Abstract
Purpose
Transplantation of mesenchymal stem cells (MSCs) is a promising therapeutic option for patients with acute myocardial infarction.
Methods
We show here that the ectopic overexpression of endothelial nitric oxide synthases (eNOS), an endothelial form of NOS, could enhance the ability of MSCs in treating ischemic heart damage after the occlusion of the coronary artery.
Results
Adenoviral delivery of human eNOS gene into mouse bone marrow-derived MSCs (BM-MSCs) conferred resistance to oxygen glucose deprivation (OGD)-induced cell death in vitro, and elevated the bioavailability of nitric oxide when injected into the myocardium in vivo. In a rat model of acute myocardial infarction, the transplantation of eNOS-overexpressing BM-MSCs significantly reduced myocardial infarct size, corrected hemodynamic parameters and increased capillary density. We also found that the synergistic effects were consistently better than either treatment alone.
Conclusions
These findings reveal a positive role of elevated eNOS expression in cardiac repair, and suggest the combination of eNOS and MSC transplant therapy as a potential approach for treating myocardial infarction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart disease is the leading cause of death around the world, which is often featured by reduced cardiac functions mainly due to a decrease in myocardial contractility. Caused by obstruction of coronary arteries or chronic high blood pressure, the heart can be greatly damaged beyond a critical threshold, and a severe loss of cardiomyocytes occurs when congestive heart failure develops. As a consequence, an efficient treatment for myocardial infarction (MI) patients requires both preventing the progression of cardiac deterioration and promoting the regeneration of healthy cardiomyocytes. A promising therapeutic option for heart failure involves the use of mesenchymal stem cells (MSCs) due to their multipotent properties. It is reported that autologous MSC transplantation is able to attenuate ischemic cardiac impairment and improve cardiac performance [1–3]. The reported benefits of engrafted MSCs into host myocardium include stimulation of vascular regrowth [4], differentiation of myogenic population [5], maintenance of optimal milieu for stress protection [6] and inhibition of pathological ventricular remodeling [7]. Both animal studies and clinical trials have supported the feasibility and efficacy of MSC transplant in treating MI. However, challenges still remain for MSC-based approaches in cardiac repair, including source of donor cells, methods of cell delivery and survival of transplanted cells in vivo. As MSCs derived from bone marrow can be readily obtained and easily expanded in culture, bone marrow-derived mesenchymal stem cells (BM-MSC) have become a major focus of research into recovery of cardiac function in MI. In addition, genetically modified BM-MSCs have been reported with a superior capacity for cell therapy of MI [4, 7].
Nitric oxide (NO) is an essential chemical signaling factor for cardiovascular homeostasis and is generated from the enzymatic activities of a family of NO synthases (NOS). The endothelium-derived NO mainly depends on the constitutive endothelial NOS (eNOS) that produces NO from the amino acid L-arginine [8]. eNOS could potentially prevent the decline in systolic function seen in MI patients through smooth muscle relaxation, which can improve vascular perfusion and reduce blood pressure. Previous reports indicate that the polymorphisms in the eNOS gene are significantly associated with coronary artery disease and acute MI [9–12]. Studies in animal models using both loss-of-function and gain-of-function approaches have revealed important roles of eNOS protein in MI. Drug-induced improvement of left ventricular dysfunction, interstitial fibrosis and survival following MI is greatly diminished in eNOS knockout mice [13]. Cardiomyocyte-specific eNOS overexpression ameliorates myocyte hypertrophy and enhances left ventricular performance [14]. The cardioprotective role of eNOS has also been demonstrated in MI animals that received the successful gene delivery of eNOS [15, 16]. Through systemic or local transduction, eNOS gene delivery can increase the levels of NO bioavailability, stimulate neovascularization, attenuate cardiac remodeling and suppress oxidative stress-associated apoptosis [15, 16].
A supportive role of eNOS in MSC function is possible and the combination of eNOS overexpression and transplantation of stem cells in treating infarcted hearts has been reported [17–19]. The therapeutic potential of using eNOS-modified BM-MSCs, however, has not yet been explored. In the current study, we used the adenoviral system to overexpress eNOS in BM-MSCs, which were then transplanted into rats with MI. We show that MI animals treated with eNOS-overexpressing MSCs have a reduced infarcted area, improved cardiac parameters and increased neovascularization. Our results suggest a potential therapy for the treatment of MI using a combination of eNOS gene delivery and BM-MSC transplant.
Methods & Materials
Mouse Bone Marrow-Derived MSC Culture
We used 8 week-old male C57/BL6 mice and harvested bone marrow stromal cells from the femur and tibia. The bone marrow nucleated cells were then collected with a Ficoll-paque density gradient (2500×g for 30 min at room temperature). We collected the nucleated cells at the Ficoll-HBSS interface and plated in culture dishes and incubated in Mesenchymal Stem Cell Growth Medium (Lonza, USA, Basel, Switzerland). Low passage MSCs (less than 5) were used in this study. To test the mesenchymal differentiation potential, we examined the differentiation of BM-MSC towards the myogenic or adipogenic lineages in vitro. For the myogenic differentiation, we seeded the cells first in DMEM-F12 supplemented with 15% fetal bovine serum (FBS). The medium was removed after 48 h and the cells were then cultured in DMEM-F12 supplemented with 5% FBS containing 0.1% of ITS (Insulin Transferrin Selenium, Invitrogen, Pleasanton, CA, USA) and 0.2 mg/ml EGF (Epidermal Growth Factor, Sigma, St. Louis, MO, USA) for 7 days [20]. For the adipogenic differentiation, we maintained cells in DMEM supplemented with 10% FBS containing 1 μM dexamethasone (Sigma), 1 μM indomethacin (Sigma), 500 μM IBMX (3-isobutyl-1-methylxantine, Sigma) and 10 μg/ml recombinant insulin (Sigma). The adipogenic induction medium was changed twice a week, and the presence of intracellular lipid vesicles was visible after 2–3 weeks. The intracellular lipid droplets were then assessed by Oil Red O staining [21].
Flow Cytometry
We characterized the murine BM-MSCs also by fluorescent-activated cell sorting (FACS) for their specific markers, which include the typical MSC surface markers (CD29 and CD44) as well as the hematopoietic cell lineage markers (CD34 and CD45). Adherent MSCs were harvested by trypsinization, and the resuspended cells were stained by antibodies in PBS containing 4% fetal bovine serum. The FITC- or PE-conjugated antibodies (eBioscience, San Diego, CA, USA) were used. We used the cells incubated with isotype control antibodies as negative controls to optimize parameters during the analysis using BD-FACScan (Franklin Lakes, NJ, USA). The data analysis was performed by Flowjo software.
Adenoviral Transduction of MSC in Vitro
Adenoviral delivery of eNOS into MSC was performed by using adenovirus carrying the human eNOS cDNA that is under the control of the CMV (cytomegalovirus) promoter. The adenoviral constructs including vector control (Ad.null) were the gifts from Dr. Julie Chao. High titers of virus were generated in 293 cells and purified at 2 × 1010 pfu/ml in PBS. Confluent MSC was incubated with 100 μl of virus solution for 2 days before the transplantation.
RNA Extraction and Real-Time PCR
Quantitative analysis of gene expression was carried out using the SYBR Green PCR Master Mix system (Applied Biosystems, Waltham, MA, USA). Total RNA was harvested from the transduced MSC or tissues of peri-infarct regions using the RNeasy kit (Qiagen, Valencia, CA, USA) and was reverse transcribed to complementary cDNAs using Superscript II (Biorad, Hercules, CA, USA) according to manufacturer’s instructions. Specific primers used for rat gene transcripts are described as following: human eNOS, forward: 5′-TGATGGCGAAGCGAGTGAAG-3′ and reverse: 5′-ACTCATCCATACACAGGACCC-3′; GAPDH, forward: 5′- TGCACCACCAACTGCTTA-3′, reverse: 5′-GGATGCAGGGATGATGTTC-3′. A series of duplicate dilutions of cDNA from control samples were used to optimize the standard curve and validate the melting curves for each primer set. GAPDH was used as a housekeeping gene for normalization of the expression levels.
Western Blotting
Protein analysis was also performed in the transduced MSC or tissues of peri-infarct regions by Western blotting. The same amounts of protein lysates were run on sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) followed by an electric transfer to a PVDF membrane. The blots after blocking were incubated with primary antibodies overnight at 4 °C. The antibodies for eNOS (BD bioscience) and GAPDH (Cell signaling, Danvers, MA, USA) were used and signals were detected by Electro-Chemi-Luminescence (ECL)-based imaging system after the incubation of blots with HRP conjugated secondary antibodies. The bands were quantified in ImageJ software by using GAPDH as a loading control.
Oxygen-Glucose Deprivation (OGD) of Ischemia Injury Model in Vitro
OGD was performed as previously described [22]. We washed the cells with deoxygenated and glucose-free solution and then incubated them in glucose-free media that was flushed with 95% nitrogen/5% CO2. The cells were cultured in anaerobic conditions (0.3–0.5%) for four hours while controls cells were placed in a normoxic incubator with 5.5 mM glucose. Following OGD, the cells and culture supernatants were quickly collected and subjected to the analysis for MTT, LDH (lactate dehydrogenase), and NO, respectively. MTT assay was done by a cell proliferation assay kit (Promega, Madison, WI, USA). Briefly, the treated cells with various conditions as indicated were incubated with a premixed optimized dye solution, and living cells were determined by the conversion of the tetrazolium component of the dye solution into a formazan product that can be measured at 570 nm absorbance by spectrometry. The released LDH in culture supernatants was measured by a cytotoxicity assay kit (Promega) which uses an enzymatic reaction with a color change assayed by spectrometry at a wavelength of 490 nm. The levels of NO were measured by a NO detection kit (Enzo Life, Shanghai, China) that is based on the enzymatic conversion of nitrate to nitrite by the enzyme nitrate reductase, followed by the Griess reaction to form a colored azo dye product. Quantification is performed by measuring absorption at 540–570 nm.
Myocardial Infarction and MSC Transplantation
We used a rat model of MI induced by occlusion of the left coronary artery [17]. The protocol was approved by the Committee on the Ethics of Animal Experiments of the First Affiliated Hospital of Nanjing Medical University. All surgery was performed under sodium pentobarbital anesthesia to minimize pains. In brief, animals were anesthetized and the chest was opened followed by LAD (left anterior descending artery)-ligation. The position was 1 to 2 mm distal to the line between the left border of the pulmonary conus and the right border of left atrial appendage. A total of 2 × 106 cells (100 μl) were transplanted into the ischemic area 1 h after the ligation by intramyocardial injection. Four groups were treated as follows: vehicle (PBS only), Ad.eNOS (direct injection of eNOS adenovirus), MSC (MSC without adenoviral treatment), and Ad.eNOS-MSC (MSC that was pretreated with eNOS adenovirus).
Hemodynamic and Echocardiographic Studies
We performed both analyses in separated animals 1 and 4 weeks after the MI induction. For hemodynamic studies, a micromanometer-tipped catheter was put into the left ventricle (LV) for the measurement of LV end-diastolic pressure (LVEDP), and the hemodynamic variables were measured using a pressure transducer (Gould Instruments, Cleveland, OH, USA), including the maximal rates of LV pressure rise and decline. Ejection fraction (EF) was measured as described previously [23]. The LV fractional shortening was measured by echocardiography by which 2-dimensional targeted M-mode traces were obtained using an echocardiographic system (Hewlett-Packard, Palo Alto, CA). LV fractional shortening was calculated as (LVDd - LVDs)/LVDd × 100, where LVDd is LV diastolic dimension and LVDs is LV systolic dimension.
Histological Analysis
We assessed the myocardial infarct size four weeks after MI induction by Masson’s trichrome staining. The left ventricle (LV) myocardium was fixed, paraffin embedded, and cut into 5 μm sections. To detect fibrosis, the sections were stained with Masson’s trichrome and assessed by a blinded pathologist and the percentages of infarct size were quantitated by a computerized morphometry system with an Olympus microscope. The capillaries were stained on the infarction area by cryosection. Optimum cutting temperature compound (O.C. T compound) embedded hearts sections (10 μm) were stained with antibodies against rat CD31 (Abcam, Cambridge, MA, USA) and detected by DAB (3, 3′-daminobenzidine)-based method. Capillary density in the infarction/peri-infarct regions was calculated as CD31+ cells per high-power field. At least five randomly fields in each section were analyzed. The data was presented as the relative percentage to the vehicle control-treated animals.
Statistics
Data are expressed as mean ± SD as indicated. We used Prism software to analyze the data for statistical significance by one-way ANOVA followed by a Tukey’s post hoc test. Significances were indicated by *p < 0.05, **p < 0.01, or #p < 0.05 versus appropriate controls.
Results
Characterization of BM-MSCs
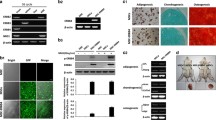
The cells used in the study were isolated from mouse bone marrow, and expanded in vitro for all experiments described below. The adherent cells were grown as normal MSCs with spindle-shaped morphology in culture dishes (Fig. 1a). We first performed flow cytometry to analyze the surface markers on these cells. As shown in Fig. 1b, the isolated cells were positive for CD44 and CD29, both of which are common markers of mouse MSCs. On the other hand, negative staining for CD34 and CD45 suggested there were no contamination of hematopoietic cell lineages in the culture. To further confirm the multipotent potential of BM-MSCs, we induced myogenic and adipogenic differentiations of these cells in vitro. The differentiation of BM-MSCs into myotube-like cells and morphological changes were observed with an inverted phase contrast microscope, while the adipogenic differentiation was verified by Oil Red O staining (Fig. 1c).
Characterization of mouse BM-MSCs in this study. (a) Phase-contrast micrographs of BM-MSCs at day 1, 3 and 5 after seeding. (b) Flow cytometric analysis indicates that BM-MSCs were positive for CD44 and CD29, but negative for CD34 and CD45. (c) BM-MSCs could differentiate into myogenic and adipogenic lineages. Myogenic differentiation was identified by phase contrast microscope, while adipogenic differentiation was verified by Oil Red O staining
Overexpression of eNOS in BM-MSCs Attenuates the Injury Induced by OGD in Vitro
To determine whether an increased eNOS expression in BM-MSCs might exert protective effects during ischemia-induced injury, we first established a protocol for eNOS overexpression through adenovirus-mediated gene transfer. The adenoviral delivery of human eNOS cDNA was performed in the BM-MSC cell culture. Following the infection, the ectopic expression of eNOS gene was evaluated by RT-PCR and Western blot. As expected, the mRNA levels of eNOS were increased in Ad.eNOS-MSCs, with approximately 8 folds increase compared to control cells (Fig. 2a). The protein level of eNOS was enhanced as well (Fig. 2b), reaching a level of 2-fold above controls (Fig. 2c). The infection using empty vector (Ad. Null) had no effects on eNOS expression. We then investigated the cells in an in vitro ischemia model of oxygen-glucose deprivation (OGD). The OGD treatments resulted in a decrease in cell survival and an increase in cell death (Fig. 3 a and b). Compared to the Ad. Null controls, Ad.eNOS-treated cells displayed enhanced MTT activity (representing living cell, Fig. 3a) and reduced LDH-release (indicating necrosis, Fig. 3b) following OGD treatment. In addition, OGD treatment reduced NO levels in controls but not in eNOS-overexpressing BM-MSCs (Fig. 3c). Together these results suggest that elevated expression of eNOS is sufficient to improve NO availability and protect BM-MSCs against cytotoxicity elicited by ischemia challenge in vitro.
Adenovirus vector-mediated eNOS over-expression in BM-MSCs. (a) Relative eNOS mRNA levels were analyzed by RT-PCR and normalized to control. GAPDH was used as an internal control. (b, c) eNOS protein expressions were analyzed by western blotting. GAPDH was used as an internal control. Experiments were repeated in triplicate. Data were presented as mean ± SD. **p < 0.01 versus control
Over-expression of eNOS protects BM-MSCs against OGD-induced impairments. Cell viability (a) and LDH release (b) in the experimental groups were measured by MTT assay and LDH release assay, respectively. (c) NO production in the mediums of the four experimental groups was measured by NO detection kit. Experiments were repeated in triplicate. Data were presented as mean ± SD. *p < 0.05 and **p < 0.01 versus control, #p < 0.05 versus Veh group
Transplantation of eNOS-Overexpressing BM-MSC Ameliorates Myocardial Infarction in Vivo
To directly explore the potential therapeutic effects using BM-MSCs with eNOS overexpression, we employed a rat model of MI in which BM-MSCs were transplanted into the injured heart (Ad.eNOS-MSC). The control groups received treatments of either PBS (vehicle), adenovirus directly (Ad.eNOS), or the BM-MSCs alone (MSCs without the preceding adenovirus infection), respectively. A slightly increased level of the serum NO was observed in the Ade.eNOS group, which is consistent with the previous report using adenoviral delivery of eNOS through intramyocardial injection [24]. As a comparison, the systemic induction of NO production was dramatically elevated in the animals receiving the transplantation of Ad.eNOS-MSC (Fig. 4a). Likewise, the expression of eNOS in the peri-infarct area was the highest in the Ad.eNOS-MSC treatment group, followed by the direct Ad.eNOS injection group (Fig. 4b-d). The fact that MSCs alone did not affect NO/eNOS in these experiments implied that the induction was specifically attributed to the transplantation of Ad.eNOS modified BM-MSCs.
Over-expression of eNOS in BM-MSCs elevated serum NO concentrations (a), eNOS mRNA levels (b) and eNOS protein expressions (c, d) in the peri-infarcted area. eNOS mRNA levels were measured by RT-PCR. GAPDH served as an internal control. eNOS protein expressions were analyzed by western blotting. GAPDH was used as an internal control. Experiments were repeated in triplicate. Data were presented as mean ± SD. *p < 0.05 and **p < 0.01 versus Veh control
At 4 weeks after MI, we observed a small but statistically significant decrease of myocardial infarct size in animals treated by MSCs alone. The histomorphometric analysis in Masson’s trichrome-stained myocardial sections also indicated a smaller size of cardiac infarction in the Ad.eNOS group. Remarkably, this effect was greatly enhanced when Ad.eNOS-MSCs was administered (Fig. 5a-b). Of note, treatments of Ad.eNOS, MSCs alone or Ad.eNOS-MSCs failed to significantly decrease infarct size in animals at 1 week post-MI (supplementary Fig. S1). Also, it was found that there were no significant differences among the experimental groups in hemodynamic parameters, including LV end-diastolic pressure, the maximal rates of LV pressure rise/decline, fractional shortening and ejection fraction (supplementary Fig. S2). Similarly, at 4 weeks after MI, the Ad.eNOS-MSC group had significantly improved hemodynamic parameters. In animals receiving Ad.eNOS-MSC transplantation, LV end-diastolic pressure was reduced (Fig. 6a), while the maximal rates of LV pressure rise/decline (Fig. 6b and c), fractional shortening (Fig. 6d; values of LVDd and LVDs in these four groups are shown in supplementary Table S1) and ejection fraction (Fig. 6e) were all significantly increased. Compared to the Veh controls, the Ad.eNOS and MSC groups also showed similar beneficial effects, both of which were less prominent than Ad.eNOS-MSC treated animals though. We also assessed the neovascularization in the heart after MI by tissue staining for endothelial cell marker CD31 (Fig. 7a). The calculated capillary densities in the infarction area were all increased compared to the Veh control group. Once again, the induced capillary density was highest in the animals receiving Ad.eNOS-MSC treatment (Fig. 7b), suggesting a synergistic effect of eNOS overexpression on MSC-mediated neovascularization after MI.
Effect of eNOS-over-expressed BM-MSC transplantation on myocardial infarct size four weeks after MI. (a) Representative Masson’s trichrome staining for myocardial sections in the experimental groups. (b) Quantitative infarct size in these groups. Experiments were repeated in triplicate. Data were presented as mean ± SD. *p < 0.05 and **p < 0.01 versus Veh control
Effect of eNOS-over-expressed BM-MSC transplantation on hemodynamic parameters at 4 weeks post-MI, including LV end-diastolic pressure (LVEDP) (a), LV maximum dP/dt (max dP/dt) (b), LV minimum dP/dt (min dP/dt) (c), LV fractional shortening (%FS) (d) and LV ejection fraction (EF%) (e). Experiments were repeated in triplicate. Data were presented as mean ± SD. *p < 0.05 and **p < 0.01 versus Veh control
Capillary densities in the infarction area in the experimental groups. (a) Representative images of CD31 staining in these groups. Scale bar, 50 μm. (b) Relative capillary densities to Veh control. Experiments were repeated in triplicate. Data were presented as mean ± SD. *p < 0.05 and **p < 0.01 versus Veh control
Discussion
The results of the current study have demonstrated that overexpressing eNOS, a critical enzyme in regulating vascular tone, could improve the therapeutic potential of BM-MSCs in cardiac protection and tissue repair. BM-MSCs with eNOS overexpression were resistant to cell death induced by OGD in vitro. Consistently, the intramyocardial injection of BM-MSCs with eNOS overexpression ameliorated ischemic injury of rats during MI, evidenced by reduced infarct region, improved hemodynamic parameters and enhanced neovascularization. These findings reveal a synergistic role of eNOS gene delivery and BM-MSC administration in modulating cardiac regeneration after MI.
In treating patients who suffer from large MIs, the most difficult part is that the adult human heart lacks sufficient regenerative capacity due to inadequate compensatory mechanisms. To overcome this problem during heart failure, a possible therapeutic option is cardiomyoplasty that aims to repopulate the region by introducing healthy muscles into the failing heart [25]. One particular method involves the transplantation of MSCs. In principle, multipotent MSCs may directly contribute to tissue regeneration through myogenic differentiation. In turn, these new populations of cells may improve systolic function and prevent the typical wall thinning and scar formation in MI patients. Though the feasibility of such MSC-mediated therapy in MI has been well established in both animal and human studies [1, 2, 26–28], it is necessary to further improve its therapeutic efficacy and specificity, both of which still limit its clinical application. Following this rationale, efforts to augment the MSC function by genetic approach have been made. Overexpression of the survival gene Akt1 in BM-MSCs has been demonstrated to modulate MSC functions in the treatment of MI [7, 29]. Furthermore, MSCs transfected with angiopoietin-1 [30], CXC chemokine receptor 4 [31], or stromal-derived factor-1 [32] have potent effects in improving cardiac function and salvaging the ischemic myocardium in animal models of MI. Similarly, eNOS expression can be introduced into the infarcted heart for boosting cardiovascular functions, which can be achieved by local delivery of eNOS through genetic techniques such as adenovirus [16, 24], although no study has used eNOS-modified MSCs. Directly introducing eNOS to the ischemic heart has beneficial effects [16, 24], and a previous study pointed to an indirect transfer into the heart mediated through adipose tissue-derived stem cells [17]. Consistent with this finding, we also found that BM-MSCs overexpressing eNOS have advantages in reducing infarct size and promoting vascular density in animals during MI. These effects were significantly better than the outcomes of using direct eNOS gene delivery, or the stem cells alone. On the other hand, two key points distinguish our current study from previous ones. First, the types of stem cell are different. Given that adipose tissue-derived stem cells and BM-MSCs have similar potential of cardiomyocyte regeneration, the differentiation efficiency and functional levels including signature gene profiles are not identical according to their distinct tissue origins [33]. The healing process could therefore be affected by cell specific mechanisms such as different stem cell niche and paracrine functions. Second, the genetic approach to overexpress eNOS gene in our study is through recombinant adenovirus which is able to achieve a substantial increase in the level of ectopic gene expression. In contrast, leniviral-mediated infection was employed in the previous study. Although it could result in a prolonged expression of eNOS, lentivirus can be integrated into the genome and cause somatic mutations during the cell therapy. For the perspective of stem cell therapy in MI, future studies are required to compare the specific needs and optimize the regimens of cell modification that are most appropriate for MI patients.
Our study also has several limitations. To minimize the numbers of animals used for investigating acute MI in vivo, we chose to omit the group that received the injection of MSCs with control virus since Ad. Null infection exhibited no effect on cell survival and NO levels in OGD (Fig. 3). The adenovirus infection alone may alter cell physiology, including immune response, although such effects should be minimal because we used adenovirus-infected cells instead of adenovirus itself in vivo [34]. In addition, we didn’t apply an autologous approach for the MSC transplantation. It is known that such heterologous transplantation is feasible due to the immunomodulatory functions of stem cells. In fact, BM-MSCs are immune evasive and have been used in allogeneic transplant for various diseases [35–37]. Last but not least, eNOS activity is regulated by multiple post-translational regulations, protein-protein interactions and phosphorylation at various sites [38], therefore delivery of the eNOS gene is just the first step for its function in repairing MI. Careful evaluations on the potential interference of stem cell transplantation with the post-translational modifications should be performed.
The recent explosion of knowledge on adult progenitor cells derived from human bone marrow [39–41] has contributed significantly to the development of cell therapy for regenerative medicine. As a promising approach to replenish the damaged cardiomyocytes, BM-MSC transplantation could be used to recover the lost cells following MI. At this moment, we were unsure if the amelioration of infarction and improved cardiac functions were attributed to newly generated cells exclusively from the injected MSCs. The induction of de novo cardiac cell formation has been suggested [42], and it would be interesting to explore the possibility if the survival/differentiation of the resident progenitor cells in the adult heart could be enhanced by eNOS expression. Pursuing these questions is potentially possible as we could take advantages of species-specific markers in a cell-lineage tracing study. In addition, the re-establishment of delicate organ structure and physiological cardiac functions often requires the integration of implanted progenitors with the resident tissues. For instance, MSCs can stimulate multiple cell types in the blood vessel that are critical for reconstructing the injured heart [1]. It is particularly important that our study demonstrated the efficacy and easiness of genetically manipulated BM-MSCs in promoting neovascularization in MI animals (Fig. 7), although the exact mechanism underlying this effect is still not clear. Given the drastically positive effects of eNOS on angiogenesis in vitro and in vivo, the local elevation of NO is clearly a key player responsible for the activation of vessel growth in the infarcted heart. The stimulated secretion of pro-angiogenic factors or enhanced recruitment of endothelial progenitor cells may contribute as well. Lastly, the in vivo MI model reported here is known to be associated with chronic inflammation. The excessive production of pro-inflammatory cytokines could elicit traumatic tissue damages and inhibit healing processes in the heart. Considering that both BM-MSCs and eNOS possess anti-inflammatory properties, it will be interesting to investigate whether the effects of this combined treatment are mediated through the collective modulation of inflammation, which could prevent extensive cell death and limited cell proliferation and renewal.
Conclusion
Vascular dysfunction may aggravate ischemic injury after MI. The combination of eNOS gene delivery and BM-MSC transplantation exhibits significantly stronger therapeutic efficacy in reducing infarct size, improving hemodynamic parameters and increasing capillary density. Our study therefore suggests that the MSCs expressing eNOS, an enzyme generating the vasoprotective molecule NO, could enhance treatment outcome of MI patients.
References
Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20.
Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–65.
Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–58.
Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229–36. discussion 36-7
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8.
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–12.
Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201.
Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37. 37a-37d
Hibi K, Ishigami T, Tamura K, Mizushima S, Nyui N, Fujita T, et al. Endothelial nitric oxide synthase gene polymorphism and acute myocardial infarction. Hypertension. 1998;32:521–6.
Colombo MG, Paradossi U, Andreassi MG, Botto N, Manfredi S, Masetti S, et al. Endothelial nitric oxide synthase gene polymorphisms and risk of coronary artery disease. Clin Chem. 2003;49:389–95.
Andrikopoulos GK, Grammatopoulos DK, Tzeis SE, Zervou SI, Richter DJ, Zairis MN, et al. Association of the 894G > T polymorphism in the endothelial nitric oxide synthase gene with risk of acute myocardial infarction. BMC Med Genet. 2008;9:43.
Abdel-Aziz TA, Mohamed RH. Association of endothelial nitric oxide synthase gene polymorphisms with classical risk factors in development of premature coronary artery disease. Mol Biol Rep. 2013;40:3065–71.
Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–9.
Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res. 2004;94:1256–62.
Smith Jr RS, Agata J, Xia CF, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery protects against cardiac remodeling and reduces oxidative stress after myocardial infarction. Life Sci. 2005;76:2457–71.
Chen LL, Yin H, Huang J. Inhibition of TGF-beta1 signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through angiogenesis and reduction of apoptosis. Cardiovasc Pathol. 2007;16:221–30.
Shi CZ, Zhang XP, Lv ZW, Zhang HL, Xu JZ, Yin ZF, et al. Adipose tissue-derived stem cells embedded with eNOS restore cardiac function in acute myocardial infarction model. Int J Cardiol. 2012;154:2–8.
Janeczek A, Zimna A, Rozwadowska N, Fraczek M, Kucharzewska P, Rucinski M, et al. Genetically modified human myoblasts with eNOS may improve regenerative ability of myogenic stem cells to infarcted heart. Kardiol Pol. 2013;71:1048–58.
Chen Q, Varga M, Wang X, Haddad DJ, An S, Medzikovic L, et al. Overexpression of nitric oxide synthase restores circulating angiogenic cell function in patients with coronary artery disease: implications for autologous cell therapy for myocardial infarction. J Am Heart Assoc. 2016;5
Gawronska-Kozak B, Manuel JA, Prpic V. Ear mesenchymal stem cells (EMSC) can differentiate into spontaneously contracting muscle cells. J Cell Biochem. 2007;102:122–35.
Scott MA, Nguyen VT, Levi B, James AW. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011;20:1793–804.
Guo J, Duckles SP, Weiss JH, Li X, Krause DN. 17beta-estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen-glucose deprivation/reperfusion. Free Radic Biol Med. 2012;52:2151–60.
Litwin SE, Katz SE, Morgan JP, Douglas PS. Serial echocardiographic assessment of left ventricular geometry and function after large myocardial infarction in the rat. Circulation. 1994;89:345–54.
Chen LL, Zhu TB, Yin H, Huang J, Wang LS, Cao KJ, et al. Inhibition of MAPK signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through reduction of inflammation. Mol Biol Rep. 2010;37:3067–72.
Chiu RC, Zibaitis A, Kao RL. Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg. 1995;60:12–8.
Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–25. discussion 26
Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–5.
Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–9.
Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, et al. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–42.
Sun L, Cui M, Wang Z, Feng X, Mao J, Chen P, et al. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun. 2007;357:779–84.
Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–9.
Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36:644–50.
Noel D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314:1575–84.
Tobias A, Ahmed A, Moon KS, Lesniak MS. The art of gene therapy for glioma: a review of the challenging road to the bedside. J Neurol Neurosurg Psychiatry. 2013;84:213–22.
Klinker MW, Wei CH. Mesenchymal stem cells in the treatment of inflammatory and autoimmune diseases in experimental animal models. World J Stem Cells. 2015;7:556–67.
MacFarlane RJ, Graham SM, Davies PS, Korres N, Tsouchnica H, Heliotis M, et al. Anti-inflammatory role and immunomodulation of mesenchymal stem cells in systemic joint diseases: potential for treatment. Expert Opin Ther Targets. 2013;17:243–54.
Zeira O, Asiag N, Aralla M, Ghezzi E, Pettinari L, Martinelli L, et al. Adult autologous mesenchymal stem cells for the treatment of suspected non-infectious inflammatory diseases of the canine central nervous system: safety, feasibility and preliminary clinical findings. J Neuroinflammation. 2015;12:181.
Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–60.
Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–86.
Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36.
Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Research Involving Animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Leilei Chen and Yuan Zhang contributed equally to this paper.
Electronic supplementary material
ESM 1
(DOCX 262 kb)
Rights and permissions
About this article
Cite this article
Chen, L., Zhang, Y., Tao, L. et al. Mesenchymal Stem Cells with eNOS Over-Expression Enhance Cardiac Repair in Rats with Myocardial Infarction. Cardiovasc Drugs Ther 31, 9–18 (2017). https://doi.org/10.1007/s10557-016-6704-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-016-6704-z