ agonists have been reported to down-regulate basal as well as TNF-α-induced RAGE expression in endothelial cells and mesangial cells and to subsequently inhibit AGE-induced MCP-1 expression [18, 19]. Since RAGE is upregulated by NF-κB activation [27] and PPAR-γ agonists reduce RAGE expression by inhibiting NF-κB expression in endothelial and mesangial cells, we speculated that rosiglitazone might suppress RAGE expression by inhibiting NF-κB activation in the myocardium, resulting in reduced CTGF expression [18, 19]. Although PPAR-γ agonists have been reported to inhibit RAGE and CTGF expression in vitro and in the glomeruli of rat kidneys, and to suppress CTGF in cardiac fibroblasts, no prior studies have defined the effect of PPAR-γ agonists on RAGE and CTGF expression in the myocardium of overt diabetic animals. Several studies have shown an association between PPAR-γ agonist treatment and extent of myocardial fibrosis. Yao et al. [33] was the first to report that a PPAR-γ agonist could reduce myocardial fibrosis in diabetic rats. In their T2D rat model study, however, the PPAR-γ agonist was administered from the age of 5 weeks, prior to the development of overt diabetes. This experimental design might confuse interpretations of the effects of the PPAR-γ agonist on the antifibrotic mechanisms in the diabetic heart because the PPAR-γ agonist has been reported to effectively prevent or delay diabetes development in diabetic rats [15]. In contrast with Yao’s report, we used a rat model with overt diabetes. Recently, Caglayan et al. [5] reported that PPAR-γ ligands attenuate angiotensin II-induced cardiac fibrosis by inhibiting myocardial macrophage infiltration. Our study further confirmed PPAR-γ agonist-mediated suppression of cardiac fibrosis, especially in diabetes-related fibrosis in vivo and also showed that PPAR-γ agonist treatment can lower myocardial expression of RAGE and CTGF, which may be involved in the process of diabetes-related cardiac fibrosis.
This study has several limitations. First, the reduction of myocardial fibrosis and RAGE expression in rosiglitazone-treated diabetic rats may result from better glycemic control with associated decreased AGE production. However, since RAGE has been reported to act independently of AGEs in cardiac remodeling [6, 24], RAGE expression status seems to be more relevant to myocardial fibrosis. Studies using heart-specific PPAR-γ knockout mice are needed to address whether the improvements in diastolic dysfunction are mediated through a direct action of rosiglitazone on the myocardial tissue or through its improvement of the metabolic milieu. Second, we did not investigate the causality between CTGF and RAGE expression reduction and decreased cardiac fibrosis after rosiglitazone treatment. Marx et al. [18] reported that the strong anti-inflammatory effect of rosiglitazone is achieved by inhibiting NF-κB activation and consequent suppression of TNF-α-induced endothelial expression of RAGE. Further studies are required to elucidate such causality.
In this study, serial echocardiographic examinations clearly showed that rosiglitazone improved LV diastolic dysfunction, an early manifestation of diabetic cardiomyopathy, which was histologically correlated to reduced cardiac fibrosis and suppression of myocardial CTGF and RAGE expression in the diabetic hearts. These results may be helpful to elucidate the mechanism of diabetic cardiomyopathy or other cardiac structural changes caused by diabetes, and support PPAR-γ activation as a potential approach to improve diastolic dysfunction caused by diabetes.
References
Asbun J, Villarreal FJ (2006) The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol 47:693–700
Bauters C, Lamblin N, McFadden EP, Van Belle E, Millaire A, de Groote P (2003) Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc Diabetol 2:1–16
Bell DS (1995) Diabetic cardiomyopathy. A unique entity or a complication of coronary artery disease? Diabetes Care 18:708–714
Blom IE, Goldschmeding R, Leask A (2002) Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol 21:473–482
Caglayan E, Stauber B, Collins AR, Lyon CJ, Yin F, Liu J, Rosenkranz S, Erdmann E, Peterson LE, Ross RS, Tangirala RK, Hsueh WA (2008) Differential roles of cardiomyocyte and macrophage peroxisome proliferator-activated receptor gamma in cardiac fibrosis. Diabetes 57:2470–2479
Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM (2003) A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res 92:785–792
Cingolani HE, Rebolledo OR, Portiansky EL, Pérez NG, Camilión de Hurtado MC (2003) Regression of hypertensive myocardial fibrosis by Na(+)/H(+) exchange inhibition. Hypertension 41:373–377
Cooper ME (2004) Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease. Am J Hypertens 17:31S–38S
Dunaeva M, Voo S, van Oosterhoud C, Waltenberger J (2009) Sonic hedgehog is a potent chemoattractant for human monocytes: diabetes mellitus inhibits Sonic hedgehog-induced monocyte chemotaxis. Basic Res Cardiol doi:10.1007/s00395-009-0047-x
El-Omar MM, Yang ZK, Phillips AO, Shah AM (2004) Cardiac dysfunction in the Goto-Kakizaki rat. A model of type II diabetes mellitus. Basic Res Cardiol 99:133–141
Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, Lonning S, Ling H, Ertl G, Bauersachs J (2008) Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol 103:485–492
Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A (2002) Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology 122:1924–1940
Kemp TJ, Aggeli IK, Sugden PH, Clerk A (2004) Phenylephrine and endothelin-1 upregulate connective tissue growth factor in neonatal rat cardiac myocytes. J Mol Cell Cardiol 37:603–606
Kim SK, Zhao ZS, Lee YJ, Lee KE, Kang SM, Choi D, Lim SK, Chung N, Lee HC, Cha BS (2003) Left-ventricular diastolic dysfunction may be prevented by chronic treatment with PPAR-alpha or -gamma agonists in a type 2 diabetic animal model. Diabetes Metab Res Rev 19:487–493
Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, Youn JH, Lee KU (2003) Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: comparison with PPAR-gamma activation. Diabetes 52:2331–2337
Koitabashi N, Arai M, Kogure S, Niwano K, Watanabe A, Aoki Y, Maeno T, Nishida T, Kubota S, Takigawa M, Kurabayashi M (2007) Increased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosis. Hypertension 49:1120–1127
Li J, Liu NF, Wei Q (2008) Effect of rosiglitazone on cardiac fibroblast proliferation, nitric oxide production and connective tissue growth factor expression induced by advanced glycation end-products. J Int Med Res 36:329–335
Marx N, Walcher D, Ivanova N, Rautzenberg K, Jung A, Friedl R, Hombach V, de Caterina R, Basta G, Wautier MP, Wautiers JL (2004) Thiazolidinediones reduce endothelial expression of receptors for advanced glycation end products. Diabetes 53:2662–2668
Matsui T, Yamagishi S, Takeuchi M, Ueda S, Fukami K, Okuda S (2009) Nifedipine, a calcium channel blocker, inhibits advanced glycation end product (AGE)-elicited mesangial cell damage by suppressing AGE receptor (RAGE) expression via peroxisome proliferator-activated receptor-gamma activation. Biochem Biophys Res Commun 385:269–272
McGowan SE, Jackson SK, Doro MM, Olson PJ (1997) Peroxisome proliferators alter lipid acquisition and elastin gene expression in neonatal rat lung fibroblasts. Am J Physiol 273:L1249–L1257
Mizushige K, Yao L, Noma T, Kiyomoto H, Yu Y, Hosomi N, Ohmori K, Matsuo H (2000) Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of a type II diabetic rat model. Circulation 101:899–907
Olivetti G, Melissari M, Balbi T, Quaini F, Sonnenblick EH, Anversa P (1994) Myocyte nuclear and possible cellular hyperplasia contribute to ventricular remodeling in the hypertrophic senescent heart in humans. J Am Coll Cardiol 24:140–149
Pathak M, Sarkar S, Vellaichamy E, Sen S (2001) Role of myocytes in myocardial collagen production. Hypertension 37:833–840
Pugliese G, Pricci F, Leto G, Amadio L, Iacobini C, Romeo G, Lenti L, Sale P, Gradini R, Liu FT, Di Mario U (2000) The diabetic milieu modulates the advanced glycation end product-receptor complex in the mesangium by inducing or upregulating galectin-3 expression. Diabetes 49:1249–1257
Sakata Y, Chancey AL, Divakaran VG, Sekiguchi K, Sivasubramanian N, Mann DL (2008) Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res Cardiol 103:60–68
Stilli D, Lagrasta C, Berni R, Bocchi L, Savi M, Delucchi F, Graiani G, Monica M, Maestri R, Baruffi S, Rossi S, Macchi E, Musso E, Quaini F (2007) Preservation of ventricular performance at early stages of diabetic cardiomyopathy involves changes in myocyte size, number and intercellular coupling. Basic Res Cardiol 102:488–499
Sun C, Liang C, Ren Y, Zhen Y, He Z, Wang H, Tan H, Pan X, Wu Z (2009) Advanced glycation end products depress function of endothelial progenitor cells via p38 and ERK 1/2 mitogen-activated protein kinase pathways. Basic Res Cardiol 104:42–49
Twigg SM, Cao Z, McLennan SV, Burns WC, Brammar G, Forbes JM, Cooper ME (2002) Renal connective tissue growth factor induction in experimental diabetes is prevented by aminoguanidine. Endocrinology 143:4907–4915
Twigg SM, Chen MM, Joly AH, Chakrapani SD, Tsubaki J, Kim HS, Oh Y, Rosenfeld RG (2001) Advanced glycosylation end products up-regulate connective tissue growth factor (insulin-like growth factor-binding protein-related protein 2) in human fibroblasts: a potential mechanism for expansion of extracellular matrix in diabetes mellitus. Endocrinology 142:1760–1769
Van Linthout S, Seeland U, Riad A, Eckhardt O, Hohl M, Dhayat N, Richter U, Fischer JW, Böhm M, Pauschinger M, Schultheiss HP, Tschöpe C (2008) Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol 103:319–327
Way KJ, Isshiki K, Suzuma K, Yokota T, Zvagelsky D, Schoen FJ, Sandusky GE, Pechous PA, Vlahos CJ, Wakasaki H, King GL (2002) Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes 51:2709–2718
Westermann D, Van Linthout S, Dhayat S, Dhayat N, Schmidt A, Noutsias M, Song XY, Spillmann F, Riad A, Schultheiss HP, Tschöpe C (2007) Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol 102:500–507
Yao L, Mizushige K, Noma T, Murakami K, Ohmori K, Matsuo H (2000) Troglitazone decreases collagen accumulation in prediabetic stage of a type II diabetic rat model. Heart 84:209
Acknowledgments
This work was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD)” (KRF-2005-E00093), in which main calculations were performed by using the supercomputing resource of the Korea Institute of Science and Technology Information (KISTI).
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ihm, SH., Chang, K., Kim, HY. et al. Peroxisome proliferator-activated receptor-γ activation attenuates cardiac fibrosis in type 2 diabetic rats: the effect of rosiglitazone on myocardial expression of receptor for advanced glycation end products and of connective tissue growth factor. Basic Res Cardiol 105, 399–407 (2010). https://doi.org/10.1007/s00395-009-0071-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-009-0071-x