Abstract
Cell transplantation has recently emerged as a novel therapy for ischemic heart disease. The presented study investigated the effect of intramyocardial transfer of human endothelial progenitor cells (EPCs) and stromal-cell derived factor-1α (SDF-1α) on left ventricular function in a chronic setting after myocardial infarction in cyclosporine treated rats. BrdU-labeled EPCs (106), 10 µg SDF-1α, EPCs+SDF-1α or placebo medium were injected directly into the border infarct zone 4 weeks after acute myocardial infarction. Eight weeks after transplantation, echocardiography identified significantly improved fractional shortening after EPC or EPCs+SDF-1α injection as compared with injection of placebo medium. Investigating isolated hearts revealed a significant increase in left ventricular developing pressure after transplantation of SDF-1α or EPCs+SDF-1α. Furthermore, coronary flow rates were significantly elevated, especially after transplantation of EPCs+SDF-1α (under catecholamine stress 24.2 ± 1.55 ml/min vs. 13.1 ± 1 ml/min in the control) correlating with increased density of CD31+ vessel structures in the EPC as well as EPCs+SDF-1α groups, thus defining a higher rate of neovascularization. Notably, SDF-1α injected hearts showed only a trend towards improvement in coronary flow. BrdU+ signals were detected in infarct areas, partially integrating into vascular networks. The rate of apoptotic cells as well as the amount of inflammatory cells was significantly elevated in the placebo control group. In conclusion, transplantation of EPCs as well as EPCs+SDF-1α associated with improvement in cardiac function after infarction, which was attributable to enhanced neovascularization and decreased inflammation. These results imply a combined benefit of EPCs+SDF-1α in the treatment of myocardial infarction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Acute myocardial infarction is still associated with a significant mortality in humans. In the industrialized world congestive heart failure after myocardial infarction represents the leading cause of hospitalization and death, and the extent of irreversibly damaged myocardium serves as an important predictor of clinical outcome. The loss of viable myocardium initiates adverse left ventricular (LV) remodeling with ventricular dilatation and contractile dysfunction.
As recent studies revealed, regeneration of the adult heart may occur by resident stem/progenitor cells [17]. In addition, several types of transplanted cells are effective in functional improvement after myocardial infarction. For instance, transplantation of fetal cardiomyocytes, skeletal myoblasts, smooth muscle cells, human umbilical vein endothelial cells (HUVEC), or bone marrow mononuclear cells led to an increase in global LV ejection fraction and to regression of LV remodeling by stimulating regeneration of cardiac myocytes but also by inducing neovascularization within the ischemic myocardium [11, 15, 16, 18, 21, 27, 29]. These effects were well documented even in long-term experiments [4, 27].
Recent reports suggest that local or systemic administration of endothelial progenitor cells (EPCs) enhances ischemic neovascularization and improves the function of ischemic tissues in animals with hindlimb or myocardial ischemia [1, 8, 13, 19]. Under physiological conditions EPCs are basically present in bone marrow and peripheral blood. EPCs can be defined as progenitor cells with the capacity to differentiate into mature endothelial cells when grown under appropriate conditions [8]. In several vascular disorders the number of circulating EPCs has been shown to associate with endothelial regeneration but also with endothelial dysfunction [5, 6, 25, 26, 31, 32]. Especially, in patients with unstable angina and after myocardial infarction a transient increase in the number of circulating EPCs was documented [5, 25]. Furthermore, it has been shown that transfer of EPCs influenced myocardial remodeling following coronary artery ligation and increased vascular perfusion in a hindlimb ischemia model [13, 19]. Encouraged by these results first clinical studies demonstrated that intracoronary infusion of autologous progenitor cells appears to be feasible and may beneficially affect the remodeling process after an acute myocardial infarction [7, 9, 23, 30].
Hence, in the presented study we characterized the effect of intramyocardial transfer of human EPCs on LV functional recovery and especially on structural changes within the myocardium after an infarction in a rat model. We furthermore attempted to augment the postulated beneficial effects of transplanted EPCs by injection of SDF-1α, a central chemokine that interacts with the CXC chemokine receptor 4 to mediate mobilization and recruitment of progenitor cells during the embryonic hemato-, vasculo-, and cardiogenesis but also along hypoxic gradients [2, 3, 24, 33]. Thus, using SDF-1α we sought to additional mobilize endogenous circulating EPCs for further improving the neovascularization.
2 Materials and methods
2.1 Generation of myocardial infarctions
Female adult Sprague–Dawley rats (200–250 g) were intubated under general anesthesia (1 ml/kg ketamine and 10 mg/kg xylasine, intraperitoneal) and positive pressure ventilation was maintained with supplemented room air, using a rodent respirator. Hearts were exposed through a 2-cm left thoracotomy and myocardial infarction was induced by suture occlusion of the left anterior descending artery (LAD) between the left atrium and the right pulmonary outflow tract using a 7/0 polyprolene snare (Ethicons). The muscle layer and skin incision were closed with a silk suture. Animal experiments were approved by local authorities and complied with German animal protection law.
2.2 Cells
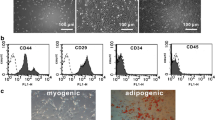
Human EPCs were isolated from 20 ml of citrate/dextran anticoagulated peripheral blood according to previously published protocols [3, 31, 32]. In brief, peripheral blood mononuclear cells (PBMCs) were separated by Biocoll (Biochrom AG) density gradient centrifugation, washed twice in phosphate buffered saline (PBS) and counted. Finally, the PBMCs were resuspended in microvascular endothelial growth medium MV2 (PromoCell) and plated in a T-25 culture flask coated with 10 µg/ml human fibronectin (Harbor Bio-Products). After 4 days non-adherent cells were removed and fresh medium was added. At day 7 adherent cells were detached with trypsin, counted, resuspended (106 in 100 µl PBS), and transplanted. On the day before transplantation, cells were incubated with BrdU (Zymed) as described by the manufacturer. Endothelial phenotype of cultured cells was confirmed by flow cytometry and fluorescence microscopy using anti-von Willebrand antibody, anti-VEGFR2 (KDR) antibody (both from Sigma), DiI-ac-LDL (Harbor Bio-Products), and lectin-FITC (Sigma) as previously described [3, 31, 32].
2.3 Cell transplantation
Transplantation was performed 4 weeks after acute myocardial infarction. The rats were anesthetized and the hearts exposed by a thoracotomy as described above. The areas for injection were visually selected by surface scarring and wall motion akinesis. EPCs were transplanted into marginal zones of the myocardial infarction by syringe injection (for 1-min injection time) at three distinct but adjacent sites. The rats are divided into four groups: the first group received BrdU-labeled EPCs (106, n = 12), the second group received 10 µg SDF-1α (Peprotec, Rocky Hill, NJ, n = 8), the third group received EPCs+SDF-1α (n = 8), and the fourth group (placebo control) received only culture medium (n = 12). After injection, the puncture sites were closed by suture, which served as a marker for the area of transplantation at follow-up thoracotomy. Cyclosporin A (50 mg/kg) was administrated orally in all groups by injection in the mouth daily, starting on the day of transplantation.
2.4 Echocardiography
Four weeks after acute myocardial infarction and 2 months after transplantation, rats were anesthetized and two-dimensional and m-mode measurements were performed with a model SONOS 5500 HP (Agilent, Palo Alto, CA) with a 12.5-MHz linear phased-array probe. The animals were placed in the supine or lateral position and excessive pressure on the thorax was avoided. Parasternal long-axis and short-axis views were obtained, ensuring that the mitral and aortic valves and apex were well visualized and recorded. Measurements of LV end-diastolic and end-systolic dimensions were obtained in M-mode from more than three beats and fractional shortening (FS) was calculated in percentage as follows: (LVIDd−LVIDs)/LVIDd × 100. LVIDd/s indicates the LV internal dimension in diastole (d) and systole (s), respectively.
2.5 Langendorff perfusion and assessment of infarct size
Two months after transplantation, rats were anesthetized and hearts removed. Heart function was measured with a Langendorff device with filtered Krebs–Henseleit buffer at a pressure of 65 mmHg equilibrated with 5% CO2 and 95% O2. A latex balloon was placed into the left ventricle to record pressure and heart rate. After 30 min of stabilization, coronary flow was measured by timed collection. The balloon size was increased by adding 5 µl increments up to a 30 µl volume. The systolic and diastolic pressures were recorded at each balloon size and developed pressure was calculated. Isoproterenol (70 nmol/l) was added and LV pressure development was measured. The scar size of LV-free wall was measured by computed planimetry (NIH Image Software) of digitized images taken from hears that were fixed in distension (30 mmHg) with 10% formalin and cut into 5 µm slices.
2.6 Cell identification and analysis of infarct area
For cell identification, cross sectional slides (5 µm) were dried overnight and stained with anti-BrdU kit (Zymed). Universal quick kit and alkaline phosphatase substrate kit (Vector Laboratories) were used with anti-CD31 antibody (Santa Cruz Biotechnology) to stain vessels in infarct areas. The CD31+ circular structures were counted and expressed as an absolute number per mm2. Nuclei undergoing apoptosis were stained with MEBSTAIN apoptosis kit II (MBL) and the TUNEL+ (i.e. apoptotic) nuclei were counted and calculated as a percentage of all nuclei (apoptotic index). Accustain trichrome stain (Masson, Sigma) was used to determine collagen content of the infarct regions. The stained areas were measured by computer-assisted planimetry (Diskus software, Hilgers) and expressed as percent from total infarct area. Macrophages and neutrophils were stained with α-naphthyl acetate esterase (Sigma) and naphthol as-d chloroacetate (Sigma), respectively. Stained cells (macrophages in yellow-brown, neutrophils in red) were counted pro mm2 in the infarct and remote area. For analyzing each heart 3–4 sections were used. Cells were counted in 3–4 fields of each section.
2.7 Statistical analysis
Data represent mean ± SD. Data analysis was performed with Prism 4 software (Graph Pad) using unpaired Student t test or one-way ANOVA followed by Newman–Keuls test. Differences with P < 0.05 were considered significant.
3 Results
Infarct size was analyzed by computed planimetry and revealed no significant difference in infarct areas between different groups as a sign of equal severity of infarctions in all groups (data not shown). BrdU- labeled EPCs (n = 12), 10 µg SDF-1α (n = 8), EPCs+SDF-1α (n = 8), and placebo medium (n = 12) were injected 4 weeks after ligation of the left coronary artery into two or three areas of infarct myocardium and adjacent border zones. The phenotype of cultured human PBMCs at day 7 after the isolation was assessed by analyzing expression of VEGFR2 (KDR) and von Willebrand factor, ac-LDL uptake as well as lectin binding (Fig. 1). Accordingly [3, 8, 31, 32], these cells were characterized as EPCs. Two months after transplantation we evaluated changes in myocardial contractility by 2D echocardiography and in isolated heart with retrograde perfusion according to Langendorff. We further assessed the presence of transplanted cells in host myocardium by immunochemistry. BrdU+ signals from transplanted areas were detected in host myocardium. These signals were identified in infarct areas, mostly integrated into host vessel (Fig. 2). The TUNEL staining for apoptotic cells demonstrated significantly reduced apoptotic index after EPC, SDF-1α, or EPCs+SDF-1α transplantation as compared with placebo medium injection (Fig. 3A).
Apoptotic cells in infarct areas were significantly decreased compared to placebo medium treated hearts (A, green: TUNEL-positive nuclei, *P < 0.05, ***P < 0.001 vs. control; insets show blue DAPI staining). Vessels/mm2 (B, red: CD31+ vessel structures, *P < 0.05) and collagen content (C, blue, *P < 0.05, **P < 0.01) also significantly differ in transplanted compared with control hearts. Representative images, insets in (C) show the whole scar area
In previous experiments we suggested that inflammation and angiogenesis are closely related with neovascularization being the prominent vascular response to chronic inflammation [14]. Vascular density in the infarction area was assessed by staining for CD31 of endothelial structures in order to examine whether transplantation of EPCs and SDF-1α might affect angiogenesis. As revealed in Fig. 3B cell transplantation and co-injection of SDF-1α displayed significantly increased number of vessels at 2 months after transplantation (337.8 ± 27.6 vessels/mm2 and 388.4 ± 18.6 vessels/mm2, respectively) as compared with placebo medium injected control hearts (246.1 ± 26.4 vessels/mm2), whereas SDF-1α injected myocardium showed only a trend towards increased vessel density (298.6 ± 9.9 vessels/mm2).
Next, collagen density was determined within the infarct area. After EPC, SDF-1α, or EPCs+SDF-1α injection significantly higher amount of collagen (37.8 ± 2.6%, 44.2 ± 4.4%, or 38.6 ± 4.5%, respectively) was detected within the infarct area as compared with the control group (25.7 ± 3.0%, Fig. 3C). As a hallmark of inflammation we assessed the macrophage/neutrophil infiltration within the infract area. In EPC (38.9 ± 11.3 macrophages/mm2, P < 0.05 vs. control), SDF-1α (78.9 ± 22.8 macrophages/mm2), or EPCs+SDF-1α (34.4 ± 4.7 macrophages/mm2, P < 0.05 vs. control) injected hearts we documented less frequently infiltrates than in placebo-injected hearts (106.8 ± 25.2 macrophages/mm2, Fig. 4). Notably, in the remote myocardium there were significantly more inflammatory cells after transplantation of EPCs (23.1 ± 4.8 macrophages/mm2) or EPCs+SDF-1α (28 ± 3.8 macrophages/mm2), as compared with placebo medium-injected hearts (11.1 ± 1.6 macrophages/mm2, Fig. 4). A similar trend was also observed for neutrophils (Fig. 4). These data suggest that 2 months after cell transplantation an inflammatory response possibly triggered by transplanted cells is still present even in the remote myocardium.
The content of neutrophils (PMNs, red) and monocytes/macrophages (yellow-brown) in infarct areas (open bars) was significantly lower in EPC, SDF-1α as well as EPCs+SDF-1α treated hearts (*P < 0.05, **P < 0.01 versus control). In contrast, within the remote myocardium (black bars) inflammatory cells were significantly more frequent after cell transplantation (*P < 0.05 vs. control). Representative images were shown
To evaluate whether EPCs are able to improve cardiac function we performed 2D echocardiography before and 2 months after transplantation. Four weeks after myocardial infarction dilated end-diastolic diameters and reduced fractional shortening were observed in all groups (data not shown). In a previous study [26] we showed in the same animal model (identical species and body weight) that the normal heart function did not significantly differ at baseline (FS 43.3 ± 5%, LVEDD 0.68 ± 0.06 cm). In the EPC as well as the EPCs+SDF-1α groups end-diastolic diameters decreased significantly 2 months after transplantation (EPCs preTX: 0.77 ± 0.08 cm, postTX: 0.56 ± 0.12 cm, EPCs+SDF-1α preTX: 0.72 ± 0.07 cm, postTX: 0.65 ± 0.06 cm, Fig. 5A) in contrast to placebo medium-treated hearts, which showed unchanged diameters (medium preTX: 0.67 ± 0.09 cm, postTX: 0.67 ± 0.2 cm, Fig. 5A). Fractional shortening also revealed a significant improvement after transplantation of EPCs or EPCs+SDF-1α, respectively (EPCs preTX: 24.2 ± 11.5%, postTX: 39 ± 11.4%, P < 0.05; EPCs+SDF-1α preTX: 26.9 ± 3.7%, postTX: 35.7 ± 8.3%, P < 0.05, Fig. 5B). In contrast, such an improvement was not observed in the placebo control group (preTX: 31.9 ± 9.75%, postTX: 29.5 ± 5.42%) and SDF-1α injection alone showed only a non-significant trend towards better heart function (Fig. 5B). For further quantification of LV function we performed isolated heart studies with retrograde perfusion according to Langendorff. We identified a significantly augmented peak in LV developed pressure (LVDP) in EPCs+SDF-1α and SDF-1α treated animals two months after transplantation (LVDP EPC+SDF- 1α 90.8 ± 23.5 mmHg, SDF-1α 90.3 ± 19.9 mmHg, Fig. 5C) as compared to placebo medium injected hearts (LVDP medium 68.7 ± 11.2 mmHg, P < 0.05, Fig. 5C). EPC injection alone showed only a trend towards improved LV function (Fig. 5C) and recording of LV compliance by measuring end-diastolic pressures in response to incremental augmentation of LV volume load did not further reveal any significant differences (data not shown). Coronary flow rates were measured in both groups by Langendorff procedure. Flow rates of EPC, SDF-1α and EPCs+SDF-1α transplanted hearts were also higher, partially significant at baseline and under catecholamine stress testing (Fig. 5D). Highest values were revealed with EPCs+SDF-1α transplanted hearts (coronary flow baseline 15.3 ± 3.6 ml/min vs. 8.8 ± 3.3 ml/min in the control, coronary flow under catecholamine stress 24.2 ± 1.55 ml/min vs. 13.1 ± 1 ml/min in the control, Fig. 5D). These results underline the histological findings, which documented a significant difference in the vessel density within infarct areas after transplantation of EPCs or EPCs+SDF-1α, Fig. 3B).
Echocardiographic parameters, LVEDD (A) and fractional shortening (FS) (B), before and after transplantation revealed significant differences towards improved LV function after transplantation of EPCs and EPCs+SDF-1α (*P < 0.05). Control groups in (A) and (B) did not show significant differences before and after transplantation. Investigating isolated heart demonstrated significantly higher baseline pressures (C, *P < 0.05) after transplantation of SDF-1α or EPCs+SDF-1α and highest coronary flow rates baseline as well as after catecholamine stimulation following transplantation of EPCs+SDF-1α (D, **P < 0.01)
4 Discussion
The presented study investigated the effect of transplanted peripheral blood-derived EPCs on functional improvement after myocardial infarction. We selected an even chronic infarction model in which the cells were transplanted 4 weeks after the acute event. After that time the acute remodelling processes including in general inflammatory reaction and increased oxidative stress has already been attenuated. Thus, it is reasonable to assume that such a microenvironment will allow a more effective survival and incorporation of transplanted cells. The study aimed in particular to analyze functional and structural changes within infarct areas and to assess potential additional benefits of SDF-1α application. Four weeks after myocardial infarction EPCs, SDF-1α, EPCs+SDF-1α, or placebo medium were injected into border zones around the infarct tissue. Two months after transplantation positive effects on LV function were evident. Histological examinations further revealed significantly increased vessel density and reduced apoptosis in the infarct area.
EPCs are able to home to areas of tissue injury, thus contributing to endothelial regeneration and neovascularization. Within the recent years considerable interest has focused on the possible therapeutic relevance of EPCs. EPCs respond to diverse angiogenic growth factors and chemokines (e.g. VEGF, SDF-1α) and their number has been altered during several vascular disorders [5, 25, 32]. Of note, patients with unstable angina or myocardial infarction [5, 25] have transient increase in the number of circulating EPCs, whereas aging and risk factors for ischemic coronary artery disease rather associated with reduced EPC number [22, 31, 32]. Notably, EPCs were able to improve the overall function in ischemic injured tissues by increasing neovascularization and attenuating organ damage [1, 13, 19]. Studies employing models of myocardial ischemia proved beneficial effects after local or systemic delivery of bone marrow cells or EPCs [9, 10–13]. In a rat model of myocardial infarction transplantation of expanded EPCs enhanced neovascularization, reduced LV dilatation, and preserved cardiac function [15].
Another relevant technique to ameliorate function of injured tissue represents the cytokine-induced mobilization. Hence, Orlic et al. [20] mobilized bone marrow cells by G-CSF and stem cell factor, which led to decreased postinfarction mortality and functional recovery as well as enhanced angiogenesis in the infarct myocardium. Another study revealed that local injection of SDF-1α stimulated homing of systemically delivered human mononuclear cells to the ischemic muscle and induced vasculogenesis in athymic nude mice with hindlimb ischemia [33]. Similar findings were presented by Askari et al. [2] after transplantation of SDF-1α transfected fibroblasts. These cells were able to induce therapeutic homing to the injured myocardium.
Secretion of pro-angiogenic cytokines in the course of EPC transplantation has been shown to contribute to new vessel formation [28]. In particular, SDF-1α, which specifically binds to the CXC chemokine receptor 4 on endothelial cells but also EPCs, has been shown to augment mobilization of EPCs into hypoxic areas [2, 3, 24, 33]. Therefore, in our study EPC transplantation was extended by additional SDF-1α protein application including a control group employing single SDF-1α injection. Indeed, our results showed higher number of vessels in infarct areas after additional application of SDF-1α as well as higher coronary flow rates. These findings suggest that homing of host EPCs contribute to increased vessel density in the infarct area. Though our results may be limited by a short lasting effect of single SDF-1α application during the transplantation process. Hence, SDF-1α would presumably be more effective after repeated application or even local expression. Additionally, the kinetics and concentration of SDF-1α may have conflicting effects on myocardial function. For example, sole catheter-based transendocardial delivery of SDF-1α in a porcine infarct model failed to improve myocardial perfusion [14]. Notably, our results further showed a higher amount of collagen in infarct areas which possibly contribute to enhanced tension stability of the ventricular wall. However, this issue remains rather controversial and open for debate given that previous studies demonstrated a lower ratio of fibrosis in infarct hearts [10, 15]. Nevertheless, the higher collagen content in our chronic post-infarction model was associated with improved LV function.
While neovascularization was increased in EPC/EPCs+SDF-1α treated infarction areas, the inflammatory cellular response remained considerably different as compared to the placebo control group. Our previous study using transplantation of HUVECs revealed that the structural and functional improvement of cardiac function was partially mediated by macrophages due to possible paracrine effects [18]. In the current study control groups showed an ongoing inflammatory response 8 weeks after transplantation within the infarct area associated with lower collagen content and a diminished LV function. After EPC transplantation, the inflammatory response was either less pronounced or already concluded. Interestingly, the surrounding regions also displayed a higher amount of cellular immune response as compared to the placebo control. These findings are further underscored by a significantly lower rate of apoptotic cells in the EPC and/or SDF-1α groups in contrast to placebo. While cell transplantation changed the structural response to myocardial injury, a postulated additional effect on LV contractility through stable integration of transplanted cells into host myocardium was only rarely detected. BrdU+ cells most commonly associated with vascular structures and the significantly higher coronary flow rate indicated that the effects observed were predominantly due to improved neovascularization in the transplanted groups.
In conclusion, the present study demonstrated that direct intramyocardial transplantation of EPCs in a chronic setting after myocardial infarction significantly improved cardiac function. Additional application of the chemokine SDF-1α further recovered LV function and supported the regenerative process. Thus, the mechanisms of altered cellular immune response, increased collagen content as well as improved coronary flow appear to play an important role in ventricular remodeling following EPC transplantation after myocardial infarction.
References
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228
Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS (2003) Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362:697–703
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10:858–864
Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA (2005) Allogeneic mesenchymal stem cell transplantation in postinfarct rat myocardium: short- and long-term effects. Circulation 112:214–223
George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, Herz I, Miller H, Keren G (2004) Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J 25:1003–1008
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348:593–600
Hristov M, Heussen N, Schober A, Weber C (2006) Intracoronary infusion of autologous bone marrow cells and left ventricular function after acute myocardial infarction: a meta-analysis. J Cell Mol Med 10:727–733
Hristov M, Weber C (2004) Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med 8:498–508
Hristov M, Weber C (2006) The therapeutic potential of progenitor cells in ischemic heart disease—past, present and future. Basic Res Cardiol 101:1–7
Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T (2006) Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation 113:1311–1325
Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Amano K, Iba O, Imada T, Iwasaka T (2002) Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol 22:1804–1810
Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T (2001) Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 104:1046–1052
Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T (2001) Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103:634–637
Koch KC, Schaefer W, Liehn EA, Rammos C, Mueller D, Schroeder J, Dimassi T, Stopinski T, Weber C (2005) Effect of catheter-based transendocardial delivery of stromal cell-derived factor 1 alpha on left ventricular function and perfusion in a porcine model of myocardial infarction. Basic Res Cardiol 100:1–9
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S (2001) Neovascularization of ischemic myocardium by human bone-marrow- derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7:430–436
Li RK, Jia ZQ, Weisel RD, Merante F, Mickle DA (1999) Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J Mol Cell Cardiol 31:513–522
Lyngbaek S, Schneider M, Hansen JL, Sheikh SP (2007) Cardiac regeneration by resident stem and progenitor cells in the adult heart. Basic Res Cardiol 102:101–114
Merx MW, Zernecke A, Liehn EA, Schuh A, Skobel E, Butzbach B, Hanrath P, Weber C (2005) Transplantation of human umbilical vein endothelial cells improves left ventricular function in a rat model of myocardial infarction. Basic Res Cardiol 100:208–216
Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T (2000) Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 105:1527–1536
Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P (2001) Mobilized bone marrow cells repair the infarct heart, improving function and survival. Proc Natl Acad Sci USA 98:10344–10349
Pouzet B, Vilquin JT, Hagege AA, Scorsin M, Messas E, Fiszman M, Schwartz K, Menasche P (2000) Intramyocardial transplantation of autologous myoblasts: can tissue processing be optimized? Circulation 102:III210–III215
Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA (2003) Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 108:457–463
Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM, REPAIR-AMI Investigators (2006) Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 355:1210–1221
Schober A, Karshovska E, Zernecke A, Weber C (2006) SDF-1α-mediated tissue repair by stem cells: a promising tool in cardiovascular medicine? Trends Cardiovasc Med 16:103–108
Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T (2001) Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103:2776–2779
Simper D, Wang S, Deb A, Holmes D, McGregor C, Frantz R, Kushwaha SS, Caplice NM (2003) Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation 108:143–149
Skobel E, Schuh A, Schwarz ER, Liehn EA, Franke A, Breuer S, Gunther K, Reffelmann T, Hanrath P, Weber C (2004) Transplantation of fetal cardiomyocytes into infarct rat hearts results in long-term functional improvement. Tissue Eng 10:849–864
Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, Jang HS, Lee JS, Byun J, Choi JH, Jeon ES, Kim DK (2005) Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells 23:1571–1578
Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE (1998) Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med 4:929–933
Templin C, Kotlarz D, Marquart F, Faulhaber J, Brendecke V, Schaefer A, Tsikas D, Bonda T, Hilfiker-Kleiner D, Ohl L, Naim HY, Foerster R, Drexler H, Limbourg FP (2006) Transcoronary delivery of bone marrow cells to the infarcted murine myocardium: feasibility, cellular kinetics, and improvement in cardiac function. Basic Res Cardiol 101:301–310
Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC (2002) Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106:2781–2786
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89:E1–E7
Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T (2003) Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107:1322–1328
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Schuh and E.A. Liehn contributed equally to this article.
Returned for 1. revision: 6 June 2007 1. Revision received: 27 August 2007 Returned for 2. revision: 24 September 2007 2. Revision received: 1 October 2007
Rights and permissions
About this article
Cite this article
Schuh, A., Liehn, E.A., Sasse, A. et al. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol 103, 69–77 (2008). https://doi.org/10.1007/s00395-007-0685-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-007-0685-9