Abstract
Background
The aim of the present work was to study the profile of the rapid delayed rectifier potassium current (I Kr) and the inward rectifier potassium current (I K1) during ventricular repolarization as a function of action potential duration and rate of repolarization.
Methods
Whole cell configuration of the patch clamp technique was used to monitor I Kr and I K1 during the action potential plateau and terminal repolarization. Action potentials recorded at various cycle lengths (0.4–5 s) and repolarizing voltage ramps having various slopes (0.5–3 V/s) were used as command signals. I Kr and I K1 were identified as difference currents dissected by E-4031 and BaCl2, respectively.
Results
Neither peak amplitudes nor mean values of I Kr and I K1 recorded during the plateau of canine action potentials were influenced by action potential duration. The membrane potential where I Kr and I K1 peaked during the terminal repolarization was also independent of action potential duration. Similar results were obtained in undiseased human ventricular myocytes, and also in canine cells when I Kr and I K1 were evoked using repolarizing voltage ramps of various slopes. Action potential voltage clamp experiments revealed that the peak values of I Kr, I K1, and net outward current during the terminal repolarization were independent of the pacing cycle length within the range of 0.4 and 5 s.
Conclusions
The results indicate that action potential configuration fails to influence the amplitude of I Kr and I K1 during the ventricular action potential in dogs and humans, suggesting that rate-dependent changes in action potential duration are not likely related to rate-dependent alterations in I Kr or I K1 kinetics in these species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The course of repolarization is set by the interplay between different voltage- and time-dependent currents. The contribution of each current strictly depends on the relation between its kinetic properties and the voltage profile during the electrical cycle, which varies with heart rate. Accordingly, action potential duration (APD) is rate-dependent: APD lengthens when the cycle length increases. Rate-dependent kinetic properties of several ion currents, including the rapid delayed rectifier potassium current (I Kr), were claimed to contribute to the rate-dependent nature of APD, however, the exact mechanism still remains to be elucidated. For instance, APD was thought to be shortened by the accumulation of I Kr at high pacing frequencies due to its slow deactivation kinetics [6]. Furthermore, previous results in guinea pig myocytes suggested that shorter repolarization causes I Kr to increase due to its peculiar gating properties, which in turn, may further shorten repolarization [15]. The inward going rectification of I Kr is attributed to the faster time course of inactivation and recovery from inactivation than that of activation and deactivation [17, 21]. Although based on a different molecular mechanism, inward rectification is also a property of the inward rectifier potassium current (I K1), which may thus behave similarly to I Kr during repolarization. The rapid binding of magnesium ion and polyamines to the channel was proposed to occur at less negative membrane potentials which very rapidly occludes, and thereby inactivates the channels mediating I K1 [11, 12]. Based on the properties outlined above, I Kr slowly activates and consecutively rapidly inactivates after the action potential upstroke. When repolarization proceeds, a large amount of recovery from inactivation can occur allowing I Kr to rise. In the case of I K1, the fast block induced by magnesium ions and polyamines during the action potential plateau becomes relieved quickly upon terminal repolarization resulting in a concomitant increase of the current.
However, data obtained on I Kr in guinea pigs is difficult to extrapolate to dogs and humans because significant differences exist in I Kr gating kinetics and rectification properties between guinea pigs and larger mammals, including dogs and humans [4, 6, 9, 10, 16]. Therefore, and due to the absence of relevant human data, we decided to study the profile of I Kr and I K1 during the canine and human ventricular action potential as a function of APD and rate of repolarization. Furthermore, action potential voltage clamp experiments were performed to study the contribution of I Kr and I K1 to terminal repolarization in a rate-dependent manner. The results indicate that amplitudes of both I Kr and I K1 are independent on APD or steepness of repolarization in canine and human ventricular myocytes, suggesting that rate-dependent changes in APD in these species can hardly be deduced from the kinetic properties of I Kr or I K1.
2 Methods
2.1 Animals
Adult mongrel dogs (8–14 kg) of either sex were used. Following anesthesia (sodium pentobarbital, 30 mg kg−1 i.v.), the hearts were rapidly removed for the purpose of cell isolation. Left ventricular myocytes were enzymatically dissociated from the hearts as described earlier in detail [20]. All experiments were carried out in compliance with the Guide for the Care and Use of Laboratory Animals (USA NIH publication NO 86-23, revised 1985), and the protocols were approved by the local ethical committee (CAR I-74-66/2005).
2.2 Human tissues
Cells were prepared from undiseased donor hearts. The hearts were obtained from general organ donors, their valves were utilized for pulmonary and aortic valve transplantation surgery. Before explantation of the hearts the patients did not receive any medication except for dobutamine, furosemide and plasma expanders. The experimental protocol complied with the ethical standards laid down in the 1964 Declaration of Helsinki and was approved by the Ethical Review Board of the Albert Szent-Györgyi Medical University (No. 51-57/1997 OEj). Proper consent was obtained for use of each individual’s tissue for experimentation. Human left ventricular myocytes were isolated by an enzymatic dissociation procedure as performed previously [9].
2.3 Electrophysiology
One drop of cell suspension was placed into a transparent recording chamber mounted on the stage of an inverted microscope (TMS, Nikon, Tokyo, Japan), and the myocytes were allowed to settle and adhere to the bottom for at least 5 min before superfusion was initiated. Only rod shaped cells with clear cross striations were used. HEPES buffered Tyrode’s solution, gassed with 100% O2, served as the normal superfusate containing (in mM): NaCl 144, NaH2PO4 0.33, KCl 4.0, CaCl2 1.8, MgCl2 0.53, Glucose 5.5, and HEPES 5.0 at pH of 7.4. Patch pipettes were fabricated from borosilicate glass capillaries (Clark, Reading, UK). These electrodes had resistances between 1.5 and 2.5 MΩ when filled with pipette solution containing (in mM): K-aspartate 100, KCl 25, ATP 3, MgCl2 1, EGTA 10 and HEPES 5. The pH of this solution was adjusted to 7.2 by KOH. Nisoldipine (1 µM, gift from Bayer AG, Leverkusen, Germany) and L-735,821 (100 nM, gift from Merck-Sharpe and Dohme, West-Point, PA, USA) were added to the external solution to eliminate L-type calcium current and slow delayed rectifier potassium current, respectively.
Membrane currents were recorded with Axopatch-1D and 200B amplifiers (Axon Instruments, Union City, CA, USA) using the whole cell configuration of the patch clamp technique. After establishing a high (1–10 GΩ) resistance seal by gentle suction, the cell membrane beneath the tip of the electrode was disrupted by suction or by application of short electrical pulses. The series resistance was typically 4–8 MΩ before compensation (50–80%, depending on the voltage protocol). Experiments where the series resistance was high, or substantially increased during measurement, were discarded. Membrane currents were digitized using a 333 kHz analog-to-digital converter (Digidata 1200, Axon Instruments) under software control (pClamp 8, Axon Instruments). Analyses were performed using pClamp 8 software after low-pass filtering at 1 kHz. All data were collected at 37°C.
Three types of voltage protocols were applied in this study. In the first series of experiments (shown in Figs. 1–3) action potentials, delivered to the preparation uniformly at a rate of 0.05 Hz, were used as command voltage pulses. These action potentials were previously collected from canine ventricular papillary muscles paced at a constant cycle length of 0.4, 0.7, 1, 2, 3, or 5 s using conventional microelectrode techniques [3]. In a further series of experiments (presented in Fig. 5) true action potential voltage clamp techniques were applied [5]. In this case the own action potential of each cell (recorded previously in current clamp mode) was applied as a voltage command at the same frequency as it was collected [1]. Finally, I Kr and I K1 were also recorded using repolarizing voltage ramps with slopes varying from 0.5 to 3 V/s (shown in Fig. 4). These ramps were preceded by depolarizations to +20 mV for 150 ms arising from the holding potential of −80 mV. In all experiments I Kr and I K1 were identified as difference currents, dissected by 1 µM E-4031 (selective blocker of I Kr) and by either 10 or 50 µM BaCl2 (selective blocker of I K1) respectively. The selectivity of BaCl2 against I Kr was tested in five canine ventricular cells: BaCl2 caused no significant alteration in I Kr tail current amplitudes (values of 65.1 ± 10.1, 62.6 ± 12.8, and 64.1 ± 14.6 pA were obtained in control, in the presence of 10 and 50 µM BaCl2, respectively).
E-4031-sensitive current (I Kr) recorded from canine ventricular myocytes during action potentials evoked at various frequencies. Transmembrane currents (a, bottom) were elicited by a series of action potentials (a, top) recorded in a previous experiment at pacing cycle lengths of 0.4, 0.7, 1, 2, 3, and 5 s, respectively, using conventional microelectrodes. Vertical dashed lines indicate temporal coincidence. Dotted lines indicate zero voltage and current levels. b I Kr current traces (taken from panel a) superimposed by horizontal shifting so that their peak values coincided. C I Kr current–voltage relationships obtained with the shortest and longest action potentials (evoked at a cycle length of 0.4 and 5 s, respectively). The momentary current was plotted against the respective isochronal membrane potential during repolarization. d Peak I Kr values (open symbols), mean I Kr values (obtained as a ratio of I Kr integral and action potential duration, filled circles), and mid-plateau I Kr values (I Kr levels measured at half-duration of the action potential, filled squares) plotted as a function of the pacing cycle length used to evoke the command action potential. e Membrane potentials where peak I Kr values occurred. Symbols and bars represent mean ± SEM values obtained in five myocytes
2.4 Statistics
Results are expressed as mean ± SEM values. Statistical differences were evaluated with ANOVA. Differences were considered significant when P was less than 0.05.
3 Results
The profile of I Kr, flowing during repolarization of canine ventricular action potentials, was studied as a function of APD. In these experiments action potentials, previously collected from canine ventricular papillary muscles at various pacing cycle lengths (0.4, 0.7, 1, 2, 3, and 5 s), were used as command voltage pulses. As expected, action potentials were longer with increasing cycle length. A series of command action potentials and the underlying E-4031-sensitive current traces, considered as I Kr, are presented in Fig. 1a. Although the development of peak I Kr occurred gradually later when APD was longer, the current profiles were similar in shape, i.e. they had practically identical time course (as indicated by Fig. 1b, where the traces were superimposed by horizontal shifting so as their peak values coincided). The current–voltage relationship of I Kr was obtained by plotting the momentary current values against the respective isochronal membrane potentials during repolarization (Fig. 1c). These phase-plane trajectories indicated that the voltage-dependence of I Kr was not affected by the duration of the command action potential: very similar I Kr I–V relationships were obtained with command action potentials evoked at 0.4 and 5 s pacing cycle lengths. The average results obtained from five myocytes are summarized in Fig. 1d, e, indicating that not only the peak value of I Kr (measured during terminal repolarization), but also its mid-plateau value (measured at half-duration of the action potential) as well as the mean value of I Kr (obtained as a ratio of I Kr integral and APD) were little influenced by the duration of the command action potential. Furthermore, the membrane potential where the maximum of I Kr appeared was also independent of the pacing cycle length which was used to evoke the command action potential.
Essentially similar results were obtained when the profile of the Ba2+-sensitive current, considered as I K1, was analyzed in the same manner in seven canine ventricular cells (Fig. 2a–e). Of course, considerable differences were seen between I Kr and I K1 both in their peak amplitudes (0.6 ± 0.08 Vs. 1.4 ± 0.17 pA/pF at 1 Hz, P < 0.05), as well as in the voltage where their peak current appeared (−48.3 ± 2.1 Vs. −64.1 ± 0.7 mV at 1 Hz, P < 0.05), however, the APD-independent behavior was a common feature of both currents.
a BaCl2-sensitive current (I K1) recorded from canine ventricular myocytes during action potentials evoked at various frequencies. The current was dissected by 10 µM BaCl2 using the action potential series identical to that showed in Fig. 1. b Superimposed I K1 current traces. C I K1 current–voltage relationships obtained with action potentials evoked at cycle lengths of 0.4 and 5 s. d and e Peak, mean, and mid-plateau levels of I K1, and membrane potentials where I K1 peaked, shown as a function of the pacing cycle length. Mean data ± SEM were obtained in seven cells
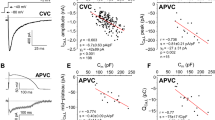
The measurements described above were repeated in ventricular myocytes isolated from three undiseased human donor hearts. As shown by the representative records presented in Fig. 3, the amplitude of I Kr was similar in human and canine myocytes, while peak amplitude of I K1 was approximately three times smaller in human than in the canine cells. In spite of this difference in I K1 amplitude, the shape of both I Kr and I K1, obtained with the same set of action potential waveforms, was similar in the two species. More importantly, the APD-independent nature of peak I Kr and I K1 values was also evident in these human cells—similarly to our findings in canine myocytes. Accordingly, no differences in the current–voltage relationships were seen when comparing I Kr or I K1 currents obtained with the shortest and longest action potentials (evoked at a cycle length of 0.4 and 5 s, respectively).
Representative set of I Kr (a) and I K1 (b) records taken from healthy human ventricular cells. The pulse protocol and experimental conditions were identical to those used in canine myocytes. c and d Set of human I Kr and I K1 records, obtained with action potentials of various durations, superimposed so as to match their peaks. e and f Current–voltage relationships obtained for human I Kr and I K1 using the shortest and longest action potentials, evoked at a cycle length of 0.4 and 5 s, respectively
Since the rate of repolarization was claimed to influence I Kr kinetics in guinea pig ventricular cells [15], I Kr and I K1 profiles during voltage ramps with various rates of repolarization were also examined (Fig. 4). Neither peak amplitudes of I Kr and I K1, nor the currents measured at 0 mV membrane potential were changed with the rate of repolarization. These experiments clearly showed that only the magnitude of membrane potential—but not the rate of its change—influenced the peak amplitude of I Kr (n = 6) and I K1 (n = 9) during repolarization.
Representative set of I Kr (a) and I K1 (c) records obtained in canine ventricular myocytes using voltage ramps with various repolarization rates (0.5, 1, 1.5, 2, 2.5, and 3 V/s). b and d Average peak currents, and current levels measured at 0 mV during the repolarizing ramps plotted against the rate of repolarization. I Kr and I K1 currents were dissected using 1 µM E-4031 and 10 µM BaCl2 in six and nine myocytes, respectively
In the previous experiments we used 10 µM of BaCl2 to dissect I K1. This low concentration was chosen to assure selectivity. However, 10 µM BaCl2 blocks only approximately 70% of I K1 at −60 mV, as it was demonstrated in Xenopus oocytes expressing Kir2.1 channels [18]. In order to directly compare the role of I Kr and I K1 in canine ventricular repolarization, 50 µM BaCl2 (which was shown to block 90% of I K1 at −60 mV) was used in further experiments. In addition, the rate-dependent properties of I Kr and I K1 were studied under action potential clamp conditions. In these experiments the own action potential, recorded previously from the studied cell in current clamp mode, was delivered as a command signal to the same cell (and at the same frequency) under voltage clamp conditions. This approach also allowed determination of the net membrane current from the action potential as the product of the membrane capacitance and the first time derivative of the action potential (I net = −C m × dV/dt). Three important observations were made. Firstly, I Kr peaked 7 ms before I net reached its maximum, while I K1 peaked 1 ms after the maximum of I net (Fig. 5a). Considering this 8 ms time lag and the approximately −2 V/s rate of repolarization at the time of maximum I net, a −16 mV difference in the membrane potential where I Kr and I K1 reach their peak values can be estimated. This is in a fairly good agreement with the values of −48.3 ± 2.1 and −64.1 ± 0.7 mV obtained for peak I Kr and I K1, respectively, when using foreign action potential waveforms. Secondly, neither I Kr nor I K1 was rate-dependent when comparing under action potential voltage clamp conditions (Fig. 5b). And thirdly, the contribution of I Kr and I K1 to I net during terminal repolarization was approximately 25% and 75%, respectively.
a I Kr, I K1, and net membrane current (I net) recorded during terminal repolarization from canine ventricular cells under action potential voltage clamp conditions. I Kr and I K1 were dissected using 1 µM E-4031 and 50 µM BaCl2, respectively, I net was calculated from the action potential. b I Kr, I K1, and I net as a function of the pacing cycle length (0.4, 1, and 5 s) under action potential clamp conditions. Symbols and bars represent mean ± SEM values obtained in six cells
I–V relations obtained for I Kr and I K1 under conventional voltage clamp and action potential voltage clamp conditions are shown in Fig. 6a, b, respectively. In the former case the command action potential, originating from a canine papillary muscle preparation paced at 1 Hz, was delivered at a rate of 0.05 Hz (as was done in Figs. 1–3), while in the latter case the own action potential of the cell was applied as command pulse at a frequency of 1 Hz, which was identical with the pacing frequency (as was done in Fig. 5). No difference was seen between the I–V curves obtained for either I Kr or I K1 under these different experimental conditions.
Superimposed current–voltage relationships obtained for canine I Kr and I K1 at a pacing cycle length of 1 s under conventional voltage clamp conditions using action potentials as command pulses (a) and under true action potential voltage clamp conditions (b). Current amplitudes were normalized according to their peak values for the sake of better comparison
4 Discussion
Rate-dependent properties of action potential duration have been studied for long time and interpreted in terms of the frequency-dependence of the underlying ion currents. In the present work we aimed to study the role of two potassium currents, I Kr and I K1, in canine and human ventricular repolarization in a “rate-dependent” manner. Since both currents show strong inward going rectification, their profiles can hardly be studied under conventional voltage clamp conditions based on application of rectangular voltage pulses. We applied, therefore, action potentials as command pulses combined with pharmacological dissection of the currents. The results clearly showed that I Kr and I K1 were not sensitive to rate-dependent changes in action potential configuration: I Kr and I K1 were not modified by either action potential duration or rate of repolarization—in contrast to I Kr results obtained in guinea pigs [15]. On the other hand, our action potential clamp data are in a good agreement with the results of Gintant [7] obtained in dogs using voltage ramp protocols to mimic the action potential, and are also in line with previous observations obtained in HEK cells expressing HERG channels [2]. All these results indicate that I Kr is not frequency-dependent within a relatively wide range of stimulation frequencies.
It must be noted, however, that the rate-independent behavior of I Kr can be demonstrated only at normal or long pacing cycle lengths. At cycle lengths shorter than 400 ms peak I Kr significantly decreased in canine ventricular cells resulting ultimately in electrical alternans [8]. Similarly, small, statistically not significant reduction in peak I Kr was observed at shorter cycle lengths in our canine cells (shown in Fig. 1d). This may be due to the incomplete activation of I Kr during the concomitantly shorter action potential plateau. However, since activation time constant of 54 ms was obtained for I Kr in dogs [20], close to 90% of I Kr has to be activated in case of an action potential with 150 ms duration, leaving little room for further activation when the action potential becomes longer. Such reduction of I Kr at short cycle lengths was not seen in human myocytes, where the activation of I Kr is faster having an activation time constant of 31 ms [9]. Although the deactivation time constant estimated for I Kr at depolarized membrane potentials was found to be relatively long, a time constant of 34 ms was obtained at −85 mV in canine ventricular cells [8]. This implicates that—except for the extremely short cycle lengths—not much rate-dependent accumulation of this current can be expected in canine myocytes. In contrast to the frequency-independent nature of I K1 demonstrated at cycle lengths longer than 0.4 s in the present study, I K1 was also shown to be modified at very high pacing frequencies [19]. These results suggest that the mechanisms responsible for rate-dependent changes in action potential duration at fast and normal heart rates may involve different mechanisms.
Present experiments have important implications regarding the role of I Kr and I K1 in terminal repolarization. The voltage-dependence of I Kr and I K1 in the voltage range experienced by an action potential can be directly compared by superimposing their I–V relations, as was done in Fig. 6. I Kr was building up gradually from the beginning of repolarization and mediated more current than I K1 at the middle of the plateau. In contrast to I Kr, I K1 began to rise with a significant delay. Since I Kr peaked earlier and at a less negative membrane potential than I K1 did, this increase in I Kr is likely to accelerate repolarization by the “ignition” of I K1. Indeed, the distinct narrow membrane potential ranges where I Kr and I K1 peaked (−48.3 ± 2.1 and −64.1 ± 0.7 mV, respectively) suggest that the increase of both currents during repolarization is governed by voltage rather than time, providing thus a positive feedback control for terminal repolarization. These results may explain why terminal repolarization is not rate-dependent in canine ventricular cells.
As peak values of I Kr and I K1 are responsible for governing terminal repolarization, action potential duration is controlled by membrane currents flowing during the plateau. I Kr and I K1 values measured during the middle segment of the plateau (I Kr mid-plateau and I K1 mid-plateau, respectively), and the mean values of I Kr and I K1 (obtained as a ratio of current integral and action potential duration) were not altered by changing the pacing frequency of the command action potential. Similarly, I Kr and I K1 values measured at 0 mV membrane potential during the repolarizing voltage ramps were independent of the rate of the ramp. Considering these results it may be concluded that I Kr and I K1 have limited chance to induce rate-dependent changes in action potential duration at normal or low heart rates. However, one might argue that since the rise of I Kr was faster during a shorter than a longer action potential (as is evident from Fig. 1a when isochronal I Kr levels are compared), the greater I Kr amplitude at a given time after the upstroke might further accelerate repolarization. This interpretation is unlikely due to the unchanged membrane potential values temporally coinciding with the I Kr or I K1 peaks observed when duration of the command action potential was varied (see Figs. 1 and 2) indicating that the rise of these currents during the action potential plateau is determined predominantly by voltage. If it is so, they can rise faster during a shorter than a longer action potential because the critical membrane potential in the first case can be achieved sooner. Such a behavior is consistent with inward going rectification. In summary, the faster rise of I Kr during a short action potential may be the consequence—rather than the reason—of the steeper repolarization. It appears that canine and human ventricular myocardium differ from that of guinea pig regarding the rate-dependent nature of I Kr.
Since the magnitudes of both I Kr and I K1 are believed to be important determinants of incidence of cardiac arrhythmias [13, 14] it may be worthy to compare these currents in dogs and humans. While the amplitude of I Kr was similar in the two species, peak I K1 was smaller in human than in canine myocytes. Apart from this difference, the voltage-dependent and rate-independent properties of I Kr and I K1 were found to be similar in dogs and humans, therefore, results obtained in the canine cells can well be extrapolated to human.
References
Bányász T, Magyar J, Szentandrássy N, Horváth B, Birinyi P, Szentmiklósi J, Nánási PP (2007) Action potential clamp fingerprints of K+ currents in canine cardiomyocytes: their role in ventricular repolarization. Acta Physiologica 190:189–198
Berecki G, Zegers JG, Verkerk AO, Bhuiyan ZA, de Jonge B, Veldkamp MW, Wilders R, van Ginneken ACG (2005) HERG channel (dys)function revealed by dynamic action potential clamp technique. Biophys J 88:566–578
Biliczki P, Virag L, Iost N, Papp JG, Varro A (2002) Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br J Pharmacol 137:361–368
Chinn K (1993) Two delayed rectifiers in guinea-pig ventricular myocytes distinguished by tail current kinetics. J Pharmacol Exp Ther 264:553–560
Fischmeister R, DeFelice LJ, Ayer RK, Levi R, DeHaan RL (1984) Channel currents during spontaneous action potentials in embryonic chick heart sells. The action potential patch clamp. Biophys J 46:267–271
Gintant GA (1996) Two components of delayed rectifier current in canine atrium and ventricle. Does I Ks play a role in the reverse rate dependence of class III agents? Circ Res 78:26–37
Gintant GA (2000) Characterization and functional consequences of delayed rectifier current transient in ventricular repolarization. Am J Physiol Heart Circ Physiol 278:H806–H817
Hua F, Gilmour RF (2004) Contribution of I Kr to rate-dependent action potential dynamics in canine endocardium. Circ Res 94:810–819
Iost N, Virag L, Opincariu M, Szecsi J, Varro A, Papp JG (1998) Delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res 40:508–515
Li G-R, Feng J, Yue L, Carrier M, Nattel S (1996) Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res 78:689–696
Lopatin AN, Makhina EN, Nichols CG (1994) Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372:366–369
Matsuda H, Saigusa A, Irisawa H (1987) Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature 325:156–158
Milberg P, Fleischer D, Stypmann J, Osada N, Mönnig G, Engelen MA, Bruch C, Breithardt G, Haverkamp W, Eckardt L (2007) Reduced repolarization reserve due to anthracycline therapy facilitates torsade de pointes induced by I Kr blockers. Basic Res Cardiol 102:42–51
Piao L, Li J, McLerie M, Lopatin AN (2007) Transgenic upregulation of I K1 in the mouse heart is proarrhythmic. Basic Res Cardiol 102:416–428
Rocchetti M, Besana A, Gurrola GB, Possani LD, Zaza A (2001) Rate dependency of delayed rectifier currents during the guinea-pig ventricular action potential. J Physiol 534:721–732
Sanguinetti MC, Jurkiewicz NK (1990) Two components of cardiac delayed rectifier K+ current: differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol 96:195–215
Shibasaki T (1987) Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol 387:227–250
Shieh R-C, Chang J-C, Arreola J (1998) Interaction of Ba2+ with the pores of the cloned inward rectifier K+ channels Kir2.1 expressed in Xenopus oocytes. Biophys J 75:2313–2322
Shimoni Y, Clark RB, Giles WR (1992) Role of an inwardly rectifying potassium current in rabbit ventricular action potential. J Physiol 448:709–727
Varro A, Balati B, Iost N, Takacs J, Virag L, Lathrop DA, Csaba L, Talosi L, Papp JG (2000) The role of the delayed rectifier component I Ks in dog ventricular muscle and Purkinje fibre repolarization. J Physiol 523:67–81
Yang T, Snyders DJ, Roden DM (1997) Rapid inactivation determines the rectification and [K+]o dependence of the rapid component of the delayed rectifier K+ current in cardiac cells. Circ Res 80:782–789
Acknowledgments
This work was supported by grants from the Hungarian Scientific Research Fund (OTKA F-67879, K-68457, NI-61902), Hungarian Ministry of Health (T-483/2006), National Research and Development Programmes (NKFP 1A/046/2004), Hungarian Academy of Sciences, and by University of Debrecen (DEOEC Mec-14/2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Returned for 1. Revision: 5 February 2008 1. Revision received: 11 April 2008 Returned for 2. Revision: 14 May 2008 2. Revision received: 15 May 2008
Rights and permissions
About this article
Cite this article
Jost, N., Acsai, K., Horváth, B. et al. Contribution of I Kr and I K1 to ventricular repolarization in canine and human myocytes: is there any influence of action potential duration?. Basic Res Cardiol 104, 33–41 (2009). https://doi.org/10.1007/s00395-008-0730-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-008-0730-3