Abstract
Purpose
The aim of this study was to evaluate the hypocholesterolemic, immune- and microbiota-modulatory effect of a mushroom extract in hypercholesterolemic subjects.
Methods
A randomized, controlled, double-blind, and parallel clinical trial was carried out with subjects from 18 to 65 years old (n = 52) with untreated mild hypercholesterolemia. Volunteers consumed a β-d-glucan-enriched (BGE) mixture (10.4 g/day) obtained from shiitake mushrooms (Lentinula edodes) ensuring a 3.5 g/day of fungal β-d-glucans or a placebo incorporated in three different commercial creams.
Results
This mixture showed hypocholesterolemic activities in vitro and in animal studies. After eight weeks intervention, no significant differences in lipid- or cholesterol-related parameters were found compared to placebo subjects as well as before and after the BGE mixture administration. No inflammatory or immunomodulatory responses were noticed and no changes in IL-1β, IL-6, TNF-α or oxLDL were recorded. However, consumption of the BGE mixture was safe and managed to achieve the dietary fibre intake recommended as cardiovascular protective diet. Moreover, the BGE mixture modulated the colonic microbiota differently compared to placebo. Microbial community composition varied from before to after the intervention with several genera being positively or negatively correlated with some biomarkers related to cholesterol metabolism.
Conclusion
These results suggested a relation between cholesterol metabolism, microbiota and BGE administration. Nevertheless, the precise significance of this differential modulation was not fully elucidated and requires further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases (CVD) are still the leading causes of death (particularly in developed areas) inducing disability and chronic diseases. However, they are disorders that can be reduced or prevented by adopting proper dietary habits and lifestyles. To help the positive influence of healthy diets, several so-called ‘functional foods’ were developed in the last decades claiming effective lowering of cholesterol levels, hypotensive and antioxidant effects, etc. [1]. The most frequently consumed bioactive products are those including phytosterols (or derivatives) or β-d-glucans. However, there are some considerations to bear in mind when a subject regularly consumes these products and particularly β-d-glucans. On the one hand, they might not be as effective as expected since if cholesterol is not acquired with the diet, the liver activates 3-hydroxy-methylglutaryl-coenzyme A reductase (HMGCR), stimulating endogenous cholesterol biosynthesis and consequently, some consumers do not manage to reduce their cholesterol levels by consuming hypocholesterolemic food products [2, 3]. Moreover, they might influence the intestinal microbiome and immune system besides their hypocholesterolemic effect [4].
Although their mechanism of action is not completely understood, it is suggested that plant sterols impair absorption of dietary cholesterol [5]. The cholesterol-lowering effect of β-d-glucans might be related to the increase of viscosity in the intestine that stimulates the synthesis of bile acids from cholesterol to compensate for their fecal excretion after being scavenged within their complex structures and therefore, reduces circulating LDL cholesterol concentrations [6, 7]. Moreover, a few studies correlated the effect of cholesterol-lowering β-d-glucans with their potential as prebiotic modulators of colonic microbiota [8, 9]. In fact, recent publications indicate that the intestinal microbiome plays a key role in cholesterol regulation via several mechanisms but particularly by its conversion into coprostanol and further on to bile acids. The main taxa known so far carrying out the first transformation are Eubacterium and Bacteroides [10]. A wide number of genera were shown to be involved in the further modulation of bile acids (deconjugation, epimerization, oxidation, etc.), affecting bile acid solubility in physiological fluids to facilitate their reabsorption or to promote their secretion through feces. Strains of Bifidobacterium and Lactobacillus were able to stimulate bile acid deconjugation and fecal excretion, thereby promoting their hepatic biosynthesis from their main precursor (cholesterol), resulting in a decrease of cholesterol levels in serum from hypercholesterolemic subjects [10].
Besides their effect on the intestinal microbiome, β-d-glucans were also pointed as immunomodulatory compounds directly interacting with specific receptors from the gut-associated lymphoid tissue (GALT) and the intestinal enterocytes, triggering immune responses by releasing specific cytokines to activate both native and adaptive responses. β-d-Glucans were also shown to enhance GALT proper recognition of commensal microbiota and to stimulate defensive responses against pathogens (i.e. IgA secretion into intestinal lumen) [11,12,13].
Edible mushrooms, and particularly shiitake mushrooms (Lentinula edodes), contain compounds with hypocholesterolemic properties [3]. Fungal sterols were acting as plant sterols by displacing cholesterol from dietary mixed micelles [5] and by modulating the expression of cholesterol-regulation genes [14]. Shiitake β-d-glucans also scavenged bile acids in digestion models [15]. Eritadenine, an S-adenosyl-l-homocysteine hydrolase (SAHH) inhibitor, could lower cholesterol levels by altering the hepatic phospholipid metabolism [16] and some water-soluble polysaccharides showed HMGCR inhibitory activities in vitro [17]. If the latter activity could also be effective in vivo, it might indicate that mushrooms could also inhibit the cholesterol biosynthetic pathway similar to drugs, such as statins. Polysaccharides are large molecules that mostly undergo partial degradation during digestion and reach the colon. However, certain water-soluble polysaccharides might cross the intestinal barrier and enter the blood stream, although the underlying mechanism is not well understood, albeit it has been suggested that they might enter via M-cells or through other means [11].
When β-d-glucan-enriched extracts obtained from oyster mushrooms (Pleurotus ostreatus) were administrated to hypercholesterolemic mice together with lard (simulating an unhealthy diet), lowering of total cholesterol (TC) and LDL cholesterol was noticed after 4-week administration [18]. Similar results were observed when mice following a high cholesterol diet were administrated shiitake polysaccharides [19] or water-soluble polysaccharide fractions [18]. Besides, an eritadenine-containing fraction from shiitake mushroom was able to lower the atherogenic index (TC/HDL-C) in rat serum [20] and only ergosterol-enriched extracts seemed to be less effective in animal studies [21] although they modulated the expression of genes related to cholesterol metabolism in mice following a similar profile as hypocholesterolemic drugs [14]. Moreover, a mixture of extracts obtained from L. edodes, formulated to contain all the previously mentioned compounds, successfully lowered cholesterol levels in hypercholesterolemic mice after five weeks [21]. Based on these results, that fungal mixture was also tested in a clinical trial to investigate whether it could exert the noticed hypocholesterolemic effect in humans. Since the mixture contained a high β-d-glucan concentration, its effect on the cytokine profile and colonic microbiota of the volunteers was also evaluated.
Materials and methods
Biological material and reagents
Powdered Lentinula edodes S. (Berkeley) fruiting bodies (particle size < 0.5 mm, moisture < 5%) were obtained from Glucanfeed S.L. (La Rioja, Spain). Commercial asparagus and zucchini creams and Gazpacho (a traditional Spanish tomato puree) were kindly supplied by Conserves Ferrer S.A. (Barcelona, Spain). Maltodextrin (Sosa Ingredients, Moia, Spain) (5.2 g) was mixed with 1.6% food-grade brown dye (Sosa Ingredients, Moia, Spain) to prepare a placebo formula that resembled the mushroom extract in its visual appearance.
Preparation and analysis of the BGE and functionalization of the food matrices
A specific β-d-glucan-enriched (BGE) mixture of two extracts obtained from Lentinula edodes fruiting bodies was prepared using a pilot scale solid/liquid extraction unit as described in Morales et al. [22]. Briefly, the BGE was prepared by mixing different polysaccharide fractions, including a fraction (2.5%) containing water-soluble α- and β-d-glucans and fucomannogalactans (named ExA in Morales et al. [23]), a fraction (26%) extracted with hot water (98 °C), filtered through a multichannel ceramic membrane (Ceramem Corporation, Waltham, USA) and concentrated with a spiral wound Nanomax50 membrane (Millipore, Bedford, USA) (called RF2 in Morales et al. [22]), and the remaining fraction (71.5%) containing mainly insoluble β-d-glucans and chitins (indicated as R in Morales et al. [22] (69%) and ReA in Morales et al. [23] (2.5%)). The specific composition of the indicated fractions is detailed in the cited works [22, 23]. The obtained mixture included the soluble and insoluble β-d-glucans identified in Morales et al. [24] and other previously described [3]. The fractions were lyophilized, pooled in the indicated ratios and stored at −20 °C until further use. Since the BGE included a few polysaccharide-enriched extracts mixed in different ratios, its proximate composition was determined following AOAC methods. Other constituents related with their potential hypocholesterolemic activity, such as total carbohydrate, β-d-glucans, chitins, ergosterol, eritadenine and lenthionine contents, were determined following the methods described in Morales et al. [22].
The optimal procedure to add the BGE mixture into the selected food matrices was studied prior to its administration to volunteers and reported in He [25]. Briefly, the addition before or after creams and gazpacho production was first compared. Afterwards, the prepared creams and gazpacho were supplemented with the extract and submitted to a non-thermal treatment, to 100 °C during 1 h or 120 °C during 20 min. The effect of processing on β-d-glucan levels was followed using the method indicated in Morales et al. [23].
A dose of 3.5 g/day of beta-glucans was established taking into account the Scientific Opinion of health claims related to beta-glucans and maintenance of normal blood cholesterol concentrations [26, 27] where foods should provide at least 3 g/day of beta-glucans from oats, oat bran, barley, barley bran, or from mixtures of non-processed or minimally processed beta-glucans in one or more servings. The target population is adults with normal or mildly elevated blood cholesterol concentrations.
Study subjects
Fifty-two men (14) and women (38) from 18 to 65 years of age completed the study. The inclusion criteria included a BMI ≥ 18.5– < 30 kg/m2, a cardiovascular risk < 10% at 10 years measured by REGICOR, a total cholesterol ≥ 200 mg/dL and having at least one of the following cardiovascular risk factors: ≥ 45 years old for men or ≥ 55 years old for women; family history of premature CVD (CVD in first degree relative in men < 55 years old and/or in women < 65 years); HDL cholesterol < 40 mg/dL in men or < 50 mg/dL in women; triglycerides ≥ 150– < 200 mg/dL; LDL ≥ 130– < 160 mg/dL; smoker. The inclusion criteria also included willing to follow healthy eating guidelines and hyperlipidemia control but without stanols, sterols and yeast dietary supplements as well as no family or social environment that avoid the compliance with dietary treatment. All participants had to sign the informed consent. The exclusion criteria were as follows: individuals diagnosed with any of the following: Diabetes Mellitus type 1 and 2 on pharmacological treatment, dyslipidemia, hypertension, hypothyroidism; individuals > 60 years smokers with total cholesterol > 200 mg/dL or LDL > 130 mg/dL; individuals allergic to shiitake mushrooms; individuals with chronic diseases (hepatic, kidney, etc.); individuals receiving pharmacological treatment that modifies the lipid profile (for example, statins, fibrates, diuretics, corticosteroids, ADOs); individuals who have participated in the last six months in a program or clinical trial to lose weight; smokers wanting to stop smoking during the duration of the clinical trial; individuals with mental illness; individuals consuming drugs to lose weight (except if they were suspended at least 30 days before starting the study), and pregnant or breastfeeding women.
All subjects gave their informed consent to take part in the study that was approved by the Scientific Research and Ethics Committee of the HULP (La Paz University Hospital) (Code 4813) in accordance with the ethical standards of the Declaration of Helsinki [28]. The study was registered at http://clinicaltrials.gov under the number NCT03550287.
Study design
The study was a randomized, controlled, double-blind, and parallel clinical trial lasting eight weeks. Subjects (n = 57) were randomly assigned (a computerized randomization list was generated by permuted blocks of 4 for each gender maintaining the gender ratio of the sample) to one of two treatments involving a β-d-glucan-enriched mixture (Treatment Group (TG): 10.4 g/day BGE mixture ensuring in intake of β-d-glucans of 3.5 g/day) or a placebo (Control Group (CG): an isocaloric dose of brown-colored maltodextrin) incorporated in the asparagus or zucchini creams or the Gazpacho. Volunteers were instructed to consume 240 ml of cream with the mixture or placebo at lunch time and no other foods were restricted except foods containing stanols, sterols or yeast dietary supplements. The placebo visually resembled the extract and the participants randomized in one or another group did not have access to the unassigned product. The creams were part of the dietary pattern since vegetable creams are of normal consumption in the population study. Neither the researchers nor the subjects knew to which treatment group the subjects were assigned to, and the researchers were unblinded only at the end of the study.
Diet
All subjects were requested to follow a healthy eating guidelines and hyperlipidemia control without stanols, sterols and yeast dietary supplement. These guidelines and hyperlipidaemia control included measures, such as avoiding the consumption of saturated fats, precooked foods, fat meats (lamb, ribs or brisket, organ meats or sausages), sugar, pastry products, industrial pastries and confectionery, creamy ice creams, butter, cream, margarines, cured cheeses, etc. It also included recommendations as choosing milk and skimmed derivatives, lean meats (removing the skin and the visible fat), eating blue fish (at least 4 servings/week), increasing the consumption of foods rich in fibre, such as vegetables and fruits, cereals, whole grain breads or flours, legumes, etc. Guidelines also included measures as degreasing the broths or read the labelling to avoid products prepared with palm oil, coconut or trans-fatty acids, among other. Reduction of intake energy was not a requirement for the subjects during the intervention. The diet of each subject was recorded during the week prior to the beginning and end of each intervention period. All food and beverages consumed inside and outside the home were recorded over three consecutive days (including one day of the weekend) [29]. Subjects were instructed to record the weight of the food consumed or, if this was not possible, to record household measurements (spoonfuls, cups, etc.). At each visit, all records were thoroughly reviewed by a nutritionist in the presence of the subject to ensure that the information collected was complete. The energy and nutritional content of the foods and beverages consumed were then calculated using DIAL software (Alce Ingeniería, Madrid, Spain).
Anthropometric and health variables
Anthropometric measurements were taken at the beginning and end of the intervention using standard techniques, adhering to international norms set out by the WHO [30]. All measurements were made by trained personnel in the morning with the subject barefoot and wearing only underwear. Height was determined using a height meter with an accuracy of 1 mm (range, 80–200 cm). Body weight was measured using a Tanita BC-420MA balance (Bio Lógica Tecnología Médica S.L, Barcelona, Spain). BMI was calculated using the following formula: [weight (kg)/height (m)2]. Waist circumference (WC) was measured using a Seca 201 steel tape (Quirumed, Valencia, Spain). Information was collected on medical conditions and the consumption of medications. Blood pressure and heart rate were measured on the right arm using a Spot Vital Signs 420 automatic monitor (Welch Allyn, Madrid, Spain) (accuracy ± 5 mmHg).
The volunteers were instructed to maintain their habitual physical activity. This was monitored throughout the study using the International Physical Activity Questionnaire Short Form (IPAQ-SF) employing the methods posted on the IPAQ website (www.ipaq.ki.se).
Biochemical and haematological analyses
At the beginning and end of the intervention, blood samples were collected early in the morning at the Extraction Unit of La Paz University Hospital (HULP). Samples were kept at 4–6 °C until analysis, which was always performed within 48 h. Blood samples were obtained from fasting subjects at baseline and at eight weeks at the HULP biochemical lab. The biochemical parameters evaluated were total cholesterol, HDL and LDL cholesterol, triglycerides concentrations as well as glucose, basal insulin, HbA1C, apolipoproteins A1 and B, creatinine, glomerular filtrate (CKD-EPI), ureate, transaminase (ASAT, ALAT), lipase, C-reactive protein, vitamin D (as calcidiol).
Other haematological data were also measured, including leucocytes, erythrocytes, haemoglobin, hematocrit, VCM, HCM, CHCM, RDW, platelets and VPM, prothrombin time and activity, INR, fibrinogen and a leucocyte differential was also included to distinguish between neutrophils, lymphocytes, monocytes, eosinophils, basophils since one previous publication indicated that shiitake consumption increased eosinophil levels together with gastrointestinal problems in a few subjects [31]. All determinations were done with CORE Lab Systems (Siemens Healthcare®). Dimension Vista Intelligent Lab System at HULP.
Inflammation and oxidative data
The concentration of cytokines (IL1-β, IL6 and TNF-α) was determined using a Luminex® 200™ multianalyte profiling system and commercially available immunoassay panels in the HCYTMAG-60K-PX30 Milliplex Map Kit (EMP Millipore Corp., Boston, MA, USA). Plasma ox-LDL levels were measured using ELISA with human apoB-100 modified by Malondialdehyde (MDA-LDL) (Immundiagnostik AG, Bensheim, Germany).
Compliance and adverse events
Subjects received the exact number of packages (in a box packaging) required for each intervention and they were asked to return all empty and unemptied packages. Compliance was measured at the middle of the intervention and at the end by comparing the number of packages provided and the number returned. A subject was considered compliant when he/she consumed ≥ 90% of the packages provided. Adverse events were recorded on the middle and final visit of the intervention. An adverse event was defined as any unfavorable, unintended effect. All such events were recorded along with the symptoms involved (nausea, vomiting, diarrhea, constipation, etc.).
Statistic processing of clinical trial data
The sample size was selected because according to previous studies, fungal β-glucans showed effects at similar range than cereal β-d-glucans [15] and the consumption of 3 g oat β-d-glucan with a high molecular weight significantly reduced the serum LDL cholesterol in -0.21 (95%CI: -0.11, -0.30; P = 0.002) mmol/L [32]. LDL was the predictor variable (-0.21 ± 0.30 mmol/L). To convert mg/dL cholesterol to mmol/L, multiply mg/dL by 0.026. It was considered an alpha 5% (α = 0.05) and a statistical power of 80%. The test was bilateral. Finally, the sample size was 32 subjects by arm. It was calculated as a drop out of 5%. The calculation was carried out in SAS Power and Sample Size 13.1 Copyright © 2013 by SAS Institute Inc., Cary, zNC, USA. Quantitative data are presented as the means ± standard deviations (SD). Atypical data (i.e., lying more than two SDs from the mean) in asymmetric distributions were deemed to reflect true results; therefore, they were not eliminated from the analysis. Qualitative data were presented as counts and percentages. Fisher’s exact test was used in the analysis of categorical data. The Kolmogorov–Smirnov test was used to determine whether the data were normally distributed. Levene’s test was used to assess the equality of variance. When the distribution of the results was normal, the Student t test was used to compare the mean values of the studied variables recorded for the two treatment groups (independent samples) and the intragroup analysis (paired samples). The Mann–Whitney U test was used when the distribution was not normal. Multiple comparisons were adjusted using the Bonferroni method. Two-sided tests were used, and a P-value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS v. 21.0 software (SPSS, Chicago, IL).
Fecal microbiota composition
A fecal sample from all subjects was individually collected at the beginning and end of the intervention. They both were collected by the participants one or two days before their visit to the hospital and stored in a sterile container in their freezer (−20 °C). The samples were later transported on ice until reception at the hospital where they were immediately stored at −80 °C. Afterwards, samples were sent to the lab on dry ice pellets for DNA extraction and analysis. DNA extraction from human faeces (0.2 g) was performed in duplicate using the Purelink™ Microbiome DNA Purification Kit from Invitrogen (Thermo Fisher Scientific, Madrid, Spain) following the manufacturer’s instructions. Isolated DNA was quantified using a fluorimetric method with Quant-iT Picogreen Assay Kit (Thermo Fisher Scientific, Madrid, Spain). Then, obtained DNA (3 ng) was submitted to PCR (23 cycles) with Q5® Hot Start High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, USA) using the primers (50 nM) 5′-ACACTGACGACATGGTTCTACAGTGCCAGCMGCCGCGGTAA-3′ and 5′-TACGGTAGCAGAGACTTGGTCTGGACTACHVGGGTWTCTAAT-3′for amplification of the V4 region of the 16S ribosomal RNA (rRNA) gene. Afterwards, qPCR (12 cycles) was performed using the primers (400 nM): (5′-AATGATACGGCGACCACCGAGATCTACACTGACGACATGGTTCTACA-3′ and 5′-CAAGCAGAAGACGGCATACGAGAT-[10 nucleotides barcode]-TACGGTAGCAGAGACTTGGTCT-3′) of the Access Array Barcode Library for Illumina Sequencers (Fluidigm Corporation, San Francisco, USA). The obtained amplicons were validated and quantified by Bioanalyzer 2100 (Agilent, Madrid, Spain) and an equimolecular pool was purified by agarose gel electrophoresis and titrated by quantitative PCR using the Kapa-SYBR FAST qPCR kit for LightCycler® 480 (Sigma-Aldrich, Madrid, Spain) and a reference standard for quantification. The amplicon pool was denatured prior to be seeded on a flowcell, in two runs, at a density of 7.5 and 9.5 pM each, where clusters were formed and sequenced using the MiSeq Reagent Kit v3, in a 2 × 300 pair-end run on a MiSeq sequencer (Illumina, San Diego, USA).
Microbial community analysis
Raw reads were processed using the NG-Tax pipeline and SILVA database (Release 132) [33,34,35]. The resulting ASV (amplicon sequence variant) table in biom format was imported in R (v 3.6.3) for downstream analysis. Since the samples were sequenced in duplicate, a correlation analysis was done using Pearson’s correlation coefficient. Samples with an R2 < 0.90 were removed. The correlation was calculated using the Hmisc (v4.4.0) R package [36]. Overall, community level similarities/differences between replicates were visualized using principal coordinates analysis using weighted and unweighted UniFrac distances [37]. Since most samples were highly correlated, replicate one was chosen for analyses reported in this study.
Phylogenetic diversity was calculated on rarified data (sample size = 43,425) using phyloseq (v 1.30.0) and picante (v1.8.1). For specific analysis of the treatment group, the data were filtered to include either TG samples or CG samples. Multivariate distance-based redundancy analysis (dbRDA using Bray–Curtis dissimilarity) was carried out using the vegan (v2.5.5) R package [38]. For RDA, the ASV table was rarefied, aggregated to genus level and transformed using Hellinger transformation. To assess the role of parameters measured in our study, the envfit (permutations = 999) function was used. To assess the influence of intra-individual variation within a treatment, the parameters showing significant association with genus-level community structure were then used in the partial dbRDA. The significance of difference was tested using the permutest function, with 999 permutations. Association analysis for ASVs and biological parameters was done using the microbiome R package (v1.8.0) [39]. All visualizations were done using ggplot2 (v3.1.1) and ggpubr (v0.2) packages.
Results
Preliminary studies indicated that addition of the BGE together with all the ingredients to produce the creams and gazpacho showed the same β-glucan levels that if it was added after the food products were prepared [25]. Similarly, no significant differences were noticed when the already prepared gazpacho and zucchini cream were only mixed or mixed and heated. However, up to 33% β-glucan reduction was observed when the asparagus cream was submitted to 120 °C (simulating a sterilization process) and therefore, the BGE mixture or placebo was incorporated into the food matrices immediately before consumption. Mild thermal re-heating of the creams with a microwave (to reach 65 °C for a warm meal consumption) did not modify the BGE β-glucan levels.
Study population and baseline characteristics
Fifty-seven subjects were randomized into both treatment groups. However, five of the subjects randomized to the BCG group (15.2%) dropped out of the study. The final sample consisted of 52 hypercholesterolemic subjects without pharmacological treatment (38 women [74.5%], 14 men [25.5%]) and with a mean age of 50.8 ± 10 years (50.8 ± 10.3 vs. 50.8 ± 9.9 years; P = 0.998) finished the study (Fig. 1, Table 1).
The analyses were carried out with 28 participants in the TG group (19 women, 9 men) and 24 in the CG group (19 women and 5 men) with no significant differences between treatments depending on gender population (Fisher's exact test P = 0.275). The mean BMI was 25.3 ± 3.1 kg/m2 (overweight) and the waist circumference (WC) was 88.9 ± 10.2 cm (85.8 ± 9.1 cm in women and 97.1 ± 8.3 cm in men). The mean blood pressure was normal (110.4 ± 13.8/74.5 ± 11.0 mmHg). At the beginning of the study (baseline), no significant differences were observed between subjects assigned to the BGE group (TG) or placebo group (CG) in terms of their anthropometric (Table 2) and dietetic parameters (Table 3), lipid metabolism (Table 4) and variables of inflammation and oxidation (Table 5). These data confirmed an adequate randomization of the sample not detecting significant differences in the gender ratio between both treatment groups.
Compliance and adverse events
All subjects ingested ≥ 90% of the product provided. No significant differences were observed in the number of packages consumed between the different treatments. During the study, three subjects from BCG group (9.1%) dropped out of the study due to gastrointestinal problems (abundant diarrheal) and two more (6.6%) due to dislike about the product (Fig. 1). The main adverse events resulting from the intake of the BGE mixture were swelling (n = 4), heartburn (n = 3) and flatulence (n = 2), while subjects of the CG reported swelling (n = 3), flatulence (n = 2) and diarrhea (n = 2). Other safety parameters, such as transaminases, creatinine or urates, did not show significant changes after BGE mixture consumption (Table 6).
Effect of the BGE mixture on general parameters of the population
After eight weeks, TG group subjects showed a significant reduction of weight (from 72.23 ± 13.36 to 71.5 ± 13.55 kg; P < 0.05) and BMI (from 26.01 ± 3.0 to 25.73 ± 3.01 kg/m2; P < 0.05) compared to the beginning of the study (Table 2). However, this reduction was not significant when it was compared with the CG (−1.01 ± 3.21 vs. −0.73 ± 1.77 kg; −0.11 ± 1.58 vs. −0.28 ± 0.65 kg/m2).
Effect of the BGE mixture on diet and cholesterol-related parameters and on LDL oxidation
After the intervention ended, the CG showed better monitoring of healthy feeding and hyperlipemia control guidelines than the TG (Table 3). The CG significantly reduced energy intake (−338.9 ± 634.6 vs. −48.4 ± 406.7 kcal; P < 0.05), total lipid consumption (−16.5 ± 34.0 vs. 3.1 ± 31.1 g; P < 0.05) and SFA (−7.8 ± 12.8 vs. 2.7 ± 15.7 g; P < 0.05) of the diet compared to the TG. Moreover, the CG also showed significantly reduced levels of proteins (from 78.1 ± 21.9 to 67.8 ± 18.1 g; P < 0.05), carbohydrates (from 200.5 ± 62.7 to 164.8 ± 55.7 g; P < 0.05), MUFAs (from 38.3 ± 12.2 to 30.3 ± 30.3 ± 11.2 g: P < 0.01) and PUFAs (from 12.2 ± 3.6 to 9.5 ± 4.1 g; P < 0.01) compared to the start of the intervention, but these changes were not significant when compared to TG (Table 3). Cholesterol consumption was similar in both intervention groups showing no change throughout the study.
The changes observed in diet among intervention groups did not affect the lipid profile of study participants who did not show significant changes for any of the variables related to lipid metabolism, including LDL (Table 4). Similarly, no significant changes were observed in oxLDL concentrations after BGE mixture administration (Table 5).
Effect of the BGE mixture on cytokine levels
After eight weeks of intervention, no significant differences were observed in the determined cytokine concentrations between the treatment groups (Table 5).
Effect of the BGE mixture on colonic microbiota
Fecal samples from all subjects from TG and CG groups were analyzed for their microbiota composition. The variable region V4 of the 16S rRNA gene was PCR amplified in duplicate reactions for each sample and sequenced as technical replicates. In total, 15,472,993 reads were obtained with a minimum of 10,888 and a maximum of 170,682 reads per sample. These samples comprised a total of 1434 ASVs. The correlation between sequencing replicates for each sample was calculated. Ten samples, including samples from seven subjects from CG and three subjects from TG, showed a Pearson’s correlation coefficient < 0.90 between replicates (Supplementary Table 1) and were excluded. One of the replicates was chosen for further analysis. Thus, the analysis was done on samples obtained before and after the intervention from 17 subjects from CG and 25 subjects from TG which comprised a total of 1299 ASVs.
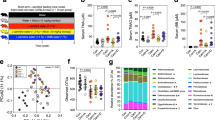
The phylogenetic diversity before and after intervention with BGE was measured, and no statistically significant difference was noticed (Wilcoxon-test, P > 0.05) in CG or in TG (Fig. 2). Inter-individual differences were observed in response to the intervention in both groups.
Afterwards, associations between variation in microbiota community structure and metadata features related to dietary intake, blood markers and cytokines were tested by envfit analysis (Supplementary Table 2). In both TG and CG, subject identity showed the highest effect on observed microbiota variation with TG showing higher association (r2 = 0.90, P = 0.001) compared to CG group (r2 = 0.74, P = 0.001). Among the continuous variables, cholesterol (r2 = 0.27, P = 0.001) and dietary fiber intake (r2 = 0.22, P = 0.001) most strongly contributed to explaining the observed microbiota variation in TG. Additionally, hematocrit, RDW, erythrocytes counts, hemoglobin, LDL, ApoB, age and systolic pressure showed significant association with observed microbiota variation in TG (Supplementary Table 2). Based on partial dbRDA, relative abundance of the genus-level taxaBacteroides, Prevotella_9 and Alistipes was positively associated with maltodextrin intervention. On the contrary, the relative abundance of Ruminococcaceae_UCG-014, Akkermansia, Erysipelotrichaceae_UCG-003 and Subdoligranulum was strongly associated with microbiota before maltodextrin intervention (Fig. 3a). In TG, the most responsive genera to intervention were [Eubacterium]_ventriosum_group, Erysipelotrichaceae_UCG-003, Akkermansia, Coprobacter, Lachnoclostridium, Bacteroides and Alistipes (Supplementary Fig. 3B).
Distance-based redundancy analysis based on Bray–Curtis dissimilarity and biological parameters for blood and immunological biomarkers and microbiota. Blue arrows indicate the biological parameters for blood and immunological biomarkers correlated to variation in gut microbiota community structure. Black arrows indicate genera associated with community variation before and after intervention, either a control group or b treatment group
Discussion
The BGE mixture obtained from Lentinula edodes contained, besides dietary fibers (β-d-glucans and chitins) other compounds with potential hypocholesterolemic activities, such as particular water-soluble polysaccharides [40], ergosterol [5, 14] and eritadenine [20] (Table 1). However, the lipid content of the BGE mixture was low, thus, ergosterol was present possibly in insufficient amounts to be effective since, when it was administrated in concentrations up to 0.45% of the diet, no lowering of serum cholesterol was noticed in mice [21]. Similarly, eritadenine intake was lower than the 10 mg/kg/day that was given to mice in experimental assays [20]. Therefore, the major compounds described as hypocholesterolemic molecules present in the extracts mixture were soluble and particularly insoluble dietary fibres [3].
Although in vitro and animal studies reported BGE mixture hypocholesterolemic activities, no significant differences in lipid- or cholesterol-related parameters were found after eight weeks of intervention when compared to placebo subjects as well as before and after the BGE mixture administration. Moreover, no changes in IL-1β, IL-6, TNF-α or oxLDL were recorded and no inflammatory or immunomodulatory responses were noticed. Nevertheless, the consumption of the BGE mixture was safe and it led to reach the dietary fibre intake recommended as cardiovascular protective diet. It also modulated the colonic microbiota differently compared to placebo, suggesting a possible relation between cholesterol markers, microbiota and BGE administration (Figs. 3, 4).
Spearman correlation analysis between microbiota and biomarkers. a ASVs showing significant correlation with biomarkers in CG. b ASVs showing significant correlation with biomarkers in TG. Significant associations (adj. P threshold > 0.05) are denoted by “ + ” sign. HDL high density lipoprotein cholesterol; LDL low density lipoprotein cholesterol; RDW red blood cell distribution width; MCH mean corpuscular hemoglobin
Regarding the effect of the BGE mixture on general parameters of the subjects, the results were similar to others studies using other mushrooms from the same order (Agaricales) where weight or BMI did not significantly change with the intake of soup containing 30 g dried oyster mushrooms (Pleurotus ostreatus) consumed over the study period of 21 days [41]. A one-year randomized clinical trial examined the effect of substituting red meat by white button mushroom (Agaricus bisporus) compared to a standard diet on weight loss in obese adults. At the end of the trial, participants on the mushroom diet lost more weight, achieved lower BMI and WC compared to the beginning of the study. However, these changes were also not significantly different when they were compared to the standard diet group [42]. On the other hand, in the present study, subjects in both intervention groups showed an increase in the systolic blood pressure from the beginning to the end of the intervention (P < 0.01) with no significant differences between treatment groups and without clinical relevance. These observations disagreed with previous reports where diabetic subjects consuming oyster mushrooms significantly reduced their systolic and diastolic blood pressure [43]. The different mushroom species tested or volunteers’ pathology might be among the reasons for the different effects. In the subjects of this study (with mild and untreated hypercholesterolemia), weight, BMI, WC, and blood pressure showed no significant differences between intervention groups.
Although the BGE mixture contained 3.5 g/day of fungal β-glucans and regular consumption of 3 g/day of oat and barley β-glucans was indicated as the effective concentration to notice a significant reduction of LDL cholesterol in serum [26, 27], no effect on total cholesterol or lipoproteins was observed. Perhaps, the different structure of shiitake β-d-glucans (see later), the BGE viscosity or its physical–chemical properties might act differently than cereal β-d-glucans in human intestine. However, these results were in line with some clinical trials where subjects were administrated fruiting bodies or extracts obtained from other mushroom species. Schneider et al. [41] noticed not significant changes in LDL and HDL cholesterol levels after eating 30 g dried oyster mushrooms over a period of 21 days compared to a placebo group. In a double-blinded, placebo-controlled, cross-over intervention study of four weeks, the supplementation with reishi (or lingzhi) mushroom (Ganoderma lucidum) did not significantly change the plasma lipids even though a small decrease was observed in total and LDL cholesterol [44]. The intravenous administration of a shiitake extract (containing lentinan, a water-soluble β-glucan) to healthy elderly subjects also failed to modify TC, LDL and HDL levels during a six-week intervention and compared to placebo subjects [45, 46]. However, Khatun et al. [43] reported reduction of total cholesterol and triglycerides with no effect on HDL during the periods where diabetic subjects were consuming oyster mushrooms in a 24-day experiment (seven days mushroom consumption, seven days no consumption and seven days restarting consumption). Thus, apparently the hypocholesterolemic effect was only noticed in diabetic patients and not in healthy individuals or subjects with mild hyperlipemia as those included in the present study.
On the other hand, the different linking patterns between cereal ((1 → 3), (1 → 4)) and fungal ((1 → 3), (1 → 6)) β-d-glucans might result in different molecular folding generating different intestinal viscosity or affinity to scavenge bile acids [45,46,47]. According to in vitro tests, fungal β-d-glucans showed similar bile acid binding capacities as a mixture of cereal β-d-glucans with cholesterol-lowering properties [15]. Therefore, the insignificant reduction of cholesterol levels in serum by fungal β-d-glucans might be related to the possible differences in intestinal viscosity. Viscosity in the intestine is also modulated by concentration, molecular weight and β-d-glucan solubility [48]. The BGE mixture contained both soluble and insoluble β-d-glucans plus chitins.
It should be mentioned that, although the TG showed a less healthy dietary pattern throughout the study, no significant variations in plasma levels of total cholesterol were observed. This could be due to the increase in total fiber consumption of the TG diet with respect to the CG (−2.8 ± 8.0 vs. 3.1 ± 7.7 g). The noticed increase in fiber consumption was mainly due to their supplementation with the BGE mixture. In fact, only the TG consumers achieved the recommendation of dietary fiber for the general population (> 25 g/d) [49]. EFSA and similar authorities recognize that diets with fiber levels higher than 25 g per day reduce the risk of coronary heart diseases and type 2 diabetes and improve weight maintenance. Therefore, consumption of the BGE mixture was favorable for that group.
Increased levels of circulating oxLDL are involved in the pathogenesis of atherosclerosis, and they are associated with clinical atherosclerotic cardiovascular disease events [50]. However, no significant changes were observed in oxLDL concentrations after BGE mixture administration. Other studies using oyster mushrooms (30 g of dried mushrooms per day) observed significant reduction of oxLDL concentrations from the beginning to the end of the mushroom diet consumption while no change was observed in the placebo group. Differences between groups of this study were not significant suggesting that dietary fiber might not be the responsible for oxLDL reduction noticed in previous study [41] or the different polysaccharide structures between the two mushroom species might be responsible for those differences.
The absence of significant differences in immunomodulatory or inflammatory responses was in concordance with a double-blind, crossover, and placebo-controlled trial carried out in 42 healthy elderly subjects (> 65 years) [45]. In this study, all participants consumed either 2.5 mg/day of an extract containing water-soluble β-d-glucans from L. edodes mycelium or placebo for six weeks. After a washout period of four weeks, the alternate supplementation was given for six weeks. At the end of the study, factors of the immune response as immunoglobulins, complement proteins and cytokines (including TNF-α) were not altered by the β-glucan consumption. These results differed from those reported by Dai et al. [51], where the pattern of cytokines secreted before and after shiitake mushroom consumption was significantly different. In their study, after four weeks, the consumption of either 5 or 10 g/day of whole fruiting body by healthy adults (21–41 years) resulted in an increased TNF-α and IL-1α levels but no changes in IL-6 or IL-1β concentrations. The secretion of the anti-inflammatory cytokines IL-4 and IL-10 was strongly stimulated after the mushrooms consumption, but pro-inflammatory TNF-α levels were also increased. However, the lack of a placebo group in the study made it impossible to unequivocally conclude whether these changes were due to the mushroom administration and to draw further comparison with the present study despite the similarities in the age range. Moreover, animal studies indicated that the immune-regulatory functions of edible mushrooms (particularly A. bisporus) in vivo are difficult to detect since they can only be noticed after a challenge. Yu and co-authors observed that after administration of dextran sodium sulfate, A. bisporus provided transient protection from colonic injury and induce a modest TNF-α secretion locally in the colon [52].
One of the side effects of undercooked shiitake mushroom consumption is a cutaneous reaction (linear erythematous eruptions); however, none of the study participants reported symptoms related to the skin (erythematous, eruptions, papules, papulovesicles or pruritus) [53]. Moreover, adverse human effects were reported after β-d-glucans consumption [54], but in this study, 3 out of 33 subjects (9.1%) dropped out the study due to gastrointestinal annoyances in the first days of the intervention suggesting that some people might show higher sensitivity to the product intake. Among the participants who completed the study, other types of milder gastrointestinal nuisances were noticed as heartburn or flatulence, probably associated with the high dietary fiber content of the product. Nevertheless, similar adverse events were noticed in CG subjects and the regular consumption of the BGE mixture did not affect safety parameters, such as transaminases, creatine or urate, or those related to glucose metabolism or inflammation. These observations were in line with another clinical study where the administration of a soluble β-d-glucan preparation (containing lentinan) from shiitake mycelium induced similar adverse events in terms of number, nature and severity in both groups [45].
A limitation of the study is that there was a dropout higher than expected and the sample size calculated to observe differences in LDL cholesterol levels was not fully achieved and therefore, it should be taken into consideration in the result interpretation and extrapolation. Another inconvenience was that although the BGE was tasted by some volunteers prior to the clinical trial, no sensory evaluation was carried out to adjust the formulation for consumer acceptance increasing the number of retracted volunteers.
Regarding microbiota studies, in CG, strong correlations were observed with hematological data, e.g. negative association was noticed between an ASV within the Christensenellaceae and leucocytes. An ASV assigned to Paraprevotella inversely correlated with erythrocyte counts but also with systolic blood pressure. The latter observations might lack significance being artefacts since the correlation was indicated for only one of the measured blood pressures although previous studies linked the genus Paraprevotella with profiles with high CVD risk [55, 56].
An ASV assigned to Faecalibacterium showed a positive association with LDL and triglyceride levels, complementing the results from Wang et al. [8] that noticed a positive correlation of this genus with BMI and a negative association with HDL. In the TG, a Dorea ASV was inversely correlated with dietary fiber intake, being in concordance with previous clinical trials including β-d-glucan or fiber administration that noticed a significant reduction of Dorea after treatment [8, 57]. One Coprococcus ASV was positively correlated with weight, while another ASV was inversely correlated with HDL (rho = -0.53, P = 0.03). Coprococcus spp. were abundant in obese children according to previous studies [58] and in rats fed with high cholesterol diets [59]. An ASV within the family Muribaculaceae was also inversely correlated with HDL (rho = -0.53, P = 0.03) and positively correlated with weight and insulin. The latter hormone was also positively associated with an ASV assigned to the Eubacterium coprostanoligenes group. Recently, Eubacterium hallii demonstrated an improvement on insulin sensitivity after oral administration to obese and diabetic mice [60]. Furthermore, an ASV assigned to Eubacterium eligens was inversely related with triglycerides levels (rho = -0.51, P = 0.048). This result was not in concordance with others that observed a positively correlation between Eubacterium rectale and triglycerides concentrations [61]. Nevertheless, most of the cholesterol-reducing bacteria isolated and characterized are members of the genus Eubacterium [10]. Previous works stated that Catenibacterium abundance was significantly increased after a high-fat and high-sugar diet [62], being in concordance with the positive correlation observed here for a Catenibacterium ASV with observed glucose levels. Total cholesterol was inversely correlated with ASVs within the Ruminococcaceae (rho = -0.55, P = 0.02) and the genus Bifidobacterium (rho = -0.57, P = 0.01). Previously, various studies reported contradictory results regarding Ruminococcaceae and lipidic profiles. Wang et al. [63] observed lower Ruminococcaceae abundance in high-fat-fed mice, whereas Sun et al. [64] noticed higher abundances in high-fat-fed hamsters. Liu et al. [65] detected positive correlations between the Ruminococcaceae and total cholesterol, LDL and LDL/HDL ratio in Japanese quail cecal microbiota. Higher consensus was achieved about the negative correlation of Bifidobacterium with cholesterol, since the ability of members of this genus to lower cholesterol levels has been repeatedly reported [10, 66, 67].
Conclusion
Although the BGE mixture showed hypocholesterolemic effects in animal studies, it did not significantly lower the cholesterol levels compared to placebo in the human trial carried out under the reported conditions. The supplement contained high concentrations of fungal β-glucans with reported immunomodulatory properties, but it did not significantly affect the cytokine profile nor the oxLDL values of the subjects. However, consumption of the BGE mixture was safe and even might be positive because even having a less healthy diet (compared to placebo subjects), consumers did not show any increase of their cholesterol levels and achieved the dietary fibre intake recommended by authorities as healthy and cardiovascular protective diet. Moreover, the BGE mixture administration during eight weeks modulated the colonic microbiota differently compared to placebo. In subjects consuming the mixture, the differences in microbiota composition from before to after the intervention correlated with some markers related to cholesterol metabolism, with a few ASVs particularly responsive to the intervention. Within the microbiota of the BGE-treated subjects, despite other groups and cholesterol-related metabolites, members of the family Ruminococcaceae and the genus Bifidobacterium were inversely associated to cholesterol levels, however, no correlation was found with any of the measured immunological parameters. Therefore, results from this study suggested a slight relation between cholesterol metabolism, microbiota and administration of the BGE mixture but still remains inconclusive, perhaps because the period of consumption or dose selected for the intervention was insufficient to achieve effective cholesterol reduction. Therefore, longer-period clinical trials would be necessary to clarify this possibility.
Code availability
Analytical code is available.
Abbreviations
- CVD:
-
Cardiovascular disease
- TC:
-
Total cholesterol
- BGE:
-
β-d-glucan-enriched mixture
- CG:
-
Control group
- TG:
-
Treatment group
- BMI:
-
Body mass index
- WC:
-
Waist circumference
References
Zawitowski J, Jones P (2015) Regulatory aspects related to plant sterol and stanol supplemented foods. J AOAC Int 98:750–756. https://doi.org/10.5740/jaoacint.SGEZawistowski
Chen J, Huang XF (2009) The effects of diets enriched in beta-glucans on blood lipoprotein concentrations. J Clin Lipidol 3:154–158. https://doi.org/10.1016/j.jacl.2009.04.054
Gil-Ramirez A, Morales D, Soler-Rivas C (2018) Molecular actions of hypocholesterolaemic compounds from edible mushrooms. Food Funct 9:53–69. https://doi.org/10.1039/C7FO00835J
Behrouzi A, Nafari AH, Sidat SD (2019) The significance of microbiome in personalized medicine. Clin Transl Med 8:16. https://doi.org/10.1186/s40169-019-0232-y
Gil-Ramirez A, Ruiz-Rodriguez A, Marin FR, Reglero G, Soler-Rivas C (2014) Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model. J Funct Foods 11:589–597. https://doi.org/10.1016/j.jff.2014.08.025
Lambeau KV, McRorie JW Jr (2017) Fiber supplements and clinically proven health benefits: how to recognize and recommend an effective fiber therapy. J Am Assoc Nurse Pract 29:216–223. https://doi.org/10.1002/2327-6924.12447
Maheshwari G, Sowrirajan S, Joseph B (2019) β-Glucan, a dietary fiber in effective prevention of lifestyle diseases—an insight. Bioact Carbohydr Diet Fibre 19:100187. https://doi.org/10.1016/j.bcdf.2019.100187
Wang Y, Ames NP, Tun HM, Tosh SM, Jones PJ, Khafipour E (2016) High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front Microbiol 7:129. https://doi.org/10.3389/fmicb.2016.00129
Volman JJ, Ramakers JD, Plat J (2008) Dietary modulation of immune function by β-glucans. Physiol Behav 94:276–284. https://doi.org/10.1016/j.physbeh.2007.11.045
Kriaa A, Bourgin M, Potiron A, Mkaouar H, Jablaoui A, Gerard P, Maguin E, Rhimi M (2019) Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J Lipid Res 60:323–332. https://doi.org/10.1194/jlr.R088989
Rice PJ, Adams EL, Ozment-Skelton T, Gonzalez AJ, Goldman MP, Lockhart BE, Barker LA, Breuel KF, DePonti WK, Kalbfleisch JH, Ensley HE, Brown GD, Gordon S, Williams DL (2005) Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J Pharmacol Exp Ther 314:1079–1086. https://doi.org/10.1124/jpet.105.085415
Petravic-Tominac V, Zechner-Krpan V, Grba S, Srecec S, Panjkota-Krbacvic I, Vidovic L (2010) Biological effects of yeast β-glucans. Agric Conspect Sci 75:149–158
Batbayar S, Lee DH, Kim HW (2012) Immunomodulation of fungal β-glucan in host defense signalling by dectin-1. Biomol Ther 20:433–445. https://doi.org/10.4062/biomolther.2012.20.5.433
Gil-Ramirez A, Caz V, Martin-Hernandez R, Marin FR, Largo C, Rodriguez-Casado A, Tabernero M, Ruiz-Rodriguez A, Reglero G, Soler-Rivas C (2016) Modulation of cholesterol-related gene expression by ergosterol and ergosterol-enriched extracts obtained from Agaricus bisporus. Eur J Nutr 55:1041–1057. https://doi.org/10.1007/s00394-015-0918-x
Palanisamy M, Aldars-Garcia L, Gil-Ramirez A, Ruiz-Rodriguez A, Marin FR, Reglero G, Soler-Rivas C (2014) Pressurized water extraction of β-glucan enriched fractions with bile acids-binding capacities obtained from edible mushrooms. Biotechnol Prog 30:391–400. https://doi.org/10.1002/btpr.1865
Yang H, Hwang I, Kim S, Hong EJ, Jeung EB (2013) Lentinus edodes promotes fat removal in hypercholesterolemic mice. Exp Ther Med 6:1409–1413. https://doi.org/10.3892/etm.2013.1333
Morales D, Smiderle FR, Villalva M, Abreu H, Rico C, Santoyo S, Iacomini M, Soler-Rivas C (2019) Testing the effect of combining innovative extraction technologies on the biological activities of obtained β-glucan-enriched fractions from Lentinula edodes. J Funct Foods 60:103446. https://doi.org/10.1016/j.jff.2019.103446
Caz V, Gil-Ramirez A, Santamaria M, Tabernero M, Soler-Rivas C, Martin-Hernandez R, Marin FR, Reglero G, Largo C (2016) Plasma cholesterol-lowering activity of lard functionalized with mushroom extracts is independent of Niemann-Pick C1-like 1 Protein and ABC sterol transporter gene expression in hypercholesterolemic mice. J Agric Food Chem 64:1686–1694. https://doi.org/10.1021/acs.jafc.5b05490
Zhu M, Nie P, Lian Y, Wang B (2013) Optimizing conditions of polysaccharide extraction from shiitake mushroom using response surface methodology and its regulating lipid metabolism. Carbohydr Polym 95:644–648. https://doi.org/10.1016/j.carbpol.2013.03.035
Morales D, Tabernero M, Largo C, Polo G, Piris AJ, Soler-Rivas C (2018) Effect of traditional and modern culinary processing, bioaccesibility, biosafety and bioavailability of eritadenine, a hypocholesterolemic compound from edible mushrooms. Food Funct 9:6360–6368. https://doi.org/10.1039/C8FO01704B
Morales D, Tejedor-Calvo E, Jurado-Chivato N, Polo G, Tabernero M, Ruiz-Rodriguez A, Largo C, Soler-Rivas C (2019) In vitro and in vivo testing of the hypocholesterolemic activity of ergosterol- and β-glucan-enriched extracts obtained from shiitake mushrooms (Lentinula edodes). Food Funct 10:7325–7332. https://doi.org/10.1039/C9FO01744E
Morales D, Smiderle FR, Piris AJ, Soler-Rivas C, Prodanov M (2019) Production of a β-D-glucan-rich extract from shiitake mushrooms (Lentinula edodes) by an extraction/microfiltration/reverse osmosis (nanofiltration) process. Innov Food Sci Emerg 51:80–90. https://doi.org/10.1016/j.ifset.2018.04.003
Morales D, Piris AJ, Ruiz-Rodriguez A, Prodanov M, Soler-Rivas C (2018) Extraction of bioactive compounds against cardiovascular diseases from Lentinula edodes using a sequential extraction method. Biotechnol Prog 34:746–755. https://doi.org/10.1002/btpr.2616
Morales D, Rutckeviski R, Villalva M, Abreu H, Soler-Rivas C, Santoyo S, Iacomini M, Smiderle FR (2020) Isolation and comparison of α- and β-D-glucans from shiitake mushrooms (Lentinula edodes) with different biological activities. Carbohyd Polym 229:115521. https://doi.org/10.1016/j.carbpol.2019.115521
He H (2019) Influencia del procesado y de la matriz alimentaria en la bioaccesibilidad de un extracto hipocolesterolémico obtenido de la seta shiitake (Lentinula edodes). Servicio de Publicaciones de la Universidad Autónoma de Madrid
EFSA (2010) Scientific opinion on the substantiation of a health claim related to oat beta glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J 8:1885
EFSA (2011) Scientific opinion on the substantiation of a health claim related to barley beta glucans and lowering of blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J 9:2471
Puri KS, Suresh KR, Gogtay NJ, Thatte UM (2009) Declaration of Helsinki, 2008: implications for stakeholders in research. J Postgrad Med 51:131–134. https://doi.org/10.4103/0022-3859.52846
Ortega RM, Requejo AM, Lopez-Sobaler AM (2006) Models of questionnaires for dietary studies in the assessment of nutritional status. In: Ortega RM, Requejo AM (eds) Nutriguía manual of clinical nutrition in primary care. Editorial Panamericana, Madrid, pp 456–467
WHO/FAO (1976) Methodology of nutritional surveillance. Report of a Joint FAO/UNICEF/WHO Expert Committee, The World Health Organization technical report series. WHO/FAO, Geneva, Switzerland
Levy AM, Kita H, Phillips SF, Schkade PA, Dyer PD, Gleich GJ, Dubravec VA (1998) Eosinophilia and gastrointestinal symptoms after ingestion of shiitake mushrooms. J Allergy Clin Immunol 101:613–620. https://doi.org/10.1016/S0091-6749(98)70168-X
Wolever TMS, Tosh SM, Gibss AL, Brand-Miller J, Duncan AM, Hart V, Lamarche B, Thomson BA, Duss R, Wood PJ (2010) Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trials. Am J Clin Nutr 92:723–732. https://doi.org/10.3945/ajcn.2010.29174
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. https://doi.org/10.1093/nar/gks1219
Ramiro-Garcia J, Hermes GDA, Giatsis C, Sipkema D, Zoetendal EG, Schaap PJ, Smidt H (2018) NG-Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes. F1000 Res 5:1791. https://doi.org/10.12688/f1000research.9227.2
Poncheewin W, Hermes GDA, van Dam JCJ, Koehorst JJ, Smidt H, Schaap PJ (2020) NG-Tax 2.0: a semantic framework for high-troghput amplicon analysis. Front Genet 10:1366. https://doi.org/10.3389/fgene.2019.01366
Harrell Jr FE, Dupont MC (2006) The Hmisc package. R Package version 2.0-0. 3:0–12
Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2011) UniFrac: an effective distance metric for microbial community comparison. ISME J 5:169–172. https://doi.org/10.1038/ismej.2010.133
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests MASS (2007) The vegan package. Commun Ecol Package 10:631–637
Lahti L, Shetty SA, Blake T, Salojarvi J (2017) Tools for microbiome analysis in R. version 1:28
Gil-Ramirez A, Caz V, Smiderle FR, Martin-Hernandez R, Largo C, Tabernero M, Marin FR, Iacomini M, Reglero G, Soler-Rivas C (2016) Water-soluble compounds from Lentinula edodes influencing the HMG-CoA reductase activity and the expression of genes involved in the cholesterol metabolism. J Agric Food Chem 64:1910–1920. https://doi.org/10.1021/acs.jafc.5b05571
Schneider I, Kressela G, Meyerb A, Kringsb U, Bergerb RG, Hahna A (2011) Lipid lowering effects of oyster mushroom (Pleurotus ostreatus) in humans. J Funct Foods 3:17–24. https://doi.org/10.1016/j.jff.2010.11.004
Poddar KH, Ames M, Hsin-Jen C (2013) Positive effect of mushrooms substituted form meat on body weight, body composition, and health parameters. A 1-year randomized clinical trial. Appetite 71:379–387. https://doi.org/10.1016/j.appet.2013.09.008
Khatun K, Mahtab H, Khanam PA, Sayeed MA, Khan KA (2007) Oyster mushroom reduced blood glucose and cholesterol in diabetic subjects. Mymensingh Med J 16:94–99. https://doi.org/10.3329/mmj.v16i1.261
Wachtel-Galor S, Tomlinson B, Benzie IF (2004) Ganoderma lucidum (“Lingzhi”), a Chinese medicinal mushroom: biomarker responses in a controlled human supplementation study. Brit J Nutr 91:264–269. https://doi.org/10.1079/BJN20041039
Gaullier JM, Sleboda J, Ojford ES, Ulvestad E, Nurminiemi M, Moe C, Tor A, Gudmunsen O (2011) Supplementation with a soluble beta-glucan exported from Shiitake medicinal mushroom, Lentinus edodes (Berk.) singer mycelium: a crossover, placebo controlled study in healthy elderly. Int J Med Mushrooms 13:319–326. https://doi.org/10.1615/intjmedmushr.v13.i4.10
Story JA, Kritchevsky D (1976) Comparison of the binding of various bile acids and bile salts in vitro by several types of fiber. J Nutr 106:1292–1294. https://doi.org/10.1093/jn/106.9.1292
Bacha U, Nasir M, Iqbal S, Anjun AA (2017) Nutraceutical, anti-inflammatory, and immune modulatory effects of β-glucan isolated from yeast. BioMed Res Int 2017:8972678. https://doi.org/10.1155/2017/8972678
Wood PJ, Beer MU, Butler G (2000) Evaluation of role of concentration and molecular weight of oat β-glucan in determining effect of viscosity on plasma glucose and insulin following an oral glucose. Brit J Nutr 84:19–23. https://doi.org/10.1017/S0007114500001185
EFSA (2017) Dietary reference values for nutrients: summary report. EFSA supporting publication e15121
Gao S, Zhao D, Wang M, Zhao F, Han X, Qi Y, Liu J (2017) Association between circulating oxidized LDL and atherosclerotic cardiovascular disease: a meta-analysis of observational studies. Can J Cardiol 33:1624–1632. https://doi.org/10.1016/j.cjca.2017.07.015
Dai X, Stanilka JM, Rowe CA, Esteves EA, Nieves C Jr, Spaiser SJ, Christman MC, Langkamp-Henken B, Percival SS (2015) Consuming Lentinula edodes (Shiitake) mushrooms daily improves human immunity: a randomized dietary intervention in healthy young adults. J Am Coll Nutr 34:478–487. https://doi.org/10.1080/07315724.2014.950391
Yu S, Weaver V, Martin K, Cantorna MT (2009) The effects of whole mushrooms during inflammation. BMC Immunol 10:12. https://doi.org/10.1186/1471-2172-10-12
Stephany MP, Chung S, Handler MZ, Handler NS, Handler GA, Schwartz RA (2016) Shiitake mushroom dermatitis: a review. Am J Clin Dermatol 17:485–489. https://doi.org/10.1007/s40257-016-0212-6
Hallfrisch J, Behall KM (2003) Physiological responses of men and women to barley and oat extracts (nu-trimX). I. Breath hydrogen, methane, and gastrointestinal symptoms. Creal Chem 80:76–79. https://doi.org/10.1094/CCHEM.2003.80.1.76
Ascher S, Reinhardt C (2018) The gut microbiota: an emerging risk factor for cardiovascular and cerebrovascular disease. Eur J Immunol 48:564–575. https://doi.org/10.1002/eji.201646879
Kjolbaek L, Benitez-Paez A, del Pulgar EMG, Brahe LK, Liebisch G, Matysik S, Rampelli S, Vermeiren J, Brigidi P, Larsen LH, Astrup A, Sanz Y (2019) Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: a randomized cross-over trial. Clin Nutr 39:67–79. https://doi.org/10.1016/j.clnu.2019.01.012
Holscher HD, Caporaso JG, Hooda S, Brulc JM, Fahey GC, Swanson KS (2014) Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am J Clin Nutr 101:55–64. https://doi.org/10.3945/ajcn.114.092064
Muruguesan S, Ulloa-Martinez M, Martinez-Rojano H, Galvan-Rodriguez FM, Miranda-Brito C, Romano MC, Piña-Escobedo A, Pizano-Zárate ML, Hoyo-Vadillo C, Garcia-Mena J (2015) Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis 34:1337–1346. https://doi.org/10.3945/10.1007/s10096-015-2355-4
Lee SM, Han HW, Yim SY (2015) Beneficial effects of soy milk and fiber on high cholesterol diet-induced alteration of gut microbiota and inflammatory gene expression in rats. Food Funct 6:492–500. https://doi.org/10.1039/C4FO00731J
Udayappan S, Manneras-Holm L, Chaplin-Scott A, Belzer C, Herrema H, Dallinga-Thie GM, Duncan SH, Stroes ESG, Groen AK, Flint HJ, Backhed F, de Vos WM, Niewdorp M (2016) Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes 2:16009. https://doi.org/10.1038/npjbiofilms.2016.9
Yang Y, Shi Y, Wiklund P, Tan X, Wu N, Zhang X, Tikkanen O, Zhang C, Munukka E, Cheng S (2017) The association between cardiorespiratory fitness and gut microbiota composition in premenopausal women. Nutrients 9:792. https://doi.org/10.3390/nu9080792
Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI (2009) The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1:6ra14. https://doi.org/10.1126/scitranslmed.3000322
Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, Wang Y, Liu C, Wang W, Wang J, Liu SJ, Liu H (2019) Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep 26:222–235. https://doi.org/10.1016/j.celrep.2018.12.028
Sun NX, Tong LT, Liang TT, Wang LL, Liu LY, Zhou XR, Zhou SM (2019) Effect of oat and tartary buckwheat-based food on cholesterol-lowering and gut microbiota in hypercholesterolemic hamsters. J Oleo Sci 68:251–259. https://doi.org/10.5650/jos.ess18221
Liu S, Bennet DC, Tun HM, Kim JE, Cheng KM, Zhang H, Leung FC (2015) The effect of diet and host genotype on cecal microbiota of Japanese quail fed a cholesterol enriched diet. Front Microbiol 6:1092. https://doi.org/10.3389/fmicb.2015.01092
Oner O, Aslim B, Aydas SB (2014) Mechanisms of cholesterol-lowering effects of lactobacilli and bifidobacteria strains as potential probiotics with their bsh gene analysis. J Mol Microbiol Biotechnol 24:12–18. https://doi.org/10.1159/000354316
Zanotti I, Turroni F, Piemontese A, Mancabelli L, Milani C, Viappiani A, Prevedini G, Sanchez B, Margolles A, Elviri L, Franco B, van Sinderen D, Ventura M (2015) Evidence for cholesterol-lowering activity by Bifidobacterium bifidum PRL2010 through gut microbiota modulation. Appl Microbiol Biotechnol 99:6813–6829. https://doi.org/10.1007/s00253-015-6564-7
Acknowledgments
This research was supported by a national R&D program from the Spanish Ministry of Science and Innovation (project AGL2014-56211-R) and a regional program from the Community of Madrid, Spain (S2013/ABI-2728). Conserves Ferrer S.A. and Glucanfeed S.L are acknowledged because of their help by providing the food matrices utilized in the clinical study. HS and SAS acknowledge support from the Netherlands Organisation for Scientific Research through the UNLOCK project (NRGWI.obrug.2018.005).
Funding
This research was supported by a national R&D program from the Spanish Ministry of Science and Innovation (project AGL2014-56211-R), a regional program from the Community of Madrid, Spain (S2013/ABI-2728) and the UNLOCK project (NRGWI.obrug.2018.005) from the Netherlands Organisation for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
The study was approved by the Scientific Research and Ethics Committee of the HULP (La Paz University Hospital) (Code 4813) in accordance with the ethical standards of the Declaration of Helsinki. The study was registered at http://clinicaltrials.gov under the number NCT03550287.
Availability of data and material
Data described in the manuscript are available.
Consent to participate
All subjects gave their informed consent to take part in the study.
Consent for publication
This manuscript is not being simultaneously submitted elsewhere and no portion of the data has been published elsewhere.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morales, D., Shetty, S.A., López-Plaza, B. et al. Modulation of human intestinal microbiota in a clinical trial by consumption of a β-d-glucan-enriched extract obtained from Lentinula edodes. Eur J Nutr 60, 3249–3265 (2021). https://doi.org/10.1007/s00394-021-02504-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02504-4