Abstract
Purpose
Recent investigations have established that the ingestion of a moderate dose of caffeine (3–6 mg kg−1) can increase exercise and sports performance in women. However, it is unknown whether the ergogenicity of caffeine is similar during all phases of the menstrual cycle. The aim of this investigation was to determine the ergogenic effects of caffeine in three phases of the menstrual cycle.
Methods
Thirteen well-trained eumenorrheic triathletes (age = 31 ± 6 years; body mass = 58.6 ± 7.8 kg) participated in a double-blind, cross-over, randomised experimental trial. In the (1) early follicular (EF); (2) preovulation (PO); (3) and mid luteal (ML) phases, participants either ingested a placebo (cellulose) or 3 mg kg−1 of caffeine in an opaque and unidentifiable capsule. After a 60-min wait for substance absorption, participants performed an incremental maximal cycle ergometer test until volitional fatigue (25 W/min) to assess peak aerobic cycling power (Wmax).
Results
In comparison to the placebo, caffeine increased Wmax in the EF (4.13 ± 0.69 vs. 4.24 ± 0.71 W kg−1, Δ = 2.7 ± 3.3%, P = 0.01), in the PO (4.14 ± 0.70 vs. 4.27 ± 0.73 W kg−1, Δ = 3.3 ± 5.0%; P = 0.03) and in the ML (4.15 ± 0.69 vs. 4.29 ± 0.67 W kg−1, Δ = 3.6 ± 5.1%; P = 0.01) phases. The magnitude of the caffeine ergogenic effect was similar during all of the menstrual cycle phases (P = 0.85).
Conclusion
Caffeine increased peak aerobic cycling power in the early follicular, preovulatory, and mid luteal phases. Thus, the ingestion of 3 mg of caffeine per kg of body mass might be considered an ergogenic aid for eumenorrheic women during all three phases of the menstrual cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current knowledge about the ergogenic effect of acute caffeine intake (3–6 mg of caffeine per kg of body mass) has been established by systematic reviews and meta-analysis investigations that primarily used male athlete populations [1,2,3,4,5,6]. In fact, a recent umbrella review that included 21 meta-analyses on the effects of caffeine on exercise performance reported that the prevalence of investigations with only male samples was from 72 to 100% [7]. This information has led to the inclusion of caffeine in the International Olympic Committee consensus on dietary supplements as one of the few substances with an adequate level of support to improve exercise and sport performance [8], although the information about the ergogenicity of caffeine in women is scarcer than in men [9].
A number of recent investigations, using women as study samples, have also found that the ingestion of a moderate dose of caffeine produces increased muscle strength [10, 11] and enhanced sports performance in cycling [12], soccer [13], rugby [14], and volleyball [15]. It has also been found that caffeine might have a similar effect in men and women in cycling [16, 17] basketball [18], endurance running [19], badminton [20], and tennis [21]. Moreover, the course of tolerance to the ergogenic effect of caffeine—when the substance is ingested for 20 days—seems independent of sex [22]. The current body of research has led to the notion that women might experience a comparable ergogenic response to caffeine to men [16, 23] but the interrelationship between the ergogenicity of caffeine and the menstrual cycle has not been thoroughly investigated.
In most of the previously mentioned investigations with women included as study samples, the ergogenic effect of caffeine was measured in a placebo-controlled experiment where trials of caffeine and a placebo were performed within a specific phase of the menstrual cycle. Participants were often tested in the follicular phase of their menstrual cycle likely to minimise the possible impacts of oestradiol and progesterone on the study outcomes [24]. In other investigations, the association of the ergogenicity of caffeine with the menstrual cycle was not examined or included in the manuscript. Interestingly, previous investigations suggest that the ergogenic effect of caffeine can be modulated by variations in female sex hormones during the menstrual cycle and thus, its ergogenic effect might vary during the follicular, ovulatory and luteal phases. The pharmacokinetics of acute caffeine intake (6 mg/kg or 300 mg) is similar in the follicular, ovulatory and luteal phases [25,26,27] in eumenorrheic women. It is also worth mentioning that the differences in serum caffeine concentration during the menstrual cycle found in some investigations are mainly the result of different caffeine intakes rather than differences in caffeine metabolism [28]. However, oral contraceptives may also interfere with caffeine metabolism and excretion [29] due to the intake of ethinylestradiol. Specifically, ethinylestradiol induces inhibitory activity of CYP1A2, an enzyme responsible for N-demethylation of caffeine [30]. Lastly it has been found that caffeine (2 mg/kg) produces greater changes in cardiovascular [31], mood, and subjective variables in the follicular phase than in the luteal phase [32, 33]. Though informative, it is worth noting that these latter investigations were carried out with non-trained adolescents.
With this background of knowledge, it is difficult to determine whether the ergogenicity of caffeine is present during all phases of the menstrual cycle. Therefore, the main aim of this study was to examine the ergogenic effect of caffeine ingestion during three different phases of the menstrual cycle in eumenorrheic females that had not used oral contraceptives. We hypothesised that women would receive a higher ergogenic effect from caffeine during the follicular phase compared to the effects found in the preovulatory and mid-luteal phases.
Methods
Participants
Thirteen well-trained women volunteered to participate in this investigation (age = 31 ± 6 year; body mass = 58.6 ± 7.8 kg; body height = 1.66 ± 0.06 m, body fatness = 14.5 ± 6.5%; maximal oxygen uptake (VO2max) = 48.1 ± 7.3 mL kg−1 min−1). All of the participants were competitive triathletes and fulfilled the following inclusion criteria: (a) age between 18 and 40 years; (b) consistent triathlon training (including running, cycling and swimming practice) of ~ 2 h day−1, at least 5 days week−1 for the previous 2 months; (c) low caffeine consumption (i.e., < 50 mg of caffeine per day in the previous 3 months), as defined by Goncalves et al. [34]; (d) regular duration of their menstrual cycle for the previous 4 months. Participants were excluded if they reported (a) any type of injury within the previous 6 months; (b) a positive smoking status; (c) medication usage within the previous month; (d) a previous history of cardiopulmonary diseases, (e) oral contraceptive use; (f) allergy to caffeine; (g) any type of menstrual disorders such as dysmenorrhea, amenorrhea, or strong symptoms associated with pre-menstrual syndrome. This information was obtained from a pre-participation screening that included a medical and training history as well as a food frequency questionnaire. One week before the start of the experimental protocol, participants were fully informed of the associated procedures and risks. Participants also signed an informed, written consent form to participate in the investigation. The study was approved by the Camilo José Cela University Research Ethics Committee. All research protocols were in accordance with the latest version of the Declaration of Helsinki.
Experimental design
A double-blind, placebo-controlled cross-over experimental design was used in this investigation and thus, participants acted as their own controls. Each participant completed two experimental trials in each of the following three phases of the menstrual cycle: (1) early follicular phase (EF); (2) preovulation phase (PO); and (3) mid luteal phase (ML), for a total of six identical experimental trials (Fig. 1). During each menstrual cycle phase, every participant ingested an opaque and unidentifiable capsule containing either caffeine (3 mg kg−1; 100% purity, Bulk Powders, United Kingdom) or a placebo (e.g., cellulose; 100% purity, Guinama, Spain) before undergoing a set of performance measurements. The order of the substances within each phase of the menstrual cycle was randomised and they were separated by at least 48 h to allow for physical recovery, testing reproducibility, and substance elimination. The first phase of the investigated menstrual cycle was randomly assigned, and a similar number of participants started in EF (5 participants), PO (4 participants) and ML (4 participants). An alphanumeric code was assigned to each trial by a person independent of the study. This was done to double-blind the participants and researchers to the trial order and substances. Menstrual cycle phase identification was conducted according to the methodological considerations proposed by Janse de Jonge [35].
Experimental design of the investigation. This image represents the protocol followed by one triathlete with a 28-day menstrual cycle. Caffeine (3 mg kg−1) or a placebo was administered in three different phases of the menstrual cycle: early follicular, preovulatory and mid-luteal. Exercise performance assessment included a graded exercise test on a cycle ergometer to volitional fatigue, 60 min after assigned capsule was ingested. After participants recorded the regularity and length of their menstrual cycle for 4 months, they then measured their basal tympanic temperature, body mass, and increases in luteinising hormone—with urine test strips—to determine the onset of each menstrual cycle phase
Standardisation
Once the participants had fulfilled the inclusion/exclusion criteria—about 2 weeks before the onset of experimental trials—they were specifically informed about the necessity of refraining from all sources of caffeine (coffee, tea, chocolate, energy drinks, etc.) until the experiment was completed. They were also encouraged to avoid nutritional supplements and to maintain their training routines and a stable state of physical fitness during the experiment. Compliance with these recommendations was certified by self-report questionnaires.
At this time, participants underwent a routine screening to ensure that they were in good health and suitable for the experiment. Participants also performed 2 days of pre-experimental trials to familiarise themselves with the testing, research procedures, and research personnel involved in the experiment. During this period, participants were nude weighed to properly calculate caffeine dosage. The day before each trial, participants performed light, standardised training and a self-selected precompetitive diet/fluid routine was kept. Fluid and diet guidelines were given to assure carbohydrate availability [36] and euhydration [37] in all experimental trials. Subjects were also required to refrain from consuming alcohol and to maintain a sleeping pattern with at least 8 h of sleep the day before each trial. Moreover, participants were encouraged to consume their habitual pre-competition meal 3 h before the start of testing and replicated this before each experimental trial. These standardisations were written in a personal journal and were repeated thoroughly for the remaining trials. Data on fluid, diet and exercise were analysed afterwards to ensure that participants fulfilled all the recommendations given. Ambient temperature and humidity were recorded at the beginning and end of each trial using a digital temperature and humidity monitor (OH1001, OH Haus, Spain). The average environmental conditions during the tests were kept constant at 21.0 ± 0.8 °C for air temperature and 42 ± 5% for relative humidity [38]. During performance testing, standardised encouragement and feedback were given to the participants in all trials by the same researcher who was blinded to the treatments. The seat and handlebar positions on the cycle ergometer were obtained during the familiarisation trials and replicated for each individual in all trials.
Menstrual cycle phase
Regularity and duration of the menstrual cycle were monitored in each participant for 4 months before the onset of the investigation through a mobile application (Mycalendar®, Period-tracker, US). With this application, participants completed a menstruation diary that included: menstrual dates, menstrual duration, and level of discomfort during the previous days leading up to menstrual activity. Menstrual cycle duration was individually assessed, and the average was 27 ± 2 days (from 24 to 31 days). During the familiarisation trials, participants were instructed to measure their own basal tympanic temperature (model HDT8208C, Nursal Ear Thermometer, China) every morning immediately after waking up. In addition, participants were supplied with reactive test strips (One Step Ovulation LH Test Strip; CVS Corporation, US) to assess any increases in luteinising hormone (LH) in the first morning urine sample. Finally, participants were provided with a digital scale (BT200, Daga, Spain) to obtain nude body mass (Fig. 1). The EF was determined by identifying the beginning of menses. It was also confirmed by previous dates of menstrual activity and menstrual cycle durations. The first EF trial was performed the day after the first signs of bleeding, and the second trial was performed 48 h later. The PO was identified by a positive LH test strip result. Tympanic temperature increases of at least 0.3 °C, body mass changes of at least 0.5%, and the information provided by the period-tracker application also helped in identifying the PO phase. The first PO trials were performed the day after a positive result on the LH test strip. The second PO trial was conducted 48 h later. The first trial ML was performed in the days equivalent to 70% and 75% of the individual menstrual cycle length (i.e., 20th to 22th from the first day of the menstrual cycle for a 28-day cycle; [35]) All these protocols helped to align the participants’ cycles so, despite different cycle lengths, participants performed the testing in the same cycle phases [28].
Experimental protocol
In all experimental trials, participants completed the same research procedures, as follows: on the day of each trial, they arrived at the laboratory (10.00 am) in a fed state (~ 3 h after their last meal) and 2 h after ingesting 7 mL/kg of water. Upon arrival at the laboratory, they were weighed, and body fat percentage was estimated by bioelectrical impedance (B-418, Tanita, Japan). After this, the assigned experimental capsule was provided in an unidentifiable bag and immediately ingested with 150 mL of water. Participants then dressed in a T-shirt, cycling shorts, and cleated shoes, and a heart rate belt (Wearlink, Polar, Finland) was attached to their chests. After that, they rested in a supine position for 45 min to allow the experimental substance to be absorbed. Resting heart rate and systolic and diastolic blood pressure (M6 Comfort, Omron, Japan) were measured three times during the last 5 min of the resting period. An average of the three blood pressure measurements was used for analysis.
Exactly 60 min after capsule ingestion, participants performed a standardised warm-up that included 15 min on a cycle ergometer (SNT Medical, Cardgirus, Spain) at 50 W. Participants then completed a ramp exercise test on the cycle ergometer, which comprised 25 W increments every minute until volitional fatigue, as previously described [22]. Pedalling frequency was individually-chosen (between 75 and 90 rpm) but maintained during the whole graded exercise test and replicated in all experimental trials. Expired gases were collected with a stationary breath-by-breath device (Metalyzer 3B, Cortex, Germany) during the test to calculate oxygen uptake (VO2) and carbon dioxide production (VCO2), among other respiratory variables. At each workload, gas exchange data were averaged every 15 s, and the last 15 s of each stage was used as a representative value of the workload. One minute after the end of the graded test, a fingertip blood sample was obtained to analyse blood lactate concentration (Lactate Pro 2, Arkay, Japan). In this test, peak cycling power (Wmax) was recorded as the exercise load on the cycle ergometer when the participants abruptly stopped pedalling or when their pedalling frequency was lower than 50 rpm. VO2max was defined as the highest VO2 value obtained during the test. VO2max was considered valid when participants rated their perceived exertion to be higher than 19 on the Borg scale, the VO2 difference between the last two consecutive workloads was less than 0.10 L min−1, respiratory exchange ratio was higher than 1.10 and heart rate was higher than 80% of the age-adjusted estimate of maximal heart rate [39]. Certified calibration gases (16.0% O2; 5.0% CO2, Cortex, Germany) and a 3-L syringe were used to calibrate the gas analyser and the flowmeter before each trial. At the end of the ramp test, participants were asked about their self-perceived endurance (1–10 arbitrary units, [a.u.]) [40] and exertion (6–20 a.u., Borg’s scale). These protocols were repeated after 48 h in each of the three menstrual cycle phases. At the end of this protocol, participants continued pedalling at 50 W for 10 min. After that they performed a 15-s Wingate test. The results of the Wingate test have not been included in this manuscript for clarity and they will be presented in a further paper.
Statistical analysis
Data were collected as previously indicated and the results of each test were blindly introduced into the statistical package SPSS v 20.0 for later analysis. Normality was tested for each variable with the Shapiro–Wilk test. All included variables in this investigation presented a normal distribution (P > 0.05) and parametric statistics were used to determine the ergogenicity of caffeine. Differences between the caffeine vs. placebo protocols were determined by two-way analysis of variance (substance × day of ingestion) with repeated measures. After a significant F test (Geisser–Greenhouse correction for the assumption of sphericity), differences between means were identified using Tukey’s HSD post hoc. As the objective of this investigation was to identify the ergogenic effect of caffeine in each phase of the menstrual cycle, only placebo-caffeine comparisons within the same phase were identified. The results are presented as group average ± standard deviation, with percentage of change with caffeine over placebo (Δ). Cohen’s effect size (d) was calculated in all statistically significant pairwise comparisons. The criteria to interpret the magnitude of effect size were: ≤ 0.2 trivial, > 0.2 to 0.6 small, > 0.6 to 1.2 moderate, > 1.2 to 2.0 large, > 2.0 very large [41]. The differences in the frequency of the participants who correctly identified the order of the trials in each phase was analysed with crosstab and Chi square tests, including adjusted standardised residuals. The significance level was set at P < 0.05.
Results
The order of the trials was correctly guessed by 38.5% of the participants in EF, 61.5% in PO and 53.8% in ML. There was no difference in the frequency of correct identification of the placebo-caffeine trials amongst the menstrual cycle phases (P = 0.49). No participant reported any serious adverse side effects during or after each testing session. Table 1 depicts resting heart rate, systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure differences between the caffeine and placebo trials. Caffeine did not affect resting heart rate in any phase of the menstrual cycle. In EF, caffeine increased diastolic and mean arterial blood pressure (both P < 0.05); in PO, caffeine increased systolic, diastolic, and mean arterial blood pressure (all at P < 0.05); and in ML, caffeine increased systolic, diastolic, and mean arterial blood pressure (all at P < 0.05).
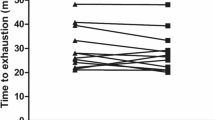
In comparison to the placebo, caffeine intake increased Wmax in EF (4.13 ± 0.69 vs. 4.24 ± 0.71 W kg−1, Δ = 2.7 ± 3.3%, d = 0.2; P = 0.01), in PO (4.14 ± 0.70 vs. 4.27 ± 0.73 W kg−1, Δ = 3.3 ± 5.0%, d = 0.2; P = 0.03) and in ML (4.15 ± 0.69 vs. 4.29 ± 0.67 W kg−1, Δ = 3.6 ± 5.1%, d = 0.2; P = 0.01). The magnitude of the ergogenic effect of caffeine was similar during all of the menstrual cycle phases (P = 0.85). In EF and ML, three participants did not increase their Wmax in response to caffeine; in PO, two participants did not increase Wmax in response to caffeine either (Fig. 2).
Peak cycling power (Wmax) during a maximal graded exercise test with the administration of 3 mg kg−1 of caffeine or a placebo in the early follicular (a), preovulatory (b) and mid luteal (c) phases of the menstrual cycle. The columns represent the group’s average and the whiskers represent 1 standard deviation. The solid lines represent individual responses for participants with increased Wmax between caffeine administration and the placebo. The dashed lines represent individual responses in individuals that decreased Wmax as a result of caffeine ingestion. (*) Caffeine different from placebo within the same menstrual cycle phase at P < 0.05
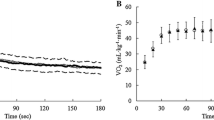
Caffeine also increased VO2max in EF (47.5 ± 7.5 vs. 49.6 ± 7.6 mL kg−1 min−1, Δ = 4.5 ± 5.7%, d = 0.3; P = 0.01). However, caffeine was ineffective at increasing VO2max in PO (48.2 ± 7.4 ~ 49.2 ± 8.4 mL kg−1 min−1, Δ = 2.1 ± 6.0%, d = 0.1; P = 0.17) and in ML (49.0 ± 7.3 ~49.7 ± 7.0 mL kg−1 min−1, Δ = 1.7 ± 4.7%, d = 0.1; P = 0.27). Nevertheless, the magnitude of the ergogenic effect of caffeine was similar during all the menstrual cycle phases (P = 0.35). In EF, one participant did not increase VO2max with caffeine; in PO, three participants did not increase VO2max with caffeine; in ML, two participants did not increase VO2max with caffeine (Fig. 3).
Maximal oxygen uptake (VO2max) during a maximal graded exercise test with the administration of 3 mg kg−1 of caffeine or a placebo in the early follicular (a), preovulatory (b) and mid luteal (c) phases of the menstrual cycle. The columns represent the group’s average and the whiskers represent 1 standard deviation. The solid lines represent individual responses for participants with increased VO2max between caffeine administration and the placebo. The dashed lines represent individual responses in individuals that decreased VO2max as a result of caffeine ingestion. (*) Caffeine different from placebo within the same menstrual cycle phase at P < 0.05
In comparison to the placebo, acute caffeine intake increased peak heart rate in PO (P = 0.05) and ML (P = 0.05, Table 2), with no caffeine-induced effect on EF. Caffeine also increased peak pulmonary ventilation in EF (P < 0.01) and ML (P < 0.01), with no statistically significant effect in PO (P = 0.22). The caffeine-induced increase in peak ventilation was the result of increased tidal volume (P < 0.05 in all three phases) because peak respiratory rate remained unchanged with caffeine (Table 2).
At the end of the graded exercise test, blood lactate concentration after the graded exercise was higher with caffeine than the placebo in EF (P = 0.03) and ML (P = 0.01). Caffeine did not produce any changes in self-perceived exertion, measured with the Borg’s scale, in any of the menstrual cycle phases where < 19 a.u. values were recorded in all tests. Similarly, caffeine did not change self-perception of endurance during the graded exercise test in any of the menstrual cycle phases (Table 2).
Figure 4 depicts the individual changes in Wmax due to the ingestion of caffeine. Except for participants number 2 and 11, all triathletes increased Wmax in response to caffeine in at least two phases of their menstrual cycle. In addition, 7 participants increased Wmax in all three phases of their menstrual cycle.
Discussion
The current body of knowledge indicates that women might experience a comparable ergogenic response to caffeine as men [16, 23]. However, no research has been conducted to determine whether the ergogenic effect of caffeine is present throughout the menstrual cycle. The aim of the current study was to assess the ergogenic effects of 3 mg kg−1 of caffeine on peak aerobic cycling power during three different phases of the menstrual cycle (EF, PO and ML) in well-trained triathletes. The following main outcomes arose from the study: (a) in comparison to the ingestion of a placebo, caffeine increased Wmax in EF, PO and ML while the magnitude of the ergogenic effect was small and comparable among phases (b) acute caffeine intake also increased VO2max in EF, but this effect was not present in PO and ML; (c) other responses to caffeine also included increased peak heart rate in PO and ML, peak pulmonary ventilation in EF and ML, and increased post-exercise blood lactate concentration in EF and ML; (d) the ergogenic response to caffeine showed a high interindividual variability with one triathlete who did not obtain benefits from acute caffeine intake in any of the menstrual cycle phases. All of this information points to caffeine being considered as a potential ergogenic aid for women during all three phases of the menstrual cycle. However, measuring individual response to a moderate dose of caffeine should be conducted before caffeine is recommended as a substance to increase performance in a female athlete.
During the menstrual cycle, serum concentrations of various female steroid hormones fluctuate. However, the effects on muscle performance or endurance capacity are limited [42]. The concentration changes in female sex hormones might also interfere with the physiological effects of caffeine because the activity of the human cytochrome P450 CYP1A2, the enzyme responsible for caffeine metabolism, might be partially inhibited by female sex hormones [41], mainly oestradiol [30]. The experimental design used in the current investigation is novel with respect to previous research because it is the only study that considers menstrual cycle phases to determine the ergogenic effect of caffeine. Previous investigations have measured the effect of caffeine on women’s performance in a myriad of exercise and sports situations [10,11,12,13,14,15], but the procedures only included one placebo-caffeine comparison during one phase of the menstrual cycle. Although the effect of the menstrual cycle on VO2max, peak heart rate [43], peak ventilation, and blood lactate [44] in eumenorrheic women is almost negligible, the possibility of interference between caffeine ergogenicity and menstrual cycle phases still exists.
Although the fluctuations of serum caffeine concentration have been measured at up to 8 different points in the menstrual cycle [28], this research focused on three different phases that coincide with three main events of the menstrual cycle: menses (i.e., early follicular), peak serum oestrogen concentration (i.e., preovulatory), and peak serum progesterone concentration (i.e., mid-luteal; [35]). Interestingly, caffeine metabolism and excretion seem similar during these three phases in eumenorrheic women [26]. Caffeine produced a positive effect on Wmax in all three phases of the menstrual cycle, while the effect size and percentage of change in aerobic cycling power was similar during the menstrual cycle (Fig. 1). This might indicate that in eumenorrheic women that have not undergone oral contraceptive treatments, caffeine is ergogenic, irrespective of the menstrual cycle phase. More research is needed to measure this effect in women undergoing oral contraceptive treatment.
The response similarities to acute caffeine intake during the menstrual cycle were not only evident in Wmax, but also in other variables as well. Before exercise, caffeine produced a comparable effect on systolic, diastolic, and mean arterial blood pressure in EL, PO and ML (Table 1) while also minimally affecting resting heart rate. Caffeine increased VO2max in EF while it induced a non-significant, but equivalent change of ~ 2% in PO and ML. It is likely that the inter-individual variation in the response to caffeine intake [45] prevented the obtaining of significant differences in VO2max in PO and ML (Fig. 2). The magnitude of changes induced by caffeine on exercise heart rate, gas exchange variables, and post-exercise blood lactate concentration was similar. However, the effect of caffeine was not statistically significant in all phases of the menstrual cycle. Finally, caffeine did not affect perceived exertion or endurance in any of the phases. The similarities in these caffeine-induced changes strongly suggest that the physiological effects of acute caffeine intake are independent of the menstrual cycle phase. This investigation disputes the idea that the menstrual cycle interferes with the pharmacological effect of caffeine, at least during exercise [29].
The necessity of analysing individual exercise responses to caffeine has been recently suggested [45], especially since a number of investigations have shown that some individuals do not obtain ergogenic effects after acute caffeine intake [20, 46, 47]. These individuals have been categorised as non-responders to the ergogenic effects of caffeine [48] and the causes for this lack of a positive physical response to caffeine have been mainly associated with genetics, caffeine tolerance, or inappropriate caffeine dosage [45]. It has been suggested that multiple caffeine-placebo comparisons must be performed, with the same dose of caffeine, to correctly identify a caffeine non-responder [49]. This stands in contrast with the protocols of previous investigations [20, 46, 47]. Following this suggestion, we have created Fig. 4 that depicts individual ergogenic responses to caffeine during the three phases of the menstrual cycle. This individual analysis reveals that participant #2 did not increase Wmax in response to caffeine in any of the phases investigated, while participant #11 only obtained an increase in Wmax during EL. With this information, participant #2 might be categorised as a non-responder to caffeine, but a more complete analysis indicates that this participant obtained an ~ 18.3% increase in mean arterial blood pressure (as an average of the changes in the three menstrual cycle phases), a ~ 2.4% increase in VO2max, and a ~ 16.7% increase in peak pulmonary ventilation. Despite the lack of effect on Wmax, it might be incorrect to classify participant #2 as a caffeine non-responder. The concept of non-response to caffeine should be revisited with a more complete analysis of all resting and exercise responses to caffeine along with the use of more than one caffeine-placebo comparison. In either case, this investigation adds new data to confirm that the ergogenic response to caffeine has great interindividual variability while the menstrual cycle is not a contributor to this variability in women.
Aside from its strengths, the current investigation presents some limitations. First, there was no measure of the serum concentration of female steroid hormones to confirm that the trials were performed in the specified phase. However, the monitoring of menstrual cycle duration for 4 months, the use of a period tracker application, and measurements of tympanic temperature, urine LH upsurge, and body mass helped to identify the phases of the menstrual cycle. Second, the relatively small sample size may have limited the ability to detect statistically significant differences in some of the investigated variables. Third, although the experiment was controlled by a placebo, double-blinded, and the order of trials and the menstrual cycle phases was randomised, participants were aware of their menstrual cycle under investigation. This could have biased some of the investigation results. In addition, only verbal confirmation was obtained to assess the fulfilment of pre-experimental hydration and nutritional standardizations, while physiological measures might have helped to confirm the correct hydration status and the dietary intake before the experimental trials. Finally, the study sample was limited to eumenorrheic women with minor symptoms associated with the pre-menstrual syndrome. Thus, the outcomes of this investigation are only applicable to women that share these characteristics. Despite these limitations, the authors believe that this experiment contributes to current knowledge about the ergogenic effects of caffeine during different phases of the menstrual cycle.
In summary, acute intake of 3 mg of caffeine per kg of body mass was effective at increasing peak cycling power during a graded exercise test in the early follicular, preovulatory, and mid luteal phases of the menstrual cycle. Caffeine intake was also accompanied by other positive changes during exercise, such as increased VO2max, increased peak heart rate, and pulmonary ventilation—even though these changes were not statistically significant in all phases investigated. The similar responses to caffeine in all three phases suggest that caffeine had a comparable ergogenic effect throughout the entire menstrual cycle. However, further investigations are necessary to determine if the ergogenic effect of caffeine on muscle performance and on anaerobic performance is comparable among the different phases of the menstrual cycle. In addition, to investigate the ergogenic effect of caffeine on athletes taking oral contraceptives and those that experience amenorrhea, dysmenorrhea, and/or other symptoms associated with pre-menstrual syndrome is essential, as a considerable prevalence of these disorders has been found in athletes [50].
References
Souza DB, Del Coso J, Casonatto J, Polito MD (2017) Acute effects of caffeine-containing energy drinks on physical performance: a systematic review and meta-analysis. Eur J Nutr 56(1):13–27. https://doi.org/10.1007/s00394-016-1331-9
Glaister M, Gissane C (2018) Caffeine and physiological responses to submaximal exercise: a meta-analysis. Int J Sports Physiol Perform 13(4):402–411. https://doi.org/10.1123/ijspp.2017-0312
Grgic J (2018) Caffeine ingestion enhances Wingate performance: a meta-analysis. Eur J Sport Sci 18(2):219–225. https://doi.org/10.1080/17461391.2017.1394371
Salinero JJ, Lara B, Del Coso J (2018) Effects of acute ingestion of caffeine on team sports performance: a systematic review and meta-analysis. Res Sports Med. https://doi.org/10.1080/15438627.2018.1552146
Warren GL, Park ND, Maresca RD, McKibans KI, Millard-Stafford ML (2010) Effect of caffeine ingestion on muscular strength and endurance: a meta-analysis. Med Sci Sports Exerc 42(7):1375–1387. https://doi.org/10.1249/MSS.0b013e3181cabbd8
Conger SA, Warren GL, Hardy MA, Millard-Stafford ML (2011) Does caffeine added to carbohydrate provide additional ergogenic benefit for endurance? Int J Sport Nutr Exerc Metab 21(1):71–84
Grgic J, Grgic I, Pickering C, Schoenfeld BJ, Bishop DJ, Pedisic Z (2019) Wake up and smell the coffee: caffeine supplementation and exercise performance-an umbrella review of 21 published meta-analyses. Br J Sports Med. https://doi.org/10.1136/bjsports-2018-100278
Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, Rawson ES, Walsh NP, Garthe I, Geyer H, Meeusen R, van Loon LJC, Shirreffs SM, Spriet LL, Stuart M, Vernec A, Currell K, Ali VM, Budgett RG, Ljungqvist A, Mountjoy M, Pitsiladis YP, Soligard T, Erdener U, Engebretsen L (2018) IOC consensus statement: dietary supplements and the high-performance athlete. Br J Sports Med 52(7):439–455. https://doi.org/10.1136/bjsports-2018-099027
Salinero JJ, Lara B, Jimenez-Ormeno E, Romero-Moraleda B, Giraldez-Costas V, Baltazar-Martins G, Del Coso J (2019) More research is necessary to establish the ergogenic effect of caffeine in female athletes. Nutrients. https://doi.org/10.3390/nu11071600
Fett CA, Aquino NM, Schantz Junior J, Brandao CF, de Araujo Cavalcanti JD, Fett WC (2018) Performance of muscle strength and fatigue tolerance in young trained women supplemented with caffeine. J Sports Med Phys Fit 58(3):249–255. https://doi.org/10.23736/s0022-4707.17.06615-4
Goldstein E, Jacobs PL, Whitehurst M, Penhollow T, Antonio J (2010) Caffeine enhances upper body strength in resistance-trained women. J Int Soc Sports Nutr 7:18. https://doi.org/10.1186/1550-2783-7-18
Astorino TA, Roupoli LR, Valdivieso BR (2012) Caffeine does not alter RPE or pain perception during intense exercise in active women. Appetite 59(2):585–590. https://doi.org/10.1016/j.appet.2012.07.008
Lara B, Gonzalez-Millan C, Salinero JJ, Abian-Vicen J, Areces F, Barbero-Alvarez JC, Munoz V, Portillo LJ, Gonzalez-Rave JM, Del Coso J (2014) Caffeine-containing energy drink improves physical performance in female soccer players. Amino Acids 46(5):1385–1392. https://doi.org/10.1007/s00726-014-1709-z
Del Coso J, Portillo J, Munoz G, Abian-Vicen J, Gonzalez-Millan C, Munoz-Guerra J (2013) Caffeine-containing energy drink improves sprint performance during an international rugby sevens competition. Amino Acids 44(6):1511–1519. https://doi.org/10.1007/s00726-013-1473-5
Perez-Lopez A, Salinero JJ, Abian-Vicen J, Valades D, Lara B, Hernandez C, Areces F, Gonzalez C, Del Coso J (2015) Caffeinated energy drinks improve volleyball performance in elite female players. Med Sci Sports Exerc 47(4):850–856. https://doi.org/10.1249/MSS.0000000000000455
Skinner TL, Desbrow B, Arapova J, Schaumberg MA, Osborne J, Grant GD, Anoopkumar-Dukie S, Leveritt MD (2019) Women experience the same ergogenic response to caffeine as men. Med Sci Sports Exerc. https://doi.org/10.1249/MSS.0000000000001885
Lane SC, Hawley JA, Desbrow B, Jones AM, Blackwell JR, Ross ML, Zemski AJ, Burke LM (2014) Single and combined effects of beetroot juice and caffeine supplementation on cycling time trial performance. Appl Physiol Nutr Metab 39(9):1050–1057. https://doi.org/10.1139/apnm-2013-0336
Puente C, Abian-Vicen J, Salinero JJ, Lara B, Areces F, Del Coso J (2017) Caffeine improves basketball performance in experienced basketball players. Nutrients 9:9. https://doi.org/10.3390/nu9091033
Prins PJ, Goss FL, Nagle EF, Beals K, Robertson RJ, Lovalekar MT, Welton GL (2016) Energy drinks improve five-kilometer running performance in recreational endurance runners. J Strength Cond Res 30(11):2979–2990. https://doi.org/10.1519/jsc.0000000000001391
Abian P, Del Coso J, Salinero JJ, Gallo-Salazar C, Areces F, Ruiz-Vicente D, Lara B, Soriano L, Munoz V, Abian-Vicen J (2015) The ingestion of a caffeinated energy drink improves jump performance and activity patterns in elite badminton players. J Sports Sci 33(10):1042–1050. https://doi.org/10.1080/02640414.2014.981849
Gallo-Salazar C, Areces F, Abian-Vicen J, Lara B, Salinero JJ, Gonzalez-Millan C, Portillo J, Munoz V, Juarez D, Del Coso J (2015) Enhancing physical performance in elite junior tennis players with a caffeinated energy drink. Int J Sports physiol Perform 10(3):305–310. https://doi.org/10.1123/ijspp.2014-0103
Lara B, Ruiz-Moreno C, Salinero JJ, Del Coso J (2019) Time course of tolerance to the performance benefits of caffeine. PLoS One 14(1):e0210275. https://doi.org/10.1371/journal.pone.0210275
Sabblah S, Dixon D, Bottoms L (2015) Sex differences on the acute effects of caffeine on maximal strength and muscular endurance. Comp Exerc Physiol 11(2):89–94. https://doi.org/10.3920/CEP150010
Bruinvels G, Burden RJ, McGregor AJ, Ackerman KE, Dooley M, Richards T, Pedlar C (2017) Sport, exercise and the menstrual cycle: where is the research? Br J Sports Med 51(6):487–488. https://doi.org/10.1136/bjsports-2016-096279
Kamimori GH, Joubert A, Otterstetter R, Santaromana M, Eddington ND (1999) The effect of the menstrual cycle on the pharmacokinetics of caffeine in normal, healthy eumenorrheic females. Eur J Clin Pharmacol 55(6):445–449
McLean C, Graham TE (2002) Effects of exercise and thermal stress on caffeine pharmacokinetics in men and eumenorrheic women. J Appl Physiol (Bethesda, Md: 1985) 93(4):1471–1478. https://doi.org/10.1152/japplphysiol.00762.2000
Magkos F, Kavouras SA (2005) Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit Rev Food Sci Nutr 45(7–8):535–562. https://doi.org/10.1080/1040-830491379245
Schliep KC, Schisterman EF, Wactawski-Wende J, Perkins NJ, Radin RG, Zarek SM, Mitchell EM, Sjaarda LA, Mumford SL (2016) Serum caffeine and paraxanthine concentrations and menstrual cycle function: correlations with beverage intakes and associations with race, reproductive hormones, and anovulation in the BioCycle Study. Am J Clin Nutr 104(1):155–163. https://doi.org/10.3945/ajcn.115.118430
Arnaud MJ (2011) Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb Exp Pharmacol 200:33–91. https://doi.org/10.1007/978-3-642-13443-2_3
Granfors MT, Backman JT, Laitila J, Neuvonen PJ (2005) Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1A2. Clin Pharmacol Ther 78(4):400–411. https://doi.org/10.1016/j.clpt.2005.06.009
Temple JL, Ziegler AM (2011) Gender differences in subjective and physiological responses to caffeine and the role of steroid hormones. J Caffeine Res 1(1):41–48. https://doi.org/10.1089/jcr.2011.0005
Temple JL, Ziegler AM, Martin C, de Wit H (2015) Subjective responses to caffeine are influenced by caffeine dose, sex, and pubertal stage. J Caffeine Res 5(4):167–175. https://doi.org/10.1089/jcr.2015.0022
Temple JL, Ziegler AM, Graczyk A, Bendlin A, Sion T, Vattana K (2014) Cardiovascular responses to caffeine by gender and pubertal stage. Pediatrics 134(1):e112–119. https://doi.org/10.1542/peds.2013-3962
Goncalves LS, Painelli VS, Yamaguchi G, Oliveira LF, Saunders B, da Silva RP, Maciel E, Artioli GG, Roschel H, Gualano B (2017) Dispelling the myth that habitual caffeine consumption influences the performance response to acute caffeine supplementation. J Appl Physiol (Bethesda, Md: 1985) 123(1):213–220. https://doi.org/10.1152/japplphysiol.00260.2017
Janse de Jonge XA (2003) Effects of the menstrual cycle on exercise performance. Sports Med 33(11):833–851. https://doi.org/10.2165/00007256-200333110-00004
Burke LM, Hawley JA, Wong SH, Jeukendrup AE (2011) Carbohydrates for training and competition. J Sports Sci 29(Suppl 1):S17–27. https://doi.org/10.1080/02640414.2011.585473
McDermott BP, Anderson SA, Armstrong LE, Casa DJ, Cheuvront SN, Cooper L, Kenney WL, O’Connor FG, Roberts WO (2017) National athletic trainers’ association position statement: fluid replacement for the physically active. J Athl Train 52(9):877–895. https://doi.org/10.4085/1062-6050-52.9.02
Ganio MS, Johnson EC, Klau JF, Anderson JM, Casa DJ, Maresh CM, Volek JS, Armstrong LE (2011) Effect of ambient temperature on caffeine ergogenicity during endurance exercise. Eur J Appl Physiol 111(6):1135–1146. https://doi.org/10.1007/s00421-010-1734-x
Edvardsen E, Hem E, Anderssen SA (2014) End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: a cross-sectional study. PLoS One 9(1):e85276. https://doi.org/10.1371/journal.pone.0085276
Salinero JJ, Lara B, Abian-Vicen J, Gonzalez-Millan C, Areces F, Gallo-Salazar C, Ruiz-Vicente D, Del Coso J (2014) The use of energy drinks in sport: perceived ergogenicity and side effects in male and female athletes. Br J Nutr 112(9):1494–1502. https://doi.org/10.1017/s0007114514002189
Batterham AM, Hopkins WG (2006) Making meaningful inferences about magnitudes. Int J Sports Physiol Perform 1(1):50–57
Jurkowski JE, Jones NL, Toews CJ, Sutton JR (1981) Effects of menstrual cycle on blood lactate, O2 delivery, and performance during exercise. J Appl Physiol 51(6):1493–1499. https://doi.org/10.1152/jappl.1981.51.6.1493
Gordon D, Scruton A, Barnes R, Baker J, Prado L, Merzbach V (2018) The effects of menstrual cycle phase on the incidence of plateau at V O2max and associated cardiorespiratory dynamics. Clin Physiol Funct Imaging 38(4):689–698. https://doi.org/10.1111/cpf.12469
Bemben DA, Salm PC, Salm AJ (1995) Ventilatory and blood lactate responses to maximal treadmill exercise during the menstrual cycle. J Sports Med Phys Fit 35(4):257–262
Pickering C, Kiely J (2018) Are the current guidelines on caffeine use in sport optimal for everyone? Inter-individual variation in caffeine ergogenicity, and a move towards personalised sports nutrition. Sports Med 48(1):7–16. https://doi.org/10.1007/s40279-017-0776-1
Jenkins NT, Trilk JL, Singhal A, O’Connor PJ, Cureton KJ (2008) Ergogenic effects of low doses of caffeine on cycling performance. Int J Sport Nutr Exerc Metab 18(3):328–342
Puente C, Abian-Vicen J, Del Coso J, Lara B, Salinero JJ (2018) The CYP1A2 -163C > A polymorphism does not alter the effects of caffeine on basketball performance. PLoS One 13(4):e0195943. https://doi.org/10.1371/journal.pone.0195943
Salinero JJ, Lara B, Ruiz-Vicente D, Areces F, Puente-Torres C, Gallo-Salazar C, Pascual T, Del Coso J (2017) CYP1A2 genotype variations do not modify the benefits and drawbacks of caffeine during exercise: a pilot study. Nutrients. https://doi.org/10.3390/nu9030269
Grgic J (2018) Are there non-responders to the ergogenic effects of caffeine ingestion on exercise performance? Nutrients 10:11. https://doi.org/10.3390/nu10111736
Takeda T, Imoto Y, Nagasawa H, Muroya M, Shiina M (2015) Premenstrual syndrome and premenstrual dysphoric disorder in Japanese collegiate athletes. J Pediatr Adolesc Gynecol 28(4):215–218. https://doi.org/10.1016/j.jpag.2014.07.006
Acknowledgements
The authors would like to thank the participants for their invaluable contribution to this research effort at investigating the effects of caffeine on female athletes. The authors are also very grateful to the Spanish Triathlon Federation for their support and help in the recruitment process.
Funding
The study was part of the CAFTRI project supported by a grant from the Spanish National Sports Council conceded to the Spanish Triathlon Federation, which covered the expenses necessary to carry out this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest derived from the outcomes of this study.
Rights and permissions
About this article
Cite this article
Lara, B., Gutiérrez-Hellín, J., García-Bataller, A. et al. Ergogenic effects of caffeine on peak aerobic cycling power during the menstrual cycle. Eur J Nutr 59, 2525–2534 (2020). https://doi.org/10.1007/s00394-019-02100-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02100-7