Abstract
Hypercholesterolemia constitutes one of the main cardiovascular risk factors and, consequently, treat it is really important to limit the risk of one of the leading causes of death in developed countries.
Therefore, an effective treatment to reduce high cholesterol is necessary: statins have proved to be the gold standard among lipid-lowering drugs, but at the cost of many unpleasant side effects.
At the same time, many nutraceuticals or functional foods were identified to lower levels of LDL cholesterol, acting according to different mechanisms of action.
Soluble fiber, glucomannan, plant sterols and probiotics inhibit the absorption of cholesterol and biliary salts by the bowel; monacolins, polycosanols, garlic and bergamot inhibit HMG-CoA reductase, limiting the hepatic synthesis of cholesterol; berberine, soy proteins and green tea act as inducers of LDL cholesterol excretion.
Clinical trials have shown that these nutraceuticals have an additive effect to statins, allowing to reduce the doses of statins without decreasing the results in terms of effectiveness in reducing total and LDL-cholesterol and significantly limiting side effects.
The results of the studies carried out have been encouraging, but further clinical trials prolonged over time are required to better assess the potential and possible long-term side effects of nutraceuticals and their combination with statins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
It is well known that statins are the gold standard among lipid-lowering drugs, both in primary and secondary prevention of cardiovascular disease; however they usually reduce LDL cholesterolemia of no more than 50–55 % at maximal dosage, the high dosages are often not well tolerated, and they have a limited efficacy on other lipid fractions like TG and HDL-C. For these reasons, their assumption could be associated to other natural or chemical drugs to improve their efficacy and tolerability, and to reach more ambitious therapeutic targets. The recent literature suggest that a number of nutraceuticals and functional foods have a significant lipid-lowering effect and potentially they could be associated to statin treatment in order to improve its efficacy. Of course, to choose which nutraceutical could be associated to statin treatment, it is needed to know the mechanism of action, the clinical efficacy, and obviously the tolerability for long-term use [18].
Recent preclinical and clinical evidence support the use of a certain number of lipid-lowering nutraceuticals in every day practical management of dyslipidemias.
Some nutraceuticals could also be associated to lipid-lowering drugs in order to potentiate the last ones or to reduce the dosage of non-fully tolerated drugs.
There have been studied over 40 lipid-lowering nutraceuticals and numerous clinical trials have confirmed their benefits on lipid metabolism and, consequently, on cardiovascular prognosis [55].
The ratio and pitfalls of combination of statins and niacin and statins and omega-3 fatty acids have been deeply described in this book, respectively in Chaps. 4 and 5. So, in this chapter the available evidence on those nutraceuticals potentially effective to improve statin efficacy in a safe way.
Natural Inhibitors of Cholesterol Absorption from the Bowel
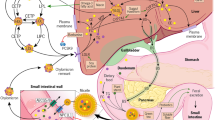
From a pharmacological point of view, the inhibition of cholesterol and biliary salt absorption by the bowel is one of the main cholesterol-lowering mechanism associated to significant reduction in statin-treated patients, typically achieved with exchange-anion resins (cholestyramin, cholestipol) or ezetimibe. Nutraceuticals with similar mechanisms of action are soluble fibers and plant sterols.
Soluble Fibers
Soluble fibers, in particular psyllium husk (but also guar, pectin, oat), lower LDL cholesterol by decreasing bowel cholesterol absorption and increasing the fractional turnover of both chenodeoxycholic acid and cholic acids [59]. Animal studies also suggest that psyllium increases activity of cholesterol 7-alpha hydroxylase, which is the rate-limiting enzyme for bile acid synthesis. Psyllium increases this activity two times faster than cellulose or oat bran and pectines [84], although this effect has never been adequately investigated in humans.
Different meta-analyses suggest that psyllium supplementation has a mild but significant dose- and time-dependent cholesterol lowering effect in hypercholesterolemic patients, with a final effect of mean decrease of LDL-cholesterolemia by 7 % for 10 g/day of supplemented fiber, without significant effect on other lipid fractions [88]. Psyllium also increases the efficacy of bile acid sequestrant drugs (even reducing their bowel side effects) [54], and phytosterols [75]. However, there are also some trials demonstrating its additive effect to the one of statins [64].
All available trials and meta-analysis confirm the overall safety of psyllium supplements. However, they could have transient gastrointestinal side effects, which are usually not severe and only mildly decrease compliance to the treatment, especially when micronized fiber is used. Entire seeds, used for the treatment of constipation, did not demonstrate lipid-lowering action, but they could exacerbate diverticulitis in patients affected by chronic diverticulosis.
A main safety concern regarding soluble fibers used as cholesterol lowering agents is the risk of interaction with the absorption of orally assumed drugs, in particular the ones with a narrow therapeutic range; some reports of reduced bioavailability are in fact available for oral antidiabetic drugs, digoxin, warfarin, lithium, iron, oral steroids, tricyclic antidepressants, carbamazepin, and other molecules [60]. For this reason it is usually suggested to assume fibers far (at least 2 h) from other medications.
Glucomannan
Glucomannan is a peculiar dietary fiber derived from tubers of the Amorphophallus konjac plant, commonly referred to as konjac root. It is found primarily in the tropical, sub-tropical, and temperate zones of Asia and contains large amounts of mannan, known as “Konjac mannan” or glucomannan. Glucomannan is an unabsorbable polysaccharide, composed of glucose and mannose in 1:1.6 ratio, bound through beta-1,4-glycosidic linkages [41]. It has been consumed in the Orient, especially in Japan, for at least 1000 years.
Like other gel forming fibers, it interferes with the motility and absorption of nutrients from the gut, slowing absorption of fats and glucose and interfering with gut hormones [65]. However, the activity of konjac mannan cannot be explained by a simple interaction with bile acids because it shows no in vitro or in vivo bile acid–binding activity. Rather, it appears to inhibit the active transport of cholesterol in the jejunum and the absorption of bile acids in the ileum, yielding improvements in plasma LDL and apolipoprotein B levels [86]. It has also been suggested that glucomannan increases the activity of 7-alpha-hydroxylase, an enzyme required for cholesterol conversion to bile acids [58].
A meta-analysis evaluating 14 randomized clinical trials including 531 patients concluded that glucomannan significantly reduces total cholesterol levels (weighted mean difference [WMD], −19.28 mg/dL; 95 % CI, −24.30 to −14.26), LDL cholesterol (WMD, −15.99 mg/dL; 95 % CI, −21.31 to −10.67), and triglycerides (WMD, −11.08 mg/dL; 95 % CI, −22.07 to 0.09) when compared to the placebo. However, it has no effect on HDL-cholesterol and blood pressure [78]. Glucomannan’s cholesterol-lowering effects have also been evaluated in children. In a clinical study of 40 children with hypercholesterolemia, patients underwent a 1-week diet run-in phase followed by randomization to either glucomannan 1–1.5 g twice daily plus diet or diet alone for 8 weeks. Treatment with glucomannan caused a significant reduction in LDL cholesterol values from baseline compared with the control group. Specifically, significant reductions were noted in favor of girls compared with boys (LDL-C: −30 % vs −9 %, P = 0.046) [57]. This gender-related effect has also been observed with other fibers and seems to be mediated by an interaction between sexual hormones and lipoprotein metabolism, although this has yet to be fully clarified [83].
Kojac glucomannan may reduce fat-soluble vitamin absorption while removing bile acids in humans. The absorption of vitamin E was reduced following administration of glucomannan. However, glucomannan did not interfere with the absorption of water soluble, fat-insoluble vitamin B-12 [41].
In one study measuring the effect of unavailable carbohydrates on the gastrointestinal absorption of calcium in rats during a 7–8 week period, calcium absorption was also compromised by nearly 20 %, partially by calcium-binding protein caused by the gastrointestinal transit of large amounts of undigested food [13].
As for psyllium, glucomannan may reduce the bioavailability of some oral medications, as well. Thus, it is recommended to take other medications 1 h before or 4 h after glucomannan administration [41]. The association of glucomannan with statin has now yet never directly evaluated in clinical trials.
Plant Sterols
Phytosterols are plant derived sterols or stanols (saturated sterols) with cholesterol-like chemical structure. Vegetable oils, cereals, breads, spreads, margarines, vegetables, and fruit are rich in plant sterols, while the intake of plant stanols depends mainly from cereals. The most commonly consumed phytosterols in the human diet are β-sitosterol, campesterol, stigmasterol, stanols sitostanol, and campestanol. Having a similar structure to cholesterol, phytosterols compete with cholesterol of dietary and biliary origin for incorporation into micelles in the gastrointestinal tract. Besides inhibition of bowel cholesterol absorption, they indirectly increase de novo hepatic synthesis of cholesterol, decrease hepatic and lipoprotein lipase activities, and increase serum lechitin:cholesterol acyl-transferase activity [62]. The average daily intake of plant stanols is 17–24 mg and that of plant sterols is 300 mg: these levels are too low for any significant LDL-lowering effect [79].
Recent results produced new information concerning relative efficacy of various mixtures and dose-response relationships [31]: a 2 g supplementation in phytosterols is sufficient to produce significant reduction of plasma LDL-C concentrations (around −10 %) both in normo- and hypercholesterolemic subjects. A 3 g supplementation in both plant sterols and stanols have a LDL-cholesterol lowering effect of 12 % [70]. However, their cholesterol lowering action increases in dose-dependent manner; phytostSerol consumption up to 9 g reduces serum LDL-cholesterol concentrations linearly up to 17.4 % [61], also in subjects affected by familial hypercholesterolemia [66]. The use of plant sterols/stanols in combination with statin therapy was evaluated in a meta-analysis of eight randomized clinical trials including a total of 306 patients: their addition to statins showed a favorable significant reduction in LDL cholesterol, but not HDL cholesterol or triglycerides [73].
The reduction of LDL-cholesterol mediated by sterols and stanols in combination with statin therapy is relevant, equivalent to doubling the dose of statin [35].
On the other side, miming the effect of dietary cholesterol, plant sterols could down-regulate the expression of the Niemann-Pick C1-like 1 protein, thus reducing the efficacy of ezetimibe [94]. On the short-term, plant sterols have been safely tested as lipid-lowering agents, also in children [32].
The main concern about phytosterols is their use in patients with very rare genetic phytosterolemia where the phytosterols are abnormally absorbed in the bowel. However, recent epidemiological data show that phytosterolemia is associated to cardiovascular disease risk in a linear manner, also for values strongly inferior to those observed in genetic phytosterolemia [89, 90]. In particular, recent preclinical data suggest that in apo E −/− mice serum phytosterols impaired endothelial vasodilation [91]. Moreover, since phytosterols mainly act on inhibition of lipid absorption and they are not very specific to cholesterol, they may also reduce absorption of carotenoids and fat-soluble vitamins [34]. Therefore, people using phytosterols may be advised to use adequate dosages (no more than 2 g/day) and to increase their diet intake of carotene-rich vegetables.
Probiotics
Probiotics could have a direct lipid-lowering effect, but also improving statin absorption in patients with inflammatory bowel disease or irritable bowel disease in inflammatory phase [8]. A recent meta-analysis of 13 clinical trials including 485 participants concluded that some probiotics could cause net changes in plasma total cholesterol by −6.40 mg/dL, LDL by −4.90 mg/dL, triglycerides by −3.95 mg/dL, and HDL −0.11 mg/dL [33]. Studies in the last decades have discussed the role of intestinal flora on energy metabolism and metabolic balance, specifically focusing on the balance between Bacteroidetes and Firmicutes species, the two dominant groups of beneficial bacteria in gut flora [8].
The cholesterol lowering activities of Lactobacillus, Enterococci and Bifidobacterium are not clearly defined. Supposed mechanisms are: (1) their co-precipitation with bile salts, (2) effects on bowel pH, (3) deconjugation of bile acids to be easily excreted as free bile acids by the body or binding to lipopolysaccharides on the surface of microorganisms, (4) incorporation of cholesterol into the cellular membrane, (5) microbial absorption of cholesterol, (6) fermentation of carbohydrates by microorganisms causing propanoic acid which inhibits hepatic cholesterol synthesis, (7) down regulation of NPC1L1 gene expression of cells, and (8) disruption of cholesterol micelles [7, 30, 43, 46]. On the other side, experimental studies suggest that specific improvement of microflora by supplementation of selected prebiotics could improve the lipid metabolism by activating the proglucagon-derived peptide (GLP2) [10]. Usually no safety concerns have been raised.
Inhibitors of Liver Cholesterol Synthesis
It is well known that about 90 % of circulating LDL-cholesterol depends on the liver cholesterol synthesis, and this is the reason why statins, inhibiting this process, are particularly efficacious LDL-cholesterol agents. Some nutraceuticals also exert some LDL-lowering effects related to liver inhibition of cholesterol synthesis as well, and they could have an additive effect to statins.
Monacolins
Monacolins are statin-like molecules derived by the mycotic fermentation of yeast rice from Monascus purpureus. Monacolins inhibit 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMGCoA) reductase. Monacolin K is in fact lovastatin. It has been used to make rice wine and as a food preservative to maintain the color and taste of fish and meat. Among all the Chinese herbal medicine with lipid-lowering activity, it is the one with the largest literature support [52].
The lipid-lowering efficacy of M. purpureus was tested in different clinical settings, from the general practice on relatively healthy subjects [15] to high-risk patients, such as those under antiretroviral therapy [42] or chronic kidney disease [27]. In a meta-analysis evaluating 93 randomized clinical trials (including 9625 participants), red yeast rice preparations showed short-time cholesterol lowering effects similar to those of low-dose statins [51]. In the same meta-analysis, no relevant side effects were highlighted. It is well tolerated also in statin intolerant subjects, at the dosage of 0.6–1.2–2.4 g/day red yeast rice form, with a 0.2 % monacolin K content [36]. Moreover, red yeast rice has pleiotropic effects similar than statins, such for instance antiinflammatory effect and improvement of serum level of vascular remodeling biomarkers [20].
Acting through a direct inhibition of the HMGCoA reductase, red yeast extract could potentially have the same side effects as the statins: myopathy, rhabdomyolysis, and hepatotoxiciy. In fact some cases reported occurrences of symptomatic myopathy, rhabdomyolisis in a renal transplant patient, and acute toxic hepatitis [68]. Other more common and relatively mild adverse reactions associated with red yeast rice consumption are headache, abdominal discomfort, heartburn, gas, bloating, muscle pain or damage, dizziness, and asthma. Those with liver damage and kidney problems, pregnant or lactating women, children, and people with bleeding tendency should avoid monacolins, due to lack of safety data. It also recommended that co-administration with gemfibrozil, cyclosporine, azole-antifungals, erythromycin, clarithromycin, and protease inhibitors be avoided [50], since monacolins are mostly metabolized by the cytochrome P3A4. The majority of these effects appear with the use of high red yeast rice dosages. Finally, issues with long-term safety, the wide variability of active ingredients in available formulations, and the potential toxic by-products such as mycotoxin citrinin in some cultivation conditions make it difficult for physicians to justify its use in treating hyperlipidemia [25].
Overall, at comparable dosages, the monacolin efficacy and safety profile is similar to that of statins. Because of the statin-like mechanism of action the possibility to add red yeast rice to statin treatment is limited because the risk to increase the dose-related statin adverse event risk and because the increase in statin dose is usually not associated to a linear improvement in LDL-reduction effect.
Policosanols
Policosanols are aliphatic primary alcohols mainly extracted from sugarcane (Saccharum officinarum L) wax. Cuban studies claim policosanol supplements significantly reduce LDL cholesterolemia by modulation of HMGCoA reductase transcription and bile acid absorption inhibition [56]. In this context, theoretically policosanols would be a favorite nutraceutical to be associated to statin treatment. However, this effect has not been confirmed by some recent randomized controlled trials carried out on more severe hypercholesterolemic patients [6]. Beyond the characteristics of the enrolled patients, one of the reasons is most likely due to the largely variant ability of the different components of policosanols to reduce cholesterol synthesis in liver cell [76].
Garlic
Several placebo-controlled randomized clinical trials have been carried out confirming the potential antihypercholesterolemic, and antihypertriglyceridemic properties of aged garlic powder. The main mechanism of action is the inhibition of the 3-hydroxy-3-methyl-coenzyme A reductase activity, with an additive effect on the statins [1]. Recent and well-designed clinical studies reveal conflicting results about the effects of commercial garlic supplementations on lipid parameters.
A recent meta-analysis, which has been performed on 22 trials reporting total cholesterol, 17 trials reporting LDL cholesterol, and 18 trials reporting HDL cholesterol, demonstrates that garlic powder intake reduces cholesterol and LDL cholesterol significantly [47].
Another meta-analysis performed on 39 clinical trials about the effect of garlic preparations on total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides shows an 8 % reduction in total serum cholesterol after 2 months of therapy, which is associated with a 38 % reduction in risk of coronary events at 50 years of age [71].
Even if in vitro and in animal experimental models garlic extract has a significant antiplatelet effect, its use has been proven to be relatively safe in patients under warfarin treatment [53]. However, it is recommended to be discontinued at least 7–10 days prior to surgical interventions. Garlic may also trigger gastroesophageal reflux in patients with a reflux tendency and may cause mild gastrointestinal side effects. Halitosis caused by allyl-methyl-sulphite is common complaint of natural garlic ingested subjects. It should also be avoided during lactation as it may also alter the odor of the milk, thus affecting infant sucking behavior. People with an allergy to plants in the allium family may also experience allergic reactions, including anaphylaxis [39]. Garlic may also interact with saquinavir and darunavir, antiretroviral drugs used in HIV therapy, decreasing their blood levels [5].
Bergamot
Bergamot (Citrus bergamia) is a citrus fruit spread in Italy in the region of Calabria; its juice is characterized by high content of some flavonoid glycosides, including neoeriocitrina, neohesperidin, and naringin. It remains unclear the mechanism by which the fraction of juice to achieve its lowering-cholesterol effect, but it is likely that some derivatives of it competitively inhibit the HMG-CoA reductase enzyme, producing a decrease in the synthesis of cholesterol.
Mollace et al. observed the cholesterol-lowering efficacy of bergamot extract in diet-induced hyperlipemia in Wistar rats and in 237 patients suffering from hyperlipemia. After 30 days of treatment, the results showed that bergamot extract reduces total and LDL cholesterol levels, increases HDL cholesterol, and reduces triglycerides [63].
Furthermore, a prospective, placebo-controlled study on 77 patients with elevated serum LDL-C and triglycerides demonstrates the significant addictive effect of bergamot extract to rosuvastatin [28].
Bergamot’s cholesterol-lowering properties were so tested with significant results, but the number of studies regarding these effects are yet limited and further studies are needed to confirm its properties.
Inducer of LDL-Cholesterol Excretion
One of the most physiological ways to improve the efficacy of statins is to increase the liver ability to re-uptake the circulating LDL-cholesterol and to increase its excretion with bile in the bowel. There are some nutraceutical with this mechanism of action, and in particular berberine.
Berberine
Berberine is a natural alkaloid with lipid-lowering, antidiabetic, antiinflammatory, and antiproliferative effects [24].
A 3-month treatment of 400 mg of berberine extracted from Coptis chinensis reduced plasma LDL-C by 25 % and TG by 35 % in a preliminary clinical trial carried out on 91 mixed hyperlipidemic subjects [45].
Another trial evaluated a 3-month treatment with berberine (500 mg/tab) and monacolins (3 mg/tab) in 84 patients with LDL-C increased above normal value after the use of at least two different oral estroprogestins treatments: the results showed that berberine and monacolins are able also to improve lipid metabolism in oral contraceptive induced hypercholesterolemia [22].
The supposed mechanism of action is the increased expression of the liver receptor for LDL. Besides its up regulation effect on the LDL receptor, berberine could also reduce triglycerides by AMP kinase activation and MAPK/ERK pathway blocking [93]. Due to its peculiar mechanism of action not directly involving the HMCGCoA reductase, berberine has been observed to increase the cholesterol-lowering action of both simvastatin and monakolines [16, 45].
The LD50 of berberine sulfate is 25 mg/kg in mice while the one of Berberis vulgare is moderately high (LD50 = 2.6 ± 0.22 g/kg b.w. in mice) [14]; this data supports the use of highly purified and concentrated berberine formulations only.
Standard doses of berberine (500–1000 mg/day) are usually well tolerated and adverse reactions are rare and mild (mainly gastrointestinal discomfort). On the contrary, high doses (>1000 mg/day) have been associated to arterial hypotension, dyspnea, flu-like symptoms, gastrointestinal discomfort, constipation, and cardiac damage [24].
The main safety issue of berberine involves the risk of some pharmacological interaction. In fact, berberine displaces bilirubin from the albumin about ten-fold more than phenylbutazone. Thus, any herb containing large amounts of berberine should be avoided in jaundiced infants and pregnant woman [11]. Thus, berberine could warfarin, thiopental, and tolbutamide from their protein binding sites, thus increasing their plasma levels [24]. However, until now, no clinical report of a significant pharmacological interaction is yet available.
Berberine can also markedly increase blood levels of cyclosporine A due to CYP3A4 and P-glycoprotein inhibition in the liver and gut wall respectively and because of an increase in gastric emptying time, thus causing increased cyclosporine A bioavailability and reduced metabolism. In renal transplant recipients taking cyclosporine 3 mg/kg twice daily, the co-administration of berberine (0.2 g/day for three times a day for 3 months) increases the mean cyclosporine A AUC by 34.5 % and its mean half-life by 2.7 h [92].
Although the main mechanism of pharmacological interaction of berberine involves CYP3A4 and intestinal P-glycoprotein, it also inhibits CYP1A1 in vitro, therefore potentially interacting with drugs metabolized by this cytochrome isoform as well [85]. The impact of this observation in clinical practice has to yet to be evaluated since the CYP1A1 metabolized drugs are relatively rare.
Soybean Proteins
Soybeans contain high-quality proteins and have been consumed for approximately 5000 years in Asian countries. The role of vegetable proteins in reducing cardiovascular risk was postulated as much as a century ago and soy products were found to be effective cholesterol-lowering agents in the last three to four decades. Several mechanisms for the lipid-lowering action of soy protein have been proposed and include increased bile acid synthesis, increased apolipoprotein B receptor activity, but also decreased cholesterol synthesis, and decreased hepatic lipoprotein secretion and cholesterol content, both associated with an increased clearance of cholesterol from the blood [29, 69]. Soy protein also reduces the insulin/glucagon ratio, which in turn down-regulates the expression of the hepatic transcription factor sterol regulatory element binding protein (SREBP)-1. The SREBP-1 reduction in turn decreases the expression of several lipogenic enzymes, thus reducing serum and hepatic triglycerides as well as LDL-C and VLDL triglycerides and liver lipotoxicity [81]. Additionally, soy components also induce the SREBP-2 regulated gene expression, which increases serum cholesterol clearance [67].
The soybean protein cholesterol lowering effect is clearly dose-related [77]. In an old, but complete, meta-analysis of 38 randomized clinical trials carried out by Anderson et al., it was estimated that, after adjustments for initial serum cholesterol concentrations and other variables were made, the ingestion of 25 or 50 g (mean 47 g/day) of soy protein per day decreases serum cholesterol by 0.23 or 0.45 mmol/dL, respectively [4]. Thus, it was recommended to include four servings of at least 6.25g each (25 g/day) of soy protein into a low saturated fat and cholesterol diet to reduce the risk of heart disease [48]. In a more recent meta-analysis of 30 randomized clinical trials including data from 2913 subjects, 25 g (range 15–40 g/day) of soy protein reduced LDL by 6 %. Further analysis, however, showed no dose response relationship between 15 and 40 g/day [37]. The smaller effect observed in this meta-analysis is most likely due to the exclusion of studies using higher soy protein dosages and including patients with higher cholesterol level. In fact, the cholesterol-lowering effect of soy protein seems to be proportional to the baseline cholesterolemia level.
Both meta-analyses conclude that soy protein can be safely used for cholesterol reduction.
In patients with lipid abnormalities, the effects of dietary proteins have to be confirmed, for formulation of dietary alternatives for the treatment of lipid disturbances [26].
Moreover, soy proteins with 0.25–0.5 g/kg body weight dosage were also safely tested in children/adolescents [87] and hemodialysis patients [12], where the safety of high dosage statins is not so clear.
The main risk associated with the use of a high dosage of isolated soy protein is the dietary unbalance due to excessive protein intake. If the overall protein intake is not adequately controlled, some patients, such as those with advanced chronic renal failure, may have excessive dietary protein load. Although the thyramine content of soy protein is not higher than 0.6 mg per serving, it should be considered in patients using monoamine oxidase inhibitor antidepressant medications [38]. Only one report suggests an interaction between soy milk and warfarin [9]. In vitro, unhydrolyzed soy extract produces very little inhibition of CYP1A2, CYP2A6, and CYP2D6 and a trend of activation of CYP3A4, while hydrolyzed soy extract shows mild inhibition of CYP2C9 and CYP3A4, but the clinical relevance of these observations is yet to be defined [3].
Green Tea
Different researches carried out on rats also suggest that green tea antihypercholesterolemic activity is related to an increased excretion of bile acids [49]. A Japanese epidemiological study carried out on 13,916 healthy workers (age = 40–69 years) concludes that high consumption of green tea (Cammelia sinensis) is associated with significantly lower serum concentration of total cholesterol [80]. Green tea may decrease the risk of coronary heart disease by inhibiting the development of atherosclerosis, protecting LDL against oxidation and foam cell formation via catechins similar to blacktea theaflavins [21]. Despite conflicting results on lipid-lowering effects of green tea catechins, in recent meta-analyses consumption of green tea catechins 145–3000 mg/day for 3–24 weeks were found to be associated with a significant reduction in total and LDL cholesterol levels (ranging from −5.46 mg/dL to −7.20 mg/dL, and −2.19 to −5.30 mg/dL compared to controls, respectively), but no effect on HDL or triglyceride levels were found [44, 95]. Besides discussions regarding effects on iron absorption [72], large doses may be related with folate deficiency in pregnancy and may lead to neural tube defects [74].
Discussion and Conclusion
The largest part of the most widely marketed lipid-lowering nutraceuticals was not clearly demonstrated to have positive additive cardiometabolic effects. The reasons could be different, among the others low bioavailability, scarce tolerability of efficacious dosages, short duration of the studies, low methodology quality of the available clinical trials. The low interest of industries to invest large amount of money in outcome study on products that could not be exclusive is probably an important reason, as well. Moreover, these compounds, usually easily available in the market, need to be long-term tested and evaluated on larger patient samples in clinical practice setting. Another concern is the efficacy and safety of lipid-lowering nutraceuticals when differently combined for marketing purposes without being directly tested in clinical trials. In fact, a perspective could be the association of more active nutraceuticals in order to improve their efficacy maintaining dosages not associated to potential side effects. In this context, an example of a good practice has been applied to the study of a registered association of Monacolins, Berberine, and Policosanols (Armolipid Plus), which has been tested for its efficacy in more than 1700 subjects in clinical practice [82] and for a period longer than 1 year [17] (Fig. 11.1), clearly showed to improve Flow-mediated vasodilation [2] and pulse wave velocity in mildly hypercholesterolemic subjects [19].
More clinical research is needed to clarify the potential role in therapy of some interesting nutraceuticals with strong preclinical evidence of efficacy, such as guggulipid (Commiphora mukul) [23] and curcumin (Curcuma longa) [40]. The most convincing evidence suggest that the association of a bowel cholesterol inhibitor nutraceutical and a cholesterol excreting natural molecule (in particular, berberine) to a statin could be an efficient and safe approach to improve cholesterolemia control in a large number of patients.
However, some nutraceuticals could exert a significant reduction in LDL-cholesterol (Table 11.1), thus clinicians should be informed about their efficacy and safety, in order to use them as preventive tools as additive tools to potentiate more conventional treatments in high-risk subjects. They should also be able to give the consumer full information about the product they are assuming. Further clinical research is advisable to individuate between the available lipid-lowering nutraceuticals with the best cost-effectiveness and risk-benefit ratio for large use in the general population, and in particular in statin treated patients.
References
Ackermann RT, et al. Garlic shows promise for improving some cardiovascular risk factors. Arch Intern Med. 2001;161:813–24.
Affuso F, et al. A nutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J Cardiol. 2012;4:77–83.
Anderson GD, et al. Drug interaction potential of soy extract and Panax ginseng. J Clin Pharmacol. 2003;43:643–8.
Anderson JW, et al. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–82.
Berginc K, et al. Garlic flavonoids and organosulfur compounds: impact on the hepatic pharmacokinetics of saquinavir and darunavir. Drug Metab Pharmacokinet. 2010;25(6):521–30.
Berthold HK, et al. Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: a randomized, controlled trial. JAMA. 2006;295:2262–9.
Brashears MM, et al. Bile salt deconjugation and cholesterol removal from media by Lactobacillus casei. J Dairy Sci. 1998;81:2103–10.
Burcelin R, et al. The gut microbiota ecology: new opportunity for the treatment of metabolic diseases? Front Biosci. 2009;14:5107–17.
Cambria-Kiely JA. Effect of soy milk on warfarin. Ann Pharmacother. 2002;36:1893–6.
Cani PD, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–103.
Chan E. Displacement of bilirubin from albumin by berberine. Biol Neonat. 1993;63:201–8.
Chen ST, et al. Variable effects of soy protein on plasma lipids in hyperlipidemic and normolipidemic hemodialysis patients. Am J Kidney Dis. 2005;46:1099–106.
Chua M, et al. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. J Ethnopharmacol. 2010;128:268–78.
Cicero AFG, Ertek S. Metabolic and cardiovascular effects of berberine: from preclinical evidences to clinical trial results. Clin Lipidol. 2009;4(5):553–63.
Cicero AFG, et al. Antihyperlipidaemic effect of a Monascus purpureus brand dietary supplement on a large sample of subjects at low risk for cardiovascular disease: a pilot study. Complement Ther Med. 2005;13:273–8.
Cicero AFG, et al. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents in humans. Arzneimittelforschung. 2007;57:26–30.
Cicero AFG, et al. Long-term efficacy and tolerability of a largely marketed multicomponent nutraceutical in overweight and normoweight dyslipidaemic patients. Nutrafoods. 2012;11(2):15–21.
Cicero AFG, et al. Tolerability and safety of commonly used dietary supplements and nutraceuticals with lipid-lowering effects. Expert Opin Drug Saf. 2012;11(5):753–66. doi:10.1517/14740338.2012.705827.
Cicero AFG, et al. Effect of a lipid-lowering nutraceutical on pulse-wave-velocity in hypercholesterolemic patients with or without chronic kidney disease. Open Hypertens J. 2013;5:18–22.
Cicero AFG, et al. Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr Res. 2013;33(8):622–8. doi:10.1016/j.nutres.2013.05.01.
Cicero AFG, et al. Nutraceuticals for metabolic syndrome management: from laboratory to benchside. Curr Vasc Pharmacol. 2014;12(4):565–71.
Cicero AFG, et al. Berberine and monacolin effects on the cardiovascular risk profile of women with oestroprogestin-induced hypercholesterolemia. High Blood Press Cardiovasc Prev. 2014;21(3):221–6. doi:10.1007/s40292-014-0052-5.
Deng R. Therapeutic effects of guggul and its constituent guggulsterone: cardiovascular benefits. Cardiovasc Drug Rev. 2007;25(4):375–90.
Derosa G, et al. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther. 2012;12(8):1113–24.
Eisenbrand G. Toxicological evaluation of red mould rice. Mol Nutr Food Res. 2006;50:322–7.
El Khoury D, Anderson GH. Recent advances in dietary proteins and lipid metabolism. Curr Opin Lipidol. 2013;24(3):207–13. doi:10.1097/MOL.0b013e3283613bb7.
Gheit O, et al. Efficacy and safety of Monascus purpureus Went rice in subjects with secondary hyperlipidemia. Eur J Intern Med. 2009;20:e57–61.
Gliozzi M, et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int J Cardiol. 2013;170(2):140–5. doi:10.1016/j.ijcard.2013.08.125.
Grieco A, et al. A cute hepatitis caused by a natural lipid-lowering product: when “alternative” medicine is no “alternative” at all. J Hepatol. 2009;50:1273–7.
Grill JP, et al. Effects of Lactobacillus amylovorus and Bifidobacterium breve on cholesterol. Lett Appl Microbiol. 2000;31:154–6.
Grundy SM. Stanol esters as a component of maximal dietary therapy in the National Cholesterol Education Program Adult Treatment Panel III report. Am J Cardiol. 2005;96(suppl):47D–50.
Guardamagna O, et al. Primary hyperlipidemias in children: effect of plant sterol supplementation on plasma lipids and markers of cholesterol synthesis and absorption. Acta Diabetol. 2011;48:127–33.
Guo Z, et al. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:844–50.
Gylling H, et al. The effect of a very high daily plant stanol ester intake on serum lipids, carotenoids, and fat soluble vitamins. Clin Nutr. 2010;29:112–8.
Gylling H, et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis. 2014;232(2):346–60. doi:10.1016/j.atherosclerosis.2013.11.043.
Halbert SC, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105(2):198–204.
Harland JI, Haffner TA. Systematic review, meta-analysis and regression of randomized controlled trials reporting an association between an intake of circa 25 g soya protein per day and blood cholesterol. Atherosclerosis. 2008;200:13–27.
Hutchins AM, et al. Hypertensive crisis associated with high dose soy isoflovane supplementation in a post-menopausal woman; a case report. BMC Womens Health. 2005;5:9.
Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs. A systematic review. Drugs. 2001;61:2163–75.
Kang Q, Chen A. Curcumin suppresses expression of low-density lipoprotein receptor, leading to the inhibition of LDL-induced activation of hepatic stellate cells. Br J Pharmacol. 2009;157:1354–67.
Keithley J, Swanson B. Glucomannan and obesity: a critical review. Altern Ther Health Med. 2005;11:30–4.
Keithley J, et al. A pilot study of the safety and efficacy of cholestin in treating HIV-related dyslipidemia. Nutrition. 2002;18:201–4.
Klaver FA, Van der Meer V. The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt –deconjugating activity. Appl Environ Microbiol. 1993;59:1120–4.
Kim A, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720–9.
Kong W, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51.
Kumar M, et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res. 2012;2012:902917.
Kwak JS, et al. Garlic powder intake and cardiovascular risk factors: a meta-analysis of randomized controlled clinical trials. Nutr Res Pract. 2014;8(6):644–54. doi:10.4162/nrp.2014.8.6.644.
Lenz TL. Therapeutic lifestyle changes and pharmaceutical care in the treatment of dyslipidemias in adults. J Am Pharm Assoc. 2005;45(4):492–9.
Li G, et al. A tea catechin, epigallocatechin-3-gallate, is a unique modulator of the farnesoid X receptor. Toxicol Appl Pharmacol. 2012;258(2):268–74.
Lin YL, et al. Biologically active components and nutraceuticals in Monascus-fermented rice: a review. Appl Microbiol Biotechnol. 2008;77:965–73.
Liu J, et al. Chinese red yeast rice (monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chin Med. 2006;1:4.
Liu ZL, et al. Chinese herbal medicines for hypercholesterolemia. Cochrane Database Syst Rev. 2011;(7):CD008305.
Macan H, et al. Aged garlic extract may be safe for patients on warfarin therapy. J Nutr. 2006;136(3 Suppl):793S–5.
Maciejko JJ, et al. Psyllium for the reduction of cholestyramine-associated gastrointestinal symptoms in the treatment of primary hypercholesterolemia. Arch Fam Med. 1994;3:955–60.
Mannarino MR, et al. Nutraceuticals for the treatment of hypercholesterolemia. Eur J Intern Med. 2014;25(7):592–9. doi:10.1016/j.ejim.2014.06.008.
Marinangeli CP, et al. Policosanols as nutraceuticals: fact or fiction. Crit Rev Food Sci Nutr. 2010;50:259–67.
Martino F, et al. Effect of dietary supplementation with glucomannan on plasma total cholesterol and low density lipoprotein cholesterol in hypercholesterolemic children. Nutr Metab Cardiovasc Dis. 2005;15:174–80.
McCarty MF. Glucomannan minimizes the postprandial insulin surge: a potential adjuvant for hepatothermic therapy. Med Hypotheses. 2002;58(6):487–90.
McGowan MP, Proulx S. Nutritional supplements and serum lipids: does anything work? Curr Atheroscler Rep. 2009;11:470–6.
Mechanick JI, et al. AACE Nutrition Guidelines Taskforce. American Association of Clinical Endocrinologists medical guidelines for the clinical use of dietary supplements and nutraceuticals. Endocr Pract. 2003;9:417–70.
Mensink RP, et al. Plant stenols dose dependently decrease LDL cholesterol concentrations but not cholesterol standardized fat soluble antioxidant concentrations, at intakes up to 9g/d. Am J Clin Nutr. 2010;92:24–33.
Mogadishian MH, et al. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apolipoprotein E-deficient mice: possible mechanisms of action. Circulation. 1999;99:1733–9.
Mollace V, et al. Hypolipemic and hypoglycaemic activity of bergamotpolyphenols: from animal models to human studies. Fitoterapia. 2011;82(3):309–16. doi:10.1016/j.fitote.2010.10.014.
Moreyra AE, et al. Effect of combining psyllium fiber with simvastatin in lowering cholesterol. Arch Intern Med. 2005;165:1161–6.
Morgan LM, et al. The effect of soluble- and insoluble-fibre supplementation on post-prandial glucose tolerance, insulin and gastric inhibitory polypeptide secretion in healthy subjects. Br J Nutr. 1990;64:103–10.
Moruisi KG, et al. Phytosterols/stanols lower cholesterol concentrations in familial hypercholesterolemic subjects: a systematic review with meta-analysis. J Am Coll Nutr. 2006;25:41–8.
Mullen E, et al. Soy isoflavones affect sterol regulatory element binding proteins (SREBPs) and SREBP-regulated genes in HepG2 cells. J Nutr. 2004;134:2942–7.
Nijjar PS, et al. Role of dietary supplements in lowering low-density lipoprotein cholesterol: a review. J Clin Lipidol. 2010;4(4):248–58.
Potter SM. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J Nutr. 1995;125(3 Suppl):606S–11.
Ras RT, et al. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies. Br J Nutr. 2014;112(2):214–9. doi:10.1017/S0007114514000750.
Ried K, et al. Effect of garlic on serum lipids: an updated meta-analysis. Nutr Rev. 2013;71(5):282–99. doi:10.1111/nure.12012.
Samman S, et al. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr. 2001;73:607–12.
Scholle JM, et al. The effect of adding plant sterols or stanols to statin therapy in hypercholesterolemic patients: systematic review and meta-analysis. J Am Coll Nutr. 2009;28:517–24.
Shirashi M, et al. Association between serum folate levels and tea consumption during pregnancy. Biosci Trends. 2010;4:225–30.
Shrestha S, et al. A combination of psyllium and plant sterols alters lipoprotein metabolism in hypercholesterolemic subjects by modifying the intravascular processing of lipoproteins and increasing LDL uptake. J Nutr. 2007;137:1165–70.
Singh DK, et al. Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J Pharmacol Exp Ther. 2006;318:1020–6.
Sirtori CR, Lovati MR. Soy proteins and cardiovascular disease. Curr Atheroscler Rep. 2001;3:47–53.
Sood N, et al. Effect of glucomannan on plasma lipid and glucose concentrations, body weight, and blood pressure: systematic review and meta-analysis. Am J Clin Nutr. 2008; 88(4):1167–75.
Stock J. Focus on lifestyle: EAS Consensus Panel Position Statement on Phytosterol-added Foods. Atherosclerosis. 2014;234(1):142–5. doi:10.1016/j.atherosclerosis.2014.01.047.
Tokunaga S, et al. Green tea consumption and serum lipids and lipoproteins in a population of healthy workers in Japan. Ann Epidemiol. 2002;12:157–65.
Torres N, et al. Regulation of lipid metabolism by soyprotein and its implication in diseases mediated by lipid disorders. J Nutr Biochem. 2006;17:365–73.
Trimarco B, et al. Clinical evidence of efficacy of red yeast rice and berberine in a large controlled study versus diet. Med J Nutrition Metab. 2011;4:133–40.
Vega-López S, et al. Sex and hormonal status modulate the effects of psyllium on plasma lipids and monocyte gene expression in humans. J Nutr. 2003;133:67–70.
Vergara-Jimenez M, et al. Hypolipidemic mechanisms of pectin and pysllium in guinea pigs fed high fat-sucrose diets: alterations on hepatic cholesterol metabolism. J Lipid Res. 1998;39:1455–65.
Vrzal R, et al. Activation of the arylhydrocarbon receptor by berberine in HepG2 and H4IIEcells: Biphasic effect on CYP1A1. Biochem Pharmacol. 2005;70:925–36.
Vuksan V, et al. Konjac-Mannan and American ginsing: emerging alternative therapies for type 2 diabetes mellitus. J Am Coll Nutr. 2001;20(S5):370S–80.
Weghuber D, Widhalm K. Effect of 3 month treatment of children and adolescents with familial and polygenic hypercholesterolemia with a soy-substituted diet. Br J Nutr. 2008;99:281–6.
Wei ZH, et al. Time- and dose-dependent effect of psylium on serum lipids in mild- to- moderate hypercholesterolemia: a meta—analysis of controlled trials. Eur J Clin Nutr. 2009;63:821–7.
Weingärtner O, et al. Vascular effects of diet supplementation with plant sterols. J Am Coll Cardiol. 2008;51(16):1553–61.
Weingärtner O, et al. Controversial role of plant sterol esters in the management of hypercholesterolaemia. Eur Heart J. 2009;30(4):404–9.
Weingärtner O, et al. Differential effects on inhibition of cholesterol absorption by plant stanol and plant sterol esters in apoE−/− mice. Cardiovasc Res. 2011;90(3):484–92.
Xin HW, et al. The effects of berberine on the pharmacokinetics of cyclosporine A in healthy volunteers. Methods Find Exp Clin Pharmacol. 2006;28:25–9.
Yin J, et al. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–7.
Yu L. The structure and function of Niemann-Pick C1-like 1 protein. Curr Opin Lipidol. 2008;19(3):263–9.
Zheng XX, et al. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr. 2011;94:601–10.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cicero, A.F.G., Colletti, A. (2015). Statins and Nutraceuticals/Functional Food: Could They Be Combined?. In: Banach, M. (eds) Combination Therapy In Dyslipidemia. Adis, Cham. https://doi.org/10.1007/978-3-319-20433-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-20433-8_11
Publisher Name: Adis, Cham
Print ISBN: 978-3-319-20432-1
Online ISBN: 978-3-319-20433-8
eBook Packages: MedicineMedicine (R0)