Abstract
Scutellaria baicalensis has been reported to improve the lipid metabolism of high-fat diet-induced liver dysfunction, but direct evidence is rare. This study aimed to explore the effects and mechanisms of S. baicalensis and its major constituent baicalin on hepatic lipotoxicity. KK-Ay mice and orotic acid (OA)-induced nonalcoholic fatty liver disease (NAFLD) rats were used to evaluate lipid metabolism regulatory effects. Sodium oleate-induced triglyceride-accumulated HepG2 cells were used for the mechanism study, pretreated with or without compound C or STO-609 or transfected with liver kinase B1 (LKB1) siRNA. In KK-Ay mice, S. baicalensis extract showed a decreased effect on serum and hepatic triglycerides, total cholesterols, and free fatty acid (FFA) levels after 8 weeks of treatment. In OA-induced NAFLD rats, 18 days of treatment with baicalin significantly inhibited hepatic lipid accumulation, attenuating hepatocyte hypertrophy, vacuolization and necrosis. S. baicalensis and baicalin treatment significantly suppressed the sterol regulatory element binding protein-1c (SREBP-1c) transcriptional program with downregulation of gene and protein expression of SREBP-1c (both precursor and mature fraction) and acetyl-CoA carboxylase, fatty acid synthase and stearoyl-CoA desaturase, and upregulation of AMP-activated protein kinase (AMPK), carnitine palmitoyl transferase 1 and nuclear respiratory factor 2 in the liver. Furthermore, activation of AMPK by baicalin was observed to be relative to the increase in phosphorylation of calmodulin-dependent protein kinase kinase. Taken together, S. baicalensis conferred preventive effects against FFA-induced lipotoxicity through the AMPK-mediated SREBP signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a form of chronic metabolic disease with a clinical symptom of excess lipid accumulation in the liver. Epidemiological research revealed that >30% of South American and Middle East people suffer from NAFLD [1]. Recently, there has been an increasing trend of NAFLD in China, where the average prevalence is 27.1% [2]. It is now clearly recognized that patients with NAFLD have an increased risk of diabetes, cardiovascular disease and liver-related mortality [3].
The occurrence and development of NAFLD in humans is closely related to lipotoxicity induced by free fatty acids (FFAs). FFAs are derived from the ingestion of excess carbohydrates (especially fructose), lipoprotein remnants and lipolysis in adipose tissue and are actively taken up by various transporters to be delivered into the liver. Storage of surplus FFAs in hepatocytes causes lipotoxicity via the induction of reactive oxygen species (ROS) release, leading to oxidation stress, inflammation, apoptosis, and promoting hepatic injury to liver fibrogenesis. An increasing attention has been paid to lowering FFA levels in NAFLD prevention and therapy [4].
The AMP-activated protein kinase (AMPK)-mediated sterol regulatory element binding protein (SREBP) signaling pathway plays an important role in FFA metabolism. SREBPs have been demonstrated as key nodes of diverse physiological and pathophysiological processes. Among them, SREBP-1c has been highlighted the significance of FFAs in cellular and systematic homeostasis [5]. In the process of initiating lipid synthesis, SREBP-1c is transported from the endoplasmic reticulum (ER) to the nucleus as homodimers [6], binds to SRE sequences and stimulates the transcription of downstream target genes, such as fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC), and stearoyl-CoA desaturase (SCD) to regulate fatty acid metabolism.
SREBP-1c is regulated by various upstream proteins, including phosphoinositide 3-kinase, mammalian target of rapamycin [7], and AMPK [8, 9]. AMPK is a key sensor of fuel and energy status, and it is currently the only protein kinase targeted in the treatment of metabolic syndrome [10]. Activation of AMPK attenuates the proteolytic processing of SREBP-1c, and results in accelerating fatty acid oxidation to abolish the abnormal accumulation of FFAs. Numerous evidences indicate that NAFLD is at least partially attributable to the AMPK-mediated SREBP signaling pathway.
The dried root of Scutellaria baicalensis belonging to Lamiaceae, is a Traditional Chinese Medicine (TCM) used for relieving heat, drying dampness, purging fire and removing toxins. Some TCM formulas containing S. baicalensis showed an ameliorating effect on NAFLD [11]. Scutellaria baicalensis extract (SBE) exerted regulating effects on hyperglycemia, hypertriglyceride, and hypercholesterolemia [12]. Baicalin, the major flavonoid in S. baicalensis, has been demonstrated to show significant anti-inflammation, anti-oxidation, and anti-cancer activities [13]. Baicalin was also reported to improve the lipid metabolism of high-fat diet-induced liver dysfunction [14, 15], and modulate calmodulin-dependent protein kinase kinase (CaMKK), AMPK and ACC [16]. However, the mechanism remains unclear without sufficient molecular biological supporting evidences. To date, there are few studies on the beneficial effects of S. baicalensis or baicalin on FFA-induced hepatic lipotoxicity in vivo and in vitro.
Here, we first applied abnormal hepatic lipid metabolism animal models to determine the consequences of SBE and baicalin on NAFLD lipid homeostasis. Second, a molecular biological method was used to confirm the relationship between baicalin and the AMPK-mediated SREBP signaling pathway. Finally, we addressed the concrete mechanism of baicalin on FFA production and degradation.
Materials and methods
Plant material
Scutellaria baicalensis roots were collected from Hebei Province, China, and identified by Dr. Feng Qiu at Tianjin University of Traditional Chinese Medicines (TUTCM). A voucher specimen was deposited at the Tianjin International Joint Academy of Biomedicine of TUTCM.
Sample preparation and baicalin content detection
The dried roots of S. baicalensis (1 kg) were extracted twice with 10 L of boiling water over a period of 2 h, and then filtered and lyophilized in a freezing dryer. The dry weight of the extract (SBE) was 628 g (yield 62.8%). Baicalin, baicalein, wogonoside, wogonin, scutellarin, scutellarein and oroxylin A were purchased from Zhong Xin Pharmaceuticals (purity >98.0%, Tianjin, China) and were used as standards. The baicalin content of the S. baicalensis was 14.0% using the HPLC method based on a report in the literature [12]. Baicalein, wogonoside, wogonin, scutellarin, scutellarein and oroxylin A in the SBE were 0.3, 3.2, 0.1, 0.4, 0.1 and 0.1% respectively.
Animals
Eight-week-old male KK-Ay mice (28–32 g) and C57BL/6J (C57) mice (24–26 g) were purchased from Beijing HFK Bioscience Ltd. Co., and four-week-old male Sprague–Dawley (SD) rats (100–120 g) were obtained from Beijing Vital River Laboratory Animal Technology Ltd. Co. All the animals were housed with two to a cage and acclimatized for 1 week before the experiments. The animals were fed with a standard diet and drink ad libitum and adapted to the experimental conditions at 22 ± 2 °C, humidity 60 ± 5%, and a 12 h light–dark cycle. The experimental animals were overseen and all protocols were approved by the Committee of the Animal Use and Care Committee of TUTCM.
Regulatory effects of SBE on hepatic lipid metabolism in KK-Ay mice
After 1 week of acclimatization with free eating and drinking, blood samples (ca. 0.2 ml) were collected from the infraorbital venous plexus before the experiment (0 day). Levels of serum triglyceride (TG) and glucose (GLU) of non-fasted KK-Ay mice were measured using commercial kits (BioSino Bio-Technology and Science Inc., China). C57 mice were assigned as the normal group. KK-Ay mice were randomly divided into five experimental groups (n = 10 in each group) based on serum TG and GLU levels as follows—control group, rosiglitazone group (20 mg/kg; Ark Pharm Inc., USA), SBE high-dose group (500 mg/kg), SBE middle-dose group (250 mg/kg), and SBE low-dose group (125 mg/kg). The maximum oral administration dose of mice was translated from human diary dose recommended in China pharmacopoeia. Test samples suspended in 5% acacia solution and vehicle (5% acacia solution) were given orally (0.1 ml/10 g body weight) to C57 and KK-Ay mice once daily, at 4:00 pm with a normal diet for 8 consecutive weeks.
Blood samples (ca. 0.2 ml) were collected from the infraorbital venous plexus once every 2 weeks after administration. Levels of serum TG, total cholesterol (TC), FFA and GLU of non-fasted KK-Ay mice were measured using commercial kits (TG, TC and GLU, BioSino Bio-Technology and Science Inc.; FFA, NEFA C-test Wako, Wako Pure Chemical Industries, Ltd., Japan). In addition, body weight, food intake, and water intake of the mice were recorded at daily intervals throughout the experimental period. The animals were fasted overnight prior to killing and blood samples were collected via canthus blood. The serum was immediately separated from the blood via centrifugation and stored at −80 °C until analysis. The mice were dissected open and then perfused with 10 ml Krebs–Henseleit bicarbonate through the abdominal vein while cutting the portal until the liver and kidneys were blanched. The tissues were dissected out and snap frozen in liquid nitrogen and stored at −80 °C for Western blot, real-time polymerase chain reaction (PCR) analysis.
Regulatory effect of baicalin on hepatic lipid metabolism in orotic acid (OA)-induced NAFLD rats
After 1 week of acclimatization with free eating and drinking, the rats were fed with diets containing 1% OA and 33% sugar to establish NAFLD mammal models [17]. The rats were randomly assigned to six experimental groups (n = 10 in each group) as follows—normal group, control group, fenofibrate group (FF, 50 mg/kg; Abbott Laboratories, USA), baicalin high-dose group (50 mg/kg), baicalin middle-dose group (25 mg/kg), and baicalin low-dose group (12.5 mg/kg). Test samples suspended in 5% acacia solution and vehicle (5% acacia solution) were given orally (0.1 ml/20 g body weight) to SD rats once daily, at 4:00 pm with a normal diet for 18 consecutive days.
Body weight, food intake, and water intake of the animals were recorded at daily intervals throughout the experimental period. The animals were fasted overnight prior to killing. They were then dissected open and perfused with 10 ml Krebs–Henseleit bicarbonate through the abdominal vein while cutting the portal until the liver and kidneys were blanched. Tissues were excised and snap frozen in liquid nitrogen and stored at −80 °C for the following experiments. The livers were also bisected and embedded into 4% paraformaldehyde for histological analysis.
Hepatic lipid extraction and thin-layer chromatography (TLC) analysis
Hepatic lipid extraction was adapted from the Folch method [18] and modified slightly. Briefly, approximately 100 mg of liver tissue was homogenized in 500 μl ice-cold saline using a motor homogenizer for 30 s in a 1.5-ml EP tube. A chloroform: methanol (2:1, v/v) mixture 1.5 ml was added to the homogenate and centrifuged for 10 min at 10,000g at 4 °C. The lower organic phase was separated and blown dry with nitrogen gas. The dried lipid was then re-dissolved with 500 μl of isopropanol, which was used for TG, TC and FFA analysis as described previously.
TLC was applied to analyze the lipid profiles. The developing system was n-heptane/isopropyl ether/acetic acid (60:40:3, v/v/v), with 10% (v/v) sulfuric acid–ethanol solution as chromogenic agent and heating at 120 °C for 2 min.
Histological assessment
For hematoxylin–eosin (H&E) staining, liver tissues were fixed in 4% paraformaldehyde, and embedded in paraffin. For Oil Red O staining, liver tissues were frozen in liquid nitrogen. The paraffin and frozen sections were stained with H&E and Oil Red O, respectively, for conventional morphological evaluation.
Real-time PCR analysis
Liver RNA isolation, cDNA synthesis and real-time PCR analysis were performed as described previously [19]. The specific primers utilized for real-time PCR of AMPK, SREBP-1c, FAS, ACCα, SCD, carnitine palmitoyl transferase (CPT)-1α, nuclear respiratory factor 2 (Nrf2) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are listed in Table 1. Relative gene expression quantification was normalized to GAPDH mRNA expression by applying the 2− △△CT methods. Analysis was carried out in triplicate.
Western blot analysis
Liver protein isolation and Western blotting were performed as described previously [19]. Protein extracts (30–50 μg) were separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. Membranes were blocked and immunoblotted with primary antibodies specific for β-actin [ab8227, Abcam Plc. UK (Ab)], AMPK (ab131512, Ab), p-AMPK Thr172 (ab133448, Ab), SREBP-1c (ab28481, Ab), p-SREBP1c Ser372 (9874s, Cell Signaling Technology Inc. MA, USA (CST)), FAS (ab128870, Ab), ACC (ab45174, Ab), p-ACC S79 (ab68191, Ab), and CPT-1 (15184-1-AP, Proteintech Group, USA). Secondary antibodies conjugated with 1:10,000 dilution of horseradish peroxidase (Zhong Shan Gold Bridge Bio. Co. Ltd., Beijing, China) were applied for 1 h. The bands were visualized by enhanced chemiluminescence kits (Millipore Co. Ltd., MA, USA).
Cell culture and treatments
The HepG2 cell line (SCSP-510) was obtained from the Chinese Academy of Science (Shanghai, China). The cells were maintained in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Corning, USA) containing 1% antibiotic/antimycotic (Gibco, Gaithersburg, MD, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco) at 37 °C in an atmosphere containing 95% air and 5% CO2.
We previously established a lipid accumulation model in HepG2 cells through the induction of sodium oleate (Sigma, St. Louis, MO, USA) [20]. When cultured with 70–80% confluence, the cells were seeded on 24-multiwell plates (Eppendorf AG, Germany) for 24 h, then treated with sodium oleate (200 μM) accompanied by different concentrations of baicalin and baicalein (0, 10, 100 μM) and 5 μM orlistat as a positive control. AMPK inhibitor compound C (1 μM; Calbiochem Co. Ltd., Tokyo, Japan) and CaMKK inhibitor STO-609 (1 μM; Calbiochem Co. Ltd., Tokyo, Japan) were used to verify the signaling pathway. After 48 h treatment, the amount of intracellular TG content was determined with a commercial TG kit at 492 nm after cell lysis.
RNA interference of liver kinase B1 (LKB1)
Small interfering RNA (siRNA) for LKB1 and control siRNA were synthesized by GenePharma Co. Ltd (Shanghai, China). The siRNA sequence for targeting LKB1 was 5′-CCAACGUGAAGAAGGAAAUTT-3′. As a negative control, a siRNA sequence targeting luciferase was used: 5′-UUCUCCGAACGUGUCACGUTT-3′. HepG2 cells were transfected with 100 nM of siRNA for 6 h using lipofectamine-2000 reagent (Invitrogen, USA) following the manufacturer’s protocol. The medium was then exchanged with fresh complete medium. After 24 h transfection, cells were incubated with sodium oleate (200 μM) accompanied with different concentrations of baicalin and baicalein (0, 10, 100 μM) and 5 μM orlistat as a positive control. After 48 h treatment, the amount of intracellular TG content was determined with a commercial TG kit at 492 nm after cell lysis.
Western blot analysis of HepG2 cells
Confluent cultures of HepG2 cells in 6-well plates (Eppendorf AG) were induced as previously described. Protein was isolated from HepG2 cells with NE-PER nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific Inc., Waltham, MA, USA), containing phenylmethyl sulfonyl fluoride and phosphatase inhibitors (100 μM). The protein concentration of the supernatant was measured using the BCA protein assay kit (Thermo Fisher Scientific Inc.) with bovine serum albumin as standard.
Equal amounts of proteins (30–50 μg) were separated via SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were blocked and immunoblotted with primary antibodies specific for β-actin, LaminB (GTX103292, GeneTex, USA), AMPK, p-AMPK Thr172, CaMKK (ab117594, Ab), p-CaMKK Ser511 (12818s, CST), LKB1 (ab15095, Ab), p-LKB1 Ser428 (3482s, Ab), SREBP-1c, p-SREBP1c Ser372, FAS, ACC, p-ACC S79, SCD 1 (ab19862, Ab), CPT-1, hormone sensitive lipase (HSL) (4107s, CST), p-HSL Ser563 (4139s, CST), p-HSL Ser565 (4137s, CST), p-HSL Ser660 (4126s, CST), and adipose triglyceride lipase (ATGL) (ab99532, Ab). Secondary antibodies conjugated with 1:10,000 dilution of the horseradish peroxidase were applied for 1 h. Bands were visualized by enhanced chemiluminescence kits.
Statistical analysis
All experiments were repeated three times. Values were expressed as mean ± SEM. The results were analyzed using an analysis of variance (ANOVA) and Tukey’s Studentized range test. A p value of < 0.05 was considered statistically significant.
Results
SBE diminished aberrant increase in liver weight index in KK-Ay mice
KK-Ay mice are spontaneous type 2 diabetic rodent models that exhibit marked obesity, impaired glucose tolerance, severe insulin resistance, and dyslipidemia [21]. To determine the effect of SBE treatment in KK-Ay mice, we kept records of body weight, and food and water intake throughout the experimental period. The C57 mouse (inbred strain with stable genetic background) was used as a normal control. Compared with C57 mice, an increase in food/water intake was found in KK-Ay mice. There was a significant decrease in food/water intake in SBE-treated mice (data not shown). It is reported in the literature that the safe dosage of S. baicalensis aqueous extract was >1 g/kg mice body weight/day [22]. This result suggests that SBE might attenuate the symptoms of diabetic excess consumption of food and water. As listed in Table 2, compared with the normal group, the liver weight index (the ratio of liver to body weight) of the control group and the rosiglitazone treatment group was remarkably increased; however, the SBE treatment significantly reduced the liver weight index by at least 35% compared to the control group. These results indicate that SBE treatment could inhibit hepatomegaly in KK-Ay mice.
SBE alleviated relevant lipid biochemical parameter levels in serum and liver of KK-Ay mice
We measured unfasted serum TG, TC, FFA, and GLU levels every 2 weeks. Compared to C57 mice, significantly increased serum TG, TC, FFA and GLU levels were observed in KK-Ay mice (p < 0.01, Fig. 1a–d). From 2 weeks to the end of SBE treatment, serum TG, TC, FFA and GLU levels were gradually lowered in SBE-treated mice compared with the control group. After the final administration, all the mice were fasted overnight. Fasting serum and liver TG, TC, and FFA levels were determined. As shown in Fig. 1e–h and Table 2, SBE treatment significantly decreased TG, TC and FFA levels in both the serum and liver of KK-Ay mice.
SBE alleviated relevant lipid biochemical parameter levels in the serum of KK-Ay mice. Unfasted serum TG (a), TC (b), FFA (c), and GLU (d) levels were measured every 2 weeks using commercial kits. Fasting serum TG (e), TC (f), FFA (g) and GLU (h) levels were quantified by corresponding assay kits. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group. N normal, C control, R rosiglitazone (i.g. 20 mg/kg). SBE was orally administered at 500, 250, and 125 mg/kg
SBE attenuated liver injury in KK-Ay mice by abolishing accumulation of FFAs through inhibiting biosynthesis and promoting oxidation
Recent studies demonstrated that SBE activated the key energy regulator, AMPK, to ameliorate obesity condition in db/db mice [12]. Mechanically, to determine whether AMPK plays a role in SBE-mediated effects on FFA accumulation in the liver, AMPK protein and mRNA levels were examined. As shown in Fig. 2a, d, administration of SBE could significantly induce AMPK phosphorylation with little change in AMPK mRNA and total protein expressions.
SBE attenuated liver injury in KK-Ay mice by abolishing accumulation of FFAs through inhibiting biosynthesis and promoting oxidation. Real-time PCR of AMPK, SREBP-1c (a), FAS, ACCα (b), SCD 2 and CPT-1α (c). d Protein expression levels in liver lysates from C57 and KK-Ay mice treated with rosiglitazone and different doses of SBE. Representative Western blotting images are shown. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group. N normal, C control, R rosiglitazone (i.g. 20 mg/kg). SBE was orally administered at 500, 250, and 125 mg/kg
It has been identified that Ser372 on SREBP-1c is a major phosphorylation site of AMPK, and Ser372 phosphorylation will inhibit SREBP-1c cleavage and translocation leading to downregulation of hepatic FFA biosynthesis [23]. We then analyzed the effects of SBE on SREBP-1c expression and inactivation. As shown in Fig. 2a, d, SBE administration could downregulate SREBP-1c gene expression at both transcriptional and translational levels. Notably, SBE increased Ser372 phosphorylation on SREBP-1c and, therefore, significantly reduced the level of its mature form.
In terms of AMPK activation and SREBP-1c downregulation, we then further tested their downstream genes associated with FFA synthesis, such as FAS, ACC and SCD. As shown in Fig. 2b–d, FAS, ACCα and SCD 2 expressions were decreased in both mRNA and protein level with SBE treatment. Consistent with AMPK activation, the phosphorylation of ACC was elevated in the treatment group. Moreover, we also observed an induction effect of SBE on CPT-1α mRNA and protein expression. These results indicate that SBE could inhibit FFA biosynthesis through AMPK-mediated SREBP-1c inactivation, and promote FFA oxidation; therefore, ameliorating lipid metabolic disorder caused by hepatic FFAs.
Baicalin alleviated relevant lipid hepatic biochemical index level in NAFLD rats
The results of KK-Ay with administration of SBE have shown good regulatory effects on serum and hepatic FFA levels. Baicalin is one of the major bioactive components in SBE. To further confirm the bioactive ingredients and relative mechanisms of S. baicalensis, we next chose OA-induced NAFLD rat models to evaluate the function of baicalin.
It has been well documented that OA induces fatty liver in a species-specific manner. The molecular mechanism regarding the fact that OA induces hepatic lipogenesis through suppressing the phosphorylation of AMPK and activating SREBP-1c in response to the AMPK-mediated SREBP signaling pathway has been investigated [17].
To evaluate the inhibitory effects of baicalin on hepatic lipid accumulation after the final administration of the test sample, all the rats were fasted overnight to measure the liver parameters. Levels of TG, TC, and FFAs were reduced in the baicalin-treated group similar to the positive group (p < 0.05, Fig. 3a–c). As shown in Fig. 3d, lipid extracts from liver homogenate were separated by TLC. The intensity of TG (shown as band I) and FFA (shown as band II) bands in the control group were higher than in the normal group. After the administration of fenofibrate or baicalin, the corresponding TG and FFA bands were much lower than those in the control group. However, there were no obvious differences in the proportion of diacylglycerols (shown as bands III and IV) among the different groups. These data demonstrate that baicalin treatment improved the abnormal accumulation of hepatic FFAs in NAFLD rats.
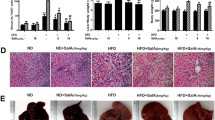
Baicalin alleviated relevant lipid hepatic biochemical index level in NAFLD rats. Liver TG (a), TC (b) and FFA (c) contents were detected using a commercial kit. d TLC method was applied to separate different lipid profiles of liver homogenate. e Histological analysis of liver sections with hematoxylin–eosin staining and Oil Red O staining, Scale bars: 100 μm. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group. N normal, C control, FF fenofibrate (i.g. 50 mg/kg). Baicalin was orally administrated at 50, 25, and 12.5 mg/kg. I, TG; II, FFAs; III, 1,3-diacylglycerol; IV, 1,2-diacylglycerol
Baicalin attenuated hepatic steatosis by abolishing FFA biosynthesis and accelerating its oxidation
There were obvious pathological changes in the liver tissue of OA-induced NAFLD rats, as evidenced by increased hepatocyte hypertrophy, vacuolization and necrosis by hepatic lipid droplets (Fig. 3e); however, baicalin treatment remarkably ameliorated OA-induced hepatic lipid injuries. Notably, the hepatic-protecting effect seen in the 50 and 25 mg/kg/day baicalin-treated groups was similar to the positive group. Consistently, the Oil Red O staining showed that 50 and 25 mg/kg/day baicalin treatment can significantly reduce hepatic lipid content and ameliorate hepatic injuries.
To further characterize the molecular mechanism of baicalin on repressing the abnormal accumulation of FFAs, we then analyzed the expression level of AMPK. As shown in Fig. 4a, d, baicalin treatment could remarkably induce AMPK phosphorylation, with limited effects on AMPK mRNA and total protein expression levels. We also detected the expression level of SREBP-1c and its downstream gene as well as genes involved in fatty acid oxidation. As shown in Fig. 4b, c, baicalin treatment could downregulate SREBP-1c expression in both mRNA and protein level. Moreover, SREBP-1c phosphorylation was increased, which was significantly accompanied by a reduction of its mature form.
Baicalin attenuated hepatic steatosis by abolishing FFA biosynthesis and accelerating its oxidation in an NAFLD rat model. Real-time PCR of AMPK, SREBP-1c (a), CPT-1α, Nrf 2 (b), FAS, ACCα, and SCD (c). d Protein expression levels in liver lysates from NAFLD rats treated with fenofibrate and different doses of baicalin. Representative Western blotting images are shown. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group. N normal, C control, FF fenofibrate (i.g. 50 mg/kg). Baicalin was orally administrated at 50, 25, and 12.5 mg/kg
Consistent with AMPK activation and SREBP-1c downregulation, FAS, ACCα, and SCD expressions were reduced in both mRNA and protein level with elevated ACC phosphorylation under baicalin treatment (Fig. 4c, d). In addition, we also observed an induction of CPT-1α protein and Nrf2 mRNA expression under baicalin treatment (Fig. 4b, d). These results suggest that baicalin treatment might suppress FFA-induced hepatic steatosis through inhibiting SREBP-1c-mediated fatty acid biosynthesis, promoting FFA oxidation and inducing antioxidative nuclear factor. To confirm the direct targets of baicalin, the mechanism by which baicalin regulates the AMPK-mediated SREBP signaling pathway was vigorously explored in vitro combined with relative pharmacological inhibitors.
Baicalin and baicalein activiated the AMPK signaling pathway to abrogate FFA accumulation in HepG2 cells
It was reported that the oral administration of baicalin was partially metabolized into baicalein; baicalin and baicalein are both major ingredients accumulated in the liver [24, 25]. Since baicalin and baicalein are the main active form in the liver after oral administration, to further define the underlying mechanism of baicalin and baicalein, we then explored their effects on lipid accumulation in HepG2 cells.
As shown in Fig. 5a, d, baicalin and baicalein suppressed sodium oleate-induced lipid accumulation. However, co-treatment with compound C could partially block the reducing effect of baicalin and baicalein on FFA-induced TG accumulation. In addition, co-treatment with STO-609, a CaMKK inhibitor, exerted a strong inhibitory function on the lipid-lowering effect of baicalin with limited effect on baicalein (Fig. 5b, e). Interestingly, we found that knockdown LKB1, another AMPK upstream gene, did not affect the TG lowering function of baicalin and baicalein in HepG2 cells (Fig. 5c, f), indicating that CaMKK is associated with baicalin-induced AMPK activation rather than LKB1.
Baicalin and baicalein activiated the CaMKK−AMPK signaling pathway in HepG2 cells. HepG2 cells pretreated with or without compound C (a, d) or STO-609 (b, e) for 1 h or transfected with LKB 1 siRNA (c, f) for 24 h prior to baicalin, baicalein and sodium oleate treatment. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group
As shown in Fig. 6a, both baicalin and baicalein could induce AMPK phosphorylation, while the increased phosphorylation of CaMKK was only observed in the baicalin-treated group. Indeed, baicalin and baicalein showed no effect on LKB1 phosphorylation. These results further confirm that activation of AMPK by baicalin is in a CaMKK-dependent manner.
Baicalin and baicalein inactivated SREBP-1c through stimulation of the CaMKK−AMPK−SREBP signaling pathway. a Western blot analysis of phosphorylated and total AMPK, CaMKK and LKB 1 in HepG2 cells treated with different concentrations of baicalin and baicalein for 48 h; b Western blot analysis of phosphorylated, precursor and mature SREBP-1c in HepG2 cells treated with different concentrations of baicalin and baicalein for 48 h; c Western blot analysis of phosphorylated and total ACC, FAS and SCD1 in HepG2 cells treated with different concentrations of baicalin and baicalein for 48 h; d Western blot analysis of CPT-1 in HepG2 cells treated with different concentrations of baicalin and baicalein for 48 h; e Western blot analysis of phosphorylated and total HSL and ATGL in HepG2 cells treated with different concentrations of baicalin and baicalein for 48 h. HepG2 cells were treated with different concentrations of baicalin and baicalein with 200 μM of sodium oleate for 48 h
Baicalin and baicalein suppressed SREBP-1c translocation to abolish FFA biosynthesis in HepG2 cells
To further confirm the function of baicalin and baicalein in CaMKK−AMPK controlled nuclear SREBP-1c translocation, we detected the phosphorylated and nuclear SREBP-1c levels in HepG2 cells. Consistent with our animal study, baicalin and baicalein could significantly induce SREBP-1c phosphorylation and reduce its nuclear translocation; however, the SREBP-1c precursor expression level was not affected (Fig. 6b).
Baicalin and baicalein suppressed fatty acid production and accelerated FFA oxidation in HepG2 cells
Next, we also investigated the effects of baicalin and baicalein on TG hydrolysis and FFA synthesis and oxidation in HepG2 cells. As expected, treatment with baicalin and baicalein led to a significant reduction of key proteins related to FFA production, including HSL, ATGL, FAS, ACC and SCD1 (Fig. 6c, e). Accordingly, the phosphorylation of ACC protein in the treatment group was increased.
Apart from suppressing FFA production, baicalin and baicalein mediated inhibitory feedbacks on FFAs and were also associated with enhanced FFA oxidation through upregulation of CPT-1 protein (Fig. 6d). Taken together, these results show that baicalin induced constitutive activation of CaMKK−AMPK leading to SREBP1c and following fatty acid synthesis gene suppression. Moreover, baicalin and baicalein also elevated FFA oxidation to suppress the aberrant accumulation of FFAs.
Discussion
NAFLD has a high incidence of hepatic disease. The common clinical pathogenic characteristic of NAFLD is hepatic steatosis associated with elevated serum FFA level [26, 27]. To date, no medication has been approved to treat NAFLD. Some medications are used for improving abnormal lipid metabolism conditions, such as pioglitazone for type 2 diabetes [28]. However, most NAFLD patients do not show high glucose levels and whole body insulin resistance; long-term anti-diabetic drug administration will lead to unexpected risks. For safety reasons, dietary supplements or complementary alternatives are choices for prevention and therapy of NAFLD.
There is concrete evidence to show the role of FFAs in NAFLD. It has been shown that hepatic accumulated FFAs and their derivatives cause lipotoxic hepatocellular injury [27], as well as overloading mitochondrial capacity leading to oxidative stress [29]. Treatment of abnormal FFA metabolism can generally be categorized into two ways—reducing production of FFAs and increasing FFA degradation.
AMPK is a critical molecule in the production of FFAs, which is activated by CaMKK and LKB1. In vivo research revealed that SBE and baicalin significantly ameliorated hepatic lipid homeostasis and upregulated phosphorylated-AMPK protein expression in liver tissue, which suggested that AMPK may be the key target for SBE and baicalin in NAFLD.
Our previous research results and other literature indicated that baicalin and baicalein are the major metabolites distributed in liver after oral administration of SBE or baicalin [30, 31]. The AMPK activation effects of baicalin and baicalein were examined using a classical sodium oleate-induced hepatocyte lipid-accumulated model. The results showed that baicalin and baicalein could decrease TG concentrations in HepG2 cells. However, AMPK inhibitor compound C significantly abrogated their TG-lowering effects. Corresponding to these results, AMPK protein expression in baicalin- and baicalein-treated cells was significantly upregulated.
AMPK is activated by upstream kinases, including LKB1 or CaMKK. Activation of AMPK in the baicalin-treated group showed increased phosphorylation of CaMKK and no effect on LKB1 phosphorylation, indicating that activation of AMPK by baicalin was in a CaMKK-dependent manner. These results were further confirmed using kinase inhibition or siRNA methods. This is consistent with previous reports in the literature that baicalin activated AMPK in response to a potential Ca2+/CaMKKβ-dependent pathway [32].
AMPK activation phosphorylates and inactivates SREBP-1c [33], leading to stimulating the oxidation of fatty acids and blockage of lipid production. During the initiation phase of the FFA synthesis process, SREBP-1c is transported from the ER to the Golgi and combined with SREBP cleavage-activating protein (SCAP), subsequently forming the SREBP−SCAP complex. After proteolytical activation by cleavage enzymes [34], SREBP-1c enters the nucleus as homodimers, binds to SRE sequences and stimulates the transcription of downstream target lipogenic genes, such as FAS, ACC and SCD.
Currently, there are few reports in the literature on the effects of baicalin on inactivation of SREBP-1c after activating AMPK [35, 36], and direct evidence is still lacking to demonstrate whether baicalin regulates phosphorylation and post-translational modification of SREBP-1. In this study, SBE and baicalin stimulated phosphorylation of SREBP-1c and prevented nucleus translocation of mature SREBP-1c in vivo. This mechanism was further confirmed using a classical sodium oleate-induced hepatocyte lipid-accumulated model. The p-SREBP1c protein expression in baicalin- and baicalein-treated cells was significantly upregulated compared with the control group, along with the downregulation of the SREBP-1c transcriptional program. Furthermore, baicalin and baicalein treatments showed inhibition of TG hydrolysis by downregulation of ATGL and p-HSL Ser563 protein expression, as well as upregulation of p-HSL Ser660 protein expression. These results indicate that baicalin and baicalein suppress FFA production through the AMPK-mediated SREBP signaling pathway.
On the other hand, increasing FFA degradation may be relative to the enhancement of FFA oxidation [37]. We also assessed the effects of SBE and baicalin in hepatic lipid disorder mammals or sodium oleate-induced hepatocytes on CPT-1 protein expression. Both in vivo and in vitro results indicated that the improving effect of baicalin was at least partly related to the elevation of CPT-1 level to increase FFA oxidation.
In conclusion, our results show that baicalin is a fine-tuner of AMPK-mediated SREBP signaling in attenuating hepatic FFA lipotoxicity. This study provided the possibility of developing new NAFLD medications from S. baicalensis and baicalin. Our next step is to carry out evidence-based clinical research to confirm the therapeutic effects on NAFLD in humans, and the chemical structural modification of baicalin to find stronger lead compounds in regulating lipid metabolism.
References
Lonardo A, Nascimbeni F, Maurantonio M, Marrazzo A, Rinaldi L, Adinolfi L (2017) Nonalcoholic fatty liver disease: evolving paradigms. World J Gastroenterol 23(36):6571–6592
Meng G, Zhang B, Yu F, Li C, Zhang Q, Liu L, Wu H, Xia Y, Bao X, Shi H, Su Q, Gu Y, Fang L, Yang H, Yu B, Sun S, Wang X, Zhou M, Jia Q, Jiao H, Wang B, Guo Q, Carvalhoa L, Sun Z, Song K, Yu M, Niu K (2017) Soft drinks consumption is associated with nonalcoholic fatty liver disease independent of metabolic syndrome in Chinese population. Eur J Nutr. https://doi.org/10.1007/s00394-017-1485-0
Benedict M, Zhang X (2017) Non-alcoholic fatty liver disease: an expanded review. World J Hepatol 9(16):715–732
Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A (2012) The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 56(4):952–964. https://doi.org/10.1016/j.jhep.2011.08.025
Shimano H, Sato R (2017) SREBP-regulated lipid metabolism: convergent physiology–divergent pathophysiology. Nat Rev Endocrinol. https://doi.org/10.1038/nrendo.2017.91
Goldstein JL, DeBose-Boyd RA, Brown MS (2006) Protein sensors for membrane sterols. Cell 124(1):35–46. https://doi.org/10.1016/j.cell.2005.12.022
Li S, Brown MS (2010) Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA 107(8):3441
Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ (2000) Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Gene Dev 14(22):2819–2830
Javary Joaquim, Allain-Courtois Nathalie, Saucisse Nicolas, Costet Pierre, Heraud Capucine, Benhamed Fadila, Pierre Rémi, Bure Corinne, Pallares-Lupon Nestor, Cruzeiro Marcio Do, Postic Catherine, Cota Daniela, Dubus Pierre, Rosenbaum Jean, Benhamouche-Trouillet S (2017) Liver Reptin/RUVBL2 controls glucose and lipid metabolism with opposite actions on mTORC1 and mTORC2 signalling. Gut. https://doi.org/10.1136/gutjnl-2017-314208
Schultze S, Hemmings B, Niessen M, Tschopp O (2012) PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Rev Mol Med 14:e1
He MY, Deng YX, Shi QZ, Zhang XJ, Lv Y (2014) Comparative pharmacokinetic investigation on baicalin and wogonoside in type 2 diabetic and normal rats after oral administration of traditional Chinese medicine Huanglian Jiedu decoction. J Ethnopharmacol 155(1):334–342. https://doi.org/10.1016/j.jep.2014.05.033
Song KH, Lee SH, Kim BY, Park AY, Kim JY (2013) Extracts of Scutellaria baicalensis reduced body weight and blood triglyceride in db/db mice. Phytother Res 27(2):244–250. https://doi.org/10.1002/ptr.4691
Li C, Lin G, Zuo Z (2011) Pharmacological effects and pharmacokinetics properties of radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos 32(8):427–445. https://doi.org/10.1002/bdd.771
Waisundara VY, Hsu A, Tan BK, Huang D (2009) Baicalin reduces mitochondrial damage in streptozotocin-induced diabetic Wistar rats. Diabetes Metab Res 25(7):671–677. https://doi.org/10.1002/dmrr.1005
Guo HX, Liu DH, Ma Y, Liu JF, Wang Y, Du ZY, Wang X, Shen JK, Peng HL (2009) Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol Sin 30(11):1505–1512. https://doi.org/10.1038/aps.2009.150
Xi Y, Wu M, Li H, Dong S, Luo E, Gu M, Shen X, Jiang Y, Liu Y, Liu H (2015) Baicalin attenuates high fat diet-induced obesity and liver dysfunction: dose-response and potential role of CaMKKbeta/AMPK/ACC pathway. Cell Physiol Biochem 35(6):2349–2359. https://doi.org/10.1159/000374037
Jung EJ, Kwon SW, Jung BH, Oh SH, Lee BH (2011) Role of the AMPK/SREBP-1 pathway in the development of orotic acid-induced fatty liver. J Lipid Res 52(9):1617–1625. https://doi.org/10.1194/jlr.M015263
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509
Zhang Y, Liu X, Han L, Gao X, Liu E, Wang T (2013) Regulation of lipid and glucose homeostasis by mango tree leaf extract is mediated by AMPK and PI3K/AKT signaling pathways. Food Chem 141(3):2896–2905. https://doi.org/10.1016/j.foodchem.2013.05.121
Gao J, Li J, An Y, Liu X, Qian Q, Wu Y, Zhang Y, Wang T (2014) Increasing effect of Tangzhiqing formula on IRS-1-dependent PI3K/AKT signaling in muscle. BMC Complement Altern Med 14:198. https://doi.org/10.1186/1472-6882-14-198
Gao Y, Gu C, Wang K, Wang H, Ruan K, Xu Z, Feng Y (2017) The effects of hypoglycemia and weight loss of total lignans from Fructus Arctii in KKAy mice and its mechanisms of the activity. Phytother Res. https://doi.org/10.1002/ptr.6003
Burnett BP, Silva S, Mesches MH, Wilson S, Jia Q (2007) Safety evaluation of a combination, defined extract of Scutellaria baicalensis and Acacia catechu. J Food Biochem 31(6):797–825
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13(4):376–388. https://doi.org/10.1016/j.cmet.2011.03.009
Qing-ming C, Xin-li H, Yan-mei L, Zhang K, Akao T, Tori MH (2001) Studies on metabolites of baicalin in human urine. China J Chin Mater Med 26(11):768–769
Zhang J, Cai W, Zhou Y, Liu Y, Wu X, Li Y, Lu J, Qiao Y (2015) Profiling and identification of the metabolites of baicalin and study on their tissue distribution in rats by ultra-high-performance liquid chromatography with linear ion trap-Orbitrap mass spectrometer. J Chromatogr B 985:91–102. https://doi.org/10.1016/j.jchromb.2015.01.018
Malhi H, Gores G (2008) Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis 28(4):360–369
Leamy A, Egnatchik R, Young J (2013) Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res 52(1):165–174
Rotman Y, Sanyal AJ (2017) Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 66(1):180–190. https://doi.org/10.1136/gutjnl-2016-312431
Neuschwander-Tetri B (2010) Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52(2):774–788
Akao T, Kawabata K, Yanagisawa E, Ishihara K, Mizuhara Y, Wakui Y, Sakashita Y, Kobashi K (2000) Baicalin, the predominant flavone glucuronide of scutellariae radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form. J Pharm Pharmacol 52(12):1563–1568
Hou Y-C, Lin S-P, Tsai S-Y, Ko M-H, Chang Y-C, Chao P-D (2010) Flavonoid pharmacokinetics and tissue distribution after repeated dosing of the roots of Scutellaria baicalensisin rats. Planta Med 77(05):455–460. https://doi.org/10.1055/s-0030-1250433
Ma Y, Yang F, Wang Y, Du Z, Liu D, Guo H, Shen J, Peng H (2012) CaMKKbeta is involved in AMP-activated protein kinase activation by baicalin in LKB1 deficient cell lines. PLoS One 7(10):e47900. https://doi.org/10.1371/journal.pone.0047900
Chen Q, Wang T, Li J, Wang S, Qiu F, Yu H, Zhang Y, Wang T (2017) Effects of natural products on fructose-induced nonalcoholic fatty liver disease (NAFLD). Nutrients. https://doi.org/10.3390/nu9020096
Li J, Liu X, Ran X, Chen J, Li X, Wu W, Huang H, Huang H, Long Y, Liang J, Cheng J, Tian H (2010) Sterol regulatory element-binding protein-1c knockdown protected INS-1E cells from lipotoxicity. Diabetes Obes Metab 12(1):35–46
Kwak DH, Lee JH, Song KH, Ma JY (2014) Inhibitory effects of baicalin in the early stage of 3T3-L1 preadipocytes differentiation by down-regulation of PDK1/Akt phosphorylation. Mol Cell Biochem 385(1–2):257–264. https://doi.org/10.1007/s11010-013-1834-0
Fang Penghua, Mei Yu, Zhang Lei, DanWan Mingyi Shi, Zhu Yan, Bo Ping, Zhang Z (2017) Baicalin against obesity and insulin resistance through activation of AKT/AS160/GLUT4 pathway. Mol Cell Endocrinol 448(2017):77–86. https://doi.org/10.1016/j.mce.2017.03.027
Zeng X, Yang J, Hu O, Huang J, Ran L, Chen M, Zhang Y, Zhou X, Zhu J, Zhang Q, Yi L, Mi M (2018) Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid Redox Sign. https://doi.org/10.1089/ars.2017.7172
Acknowledgements
This research was supported by the National Natural Science Foundation of China (81430095; 81673703; 81173524), Important Drug Development Fund, Ministry of Science and Technology of China (2017ZX09305–002).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, Q., Liu, M., Yu, H. et al. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J Nat Med 72, 655–666 (2018). https://doi.org/10.1007/s11418-018-1199-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1199-5