Abstract
Purpose
To investigate the effect of caffeine ingestion on the 3-min all-out test (3MT) performance and plasma electrolytes in athletes.
Methods
Fifteen collegiate male basketball players were recruited and completed two trials separated by at least 1 week in caffeine (CAF, 6 mg kg−1) and placebo conditions. During the first visit, participants performed an incremental cycling test to determine their 3MT resistance. After a familiarization trial, participants performed a CAF or PL trial according to a randomized crossover design. One hour after ingesting capsules, the participants performed the 3MT to estimate the end-test power (EP) and work done above EP (WEP). Blood samples for sodium (Na+), potassium (K+), pH, and lactate concentrations were drawn pretest, 1 h after ingestion, and posttest.
Results
Significant differences in WEP (CAF vs. PL, 13.4 ± 3.0 vs. 12.1 ± 2.7 kJ, P < 0.05) but not in EP (CAF vs. PL, 242 ± 37 vs. 244 ± 42 W, P > 0.05) were determined between the conditions. Compared with the PL condition, the CAF condition yielded significantly higher power outputs (60–150 s), a lower fatigue rate during the 3MT (CAF vs. PL, 0.024 ± 0.007 vs. 0.029 ± 0.006 s−1, P < 0.05), a significantly higher lactate concentration after the 3MT, and significantly lower K+ concentrations at 1 h after caffeine ingestion. There were no significant interaction effects for pH and Na+ concentrations.
Conclusions
Caffeine ingestion did not change EP but improved WEP and the rate of decline in power output during short-term, severe exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The critical power (CP) and curvature constant (W′), derived from the CP test, have been proposed to predict aerobic and anaerobic capacities, respectively (Monod and Scherrer 1965; Moritani et al. 1981). Generally, CP and W′ can be determined by requiring participants to perform multiple constant load tests until reaching exhaustion and by calculating the linear and hyperbolic relationships between the work or power outputs and the time to exhaustion (Monod and Scherrer 1965). Theoretically, CP represents the work rate upper limit that can be maintained at a metabolic steady state and demarcates the heavy from severe exercise intensity domains (Poole et al. 1988; Dekerle et al. 2008). The W′ has been defined as the total work that can be performed above CP and is associated with the immediate energy stores available to the working muscles (Monod and Scherrer 1965; Moritani et al. 1981). As a maximal steady-state work rate, the CP represents a crucial determinant of endurance performance. Previous studies have revealed that the CP is significantly correlated with endurance performance for competitive cyclists (Smith et al. 1999), rowers (Shimoda and Kawakami 2005), and runners (Kolbe et al. 1995).
Numerous studies have reported that ingestion of 3–6 mg kg−1 of caffeine can improve time to exhaustion and time trial performance (Ganio et al. 2009; Mohr et al. 2011; Desbrow et al. 2012; Black et al. 2015) as well as short-term, high-intensity exercise performance (Collomp et al. 1992; Wiles et al. 2006; Jenkins et al. 2008; Simmonds et al. 2010). Caffeine ingestion can postpone the time to exhaustion during exercise (Gaesser and Rich 1985; Dodd et al. 1991; Doherty 1998; Simmonds et al. 2010); it, therefore, may influence the CP value. Fukuda et al. (2010) examined the effect of a compound supplement comprising caffeine (approximately 1.4 mg kg−1), creatine, and amino acids. Trained participants were asked to consume this preexercise supplement prior to completing fixed-intensity exercise sessions to exhaustion. The study results suggested that this preexercise supplement had no effect on CP; however, the studied supplement contained, in addition to a relatively small amount of caffeine, numerous ingredients, making it difficult to ascertain the true effect of any single ingredient. According to our literature review, few studies have examined the effect of caffeine on CP.
Caffeine ingestion of 2–6 mg kg−1 by untrained participants does not increase the peak or mean power output in a 30-s Wingate test (Collomp et al. 1991; Bell et al. 2001; Woolf et al. 2008) or improve repeated Wingate exercise performance (Greer et al. 1998; Crowe et al. 2006; Forbes et al. 2007). In an earlier study (Collomp et al. 1992), caffeine ingestion by trained swimmers resulted in improved performance in two 100-m sprint tests; however, this effect was not found in untrained swimmers. Subsequent studies also indicated that caffeine ingestion improved 1-km cycling time trial performance (Wiles et al. 2006) and time to exhaustion (approximately 2–3 min) during supramaximal exercise (Doherty 1998; Simmonds et al. 2010) in trained individuals. Fukuda et al. (2010) determined that low dosages of caffeine increased the W′ of trained athletes. Therefore, these studies have revealed that caffeine has no significant effect on untrained individuals performing high-intensity exercises for durations of 60–180 s, but that trained athletes may benefit from the ergogenic effects of caffeine. Previous studies have found that caffeine ingestion reduces both effort sense (Doherty and Smith 2005) and leg muscle pain (Motl et al. 2003, 2006; Gliottoni and Motl 2008; Gliottoni et al. 2009) during exercise. In addition, caffeine could improve the ability of muscle to generate force by increasing motor unit recruitment (Kalmar and Cafarelli 1999; Kalmar 2005; Warren et al. 2010; Black et al. 2015). The improved effort sensation and muscular strength might contribute to the ergogenic effects of caffeine during short-term, high-intensity exercise. Recently, the 3-min all-out test (3MT) has been developed to simultaneously assess CP and W′ in a single trial (Burnley et al. 2006; Vanhatalo et al. 2007; Cheng et al. 2012; Bergstrom et al. 2013). Thus, the first objective of the present study was to examine the effect of caffeine ingestion on CP and W′ values in trained athletes after 3MT participation.
Accumulation of extracellular potassium concentration ([K+]) during intense exercise has been suggested to cause skeletal muscle fatigue and decline in force development due to impaired membrane excitability (Fitts 1994; McKenna et al. 2008). It has been suggested that caffeine ingestion might delay the development of muscle fatigue during exercise by increasing activity of sodium/potassium ATPase pumps (Astorino and Roberson 2010), thus reducing extracellular accumulation of [K+] (Lindinger et al. 1993; Crowe et al. 2006; Simmonds et al. 2010; Mohr et al. 2011). Resting values of plasma [K+] is substantially lower after caffeine ingestion (Lindinger et al. 1993; Crowe et al. 2006; Simmonds et al. 2010); however, not all studies found this effect (Greer et al. 1998; Mohr et al. 2011). Findings of changes to plasma [K+] during and after exercise were also inconsistent (Lindinger et al. 1993; Greer et al. 1998; Crowe et al. 2006; Simmonds et al. 2010; Mohr et al. 2011). Thus far, only one study (Lindinger et al. 1993) determined that caffeine improved plasma [K+] during and after exercise (at intensities of 78 and 85 % of maximal \(\dot{V}{\text{O}}_{2}\)); most studies have observed that, during extremely high-intensity exercises, the ability of caffeine to mitigate increased plasma [K+] was insignificant (Greer et al. 1998; Crowe et al. 2006; Simmonds et al. 2010; Mohr et al. 2011). Therefore, we hypothesized that caffeine ingestion would improve 3MT performance among athletes by decreasing plasma [K+] at rest, but would not influence plasma [K+] after exercise.
Methods
Participants
Fifteen Division I collegiate male basketball players (age 20 ± 2 years, height 1.88 ± 0.06 m, body mass 84 ± 12 kg, \(\dot{V}{\text{O}}_{2}\)max 52 ± 6 mL kg−1 min−1) voluntarily participated in this study. Habitual caffeine intake was determined for all participants based on caffeine-rich food and beverage recall (i.e., coffee, tea, soda, chocolate). They reported habitual caffeine intake of 50–100 mg day−1. The study was conducted during the off season of the athletes’ annual training schedule. All of the participants completed a medical history and a health questionnaire and signed informed consent forms before participating in the experiment. This study was approved by the Taipei Medical University-Joint Institutional Review Board.
Experimental design
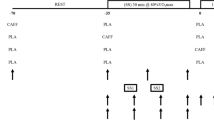
A test that used a double-blind, randomized, crossover design was conducted in this study. The testing protocol required four visits to the laboratory over a 3-week period. Participants first performed an incremental cycling test to determine the \(\dot{V}{\text{O}}_{2}\)max and gas exchange threshold (GET). During the second visit, participants performed the 3MT, which served as a familiarization trial and was not included in the data analyses. Previous studies have revealed that the 3MT had a moderate to high test–retest reliability (Burnley et al. 2006; Johnson et al. 2011; Cheng et al. 2012) and that the end-test power (EP) derived using the 3MT can appropriately determine the CP value estimated using the traditional work − time and power − 1/time CP models (Vanhatalo et al. 2007; Cheng et al. 2012). A strong correlation exists between the work done above EP (WEP), which can be inferred from the 3MT, and W′, which is estimated using traditional CP models (Vanhatalo et al. 2007).
During the following two visits (separated by at least 1 week to ensure a washout of the supplements), a randomized crossover design was used to administer gelatin capsules containing either caffeine (CAF, 6 mg kg−1 of caffeine; Sigma-Aldrich, St. Louis, MS, USA) or a placebo (PL, identical number of capsules containing cellulose; Holy Food, Taoyuan, Taiwan) with 200 mL of water to the participants. After ingesting the caffeine or placebo capsules, the participants were asked to rest in a supine position for 1 h in a semidark and quiet room. Afterwards, the participants performed the 3MT on a cycling ergometer (Avantronic Cyclus II; h/p/cosmos, Leipzig, Germany). Plasma caffeine was not measured directly in this study, but was estimated based on previous findings (Graham and Spriet 1995). This dose likely results in a plasma caffeine concentration of ~45 μM, and the 3MT was conducted 1 h later to allow sufficient time for caffeine to reach peak plasma concentrations (Graham and Spriet 1995). The blood samples were obtained before and 1 h after ingesting the caffeine or placebo, and 5 min after the 3MT. The participants completed all of the trials during the same period (±2 h) of testing days to eliminate any effect from circadian variation.
The participants were required to abstain from creatine or other nutritional supplements during the 12 weeks prior to the trials; those who did not abstain were excluded from the study. None of the participants reported using any nutritional supplements at the time of the study. The participants refrained from drinking alcohol or caffeine-containing beverages for 24 h before the experiments began and fasted at least 4 h prior to visiting the laboratory, thus reducing food interference in the experiment. In addition, the participants were required to limit caffeine consumption to less than 100 mg daily throughout the study. They recorded the contents of the meal consumed before each exercise trial and were asked to consume the same meal before each trial to reduce food inference.
Assessment of \(\dot{V}{\text{O}}_{2}\)max and GET
During each participant’s first visit to the laboratory, the cycling ergometer seat and handlebars were adjusted for comfort. These same settings were restored for each consecutive exercise trial. Before the incremental cycling test, all participants were asked to cycle at different pedaling rates of 70, 80, and 90 rpm for a while, and then chose the most comfortable one as their self-selected cadence (80 rpm, N = 13; 90 rpm, N = 2). Following a previous experimental design (Vanhatalo et al. 2007), the participants performed an incremental cycling test in which the exercise load was gradually increased. The participants first performed 3 min of unloaded baseline pedaling (0 W); the load was increased by 30 W every min thereafter until volitional exhaustion. During the incremental cycling test, the participants maintained a self-selected cadence for as long as possible. Strong verbal encouragement was provided throughout the trial. Exhaustion was defined as a pedal rate of 10 rpm lower than the self-selected cadence for 10 s or more.
Pulmonary gas exchanges were measured breath-by-breath throughout the incremental cycling test by having the participants wear a face mask (7400 Vmask series, Hans Rudolph, Kansas City, MO, USA) attached to a portable gas analysis system (Cortex Metamax 3B; Cortex Biophysik, Leipzig, Germany). Before the test, the system was calibrated according to the manufacturer’s guidelines against known concentrations of cylinder gases (15 % oxygen, 5 % carbon dioxide) and a 3-L calibration syringe (5530 series, Hans Rudolph, Kansas City, MO, USA). Heart rates were monitored using a telemetry system with a wireless chest strap (Polar S810i; Polar Electro, Inc., Oy, Kempele, Finland) and continuously measured through a link to the Cortex gas analysis system during the exercise test. The greatest \(\dot{V}{\text{O}}_{2}\) value (averaged every 30 s) measured during the incremental cycling test was recorded as the \(\dot{V}{\text{O}}_{2}\)max value. The data were reduced to 10-s averages, and three researchers separately estimated the GET values using the V-slope method (Beaver et al. 1986). If the results differed, the three researchers discussed the results until a consensus was reached.
Performance trials
The participants warmed up with 5 min of cycling at 100 W followed by 5 min of rest prior to the 3MT. The 3MT was performed according to previous studies (Vanhatalo et al. 2007). Three minutes of unloaded baseline pedaling (at 0 W and a self-selected cadence) were immediately followed by 3MT with all-out effort. In the final 5 s of unloaded baseline pedaling, the participants were asked to increase the pedal rate to 110 rpm. In the 3MT, the load used was half of the difference between the \(\dot{V}{\text{O}}_{2}\)max and GET values, as previously determined by the incremental cycling test. In other words, the load used was the GET plus 50 % ∆, where ∆ is the difference between \(\dot{V}{\text{O}}_{2}\)max and the GET. Throughout the entire trial, the participants were provided strong verbal encouragement but were not informed of the elapsed time, thus preventing pacing. To ensure that the participants exercised with all-out effort until exhaustion, they were asked to maintain a maximum pedal rate throughout the trial.
Throughout the trial, the Cortex gas analysis system and the Polar heart rate monitor were used to measure oxygen uptake and heart rate. Peak oxygen uptake (\(\dot{V}{\text{O}}_{2}\)peak) and peak heart rate (HRpeak) were defined as the highest mean \(\dot{V}{\text{O}}_{2}\) and HR values obtained in 10-s intervals throughout the 3-min effort to exhaustion. Immediately after completing the 3MT, the Borg (6–20) Scale was used to assess the rating of perceived exertion (RPE) (Borg 1970). Throughout the 3MT, the power output data were recorded by the ergometer’s built-in software. Following the trial, all of the data were transmitted to a personal computer to analyze the EP, WEP, peak power, mean power, total work, and mean cumulative power output calculated in 30-s intervals during the 3MT. EP was defined as the mean power in the final 30 s of the 3MT to exhaustion, and WEP was defined as the power–time integral above the EP in the 3MT. To assess the level of fatigue during the 3MT, the fatigue rate (FR) was calculated using the rate constant of the exponential decline of power output (Naharudin and Yusof 2013). A single-exponential model was used to characterize the power output responses to the 3MT, as described in the following formula:
where P(t) represents the power output at a given time t, P 0 represents the maximal power out, and A and k represent the amplitude and rate constant (i.e., FR), respectively. The lower FR would mean participants can maintain the power output longer throughout the 3MT period. The parameters of the nonlinear least-squares algorithm were calculated using SigmaPlot software (version 10.0, Systat Software, Inc., Germany) to fit the power output data.
Blood sampling and analysis
Capillary blood samples were taken by ear lobe puncture before and after the 3MT. The first blood samples were discarded, and the second blood samples (approximately 5 µL) were used to analyze the blood lactate concentrations using the lactate chemistry analyzer (Lactate Pro, Arkray, Inc., Japan). The Lactate Pro analyzer was calibrated using check or calibration strips provided by the manufacturer 30 min before testing the capillary blood samples, thus ensuring the correct operation and precision of the analyzer. For measuring the blood pH value and electrolytes concentrations, whole blood samples (approximately 1 mL) were drawn from the medial antecubital vein. A blood gas analyzer (OPTI CCA-TS; OPTI Medical System, Inc., USA) was used to assess the blood pH values as well as the sodium and potassium concentrations.
Statistical analysis
A previously described statistical spreadsheet (Hopkins 2006) was used to determine the study’s sample size. The spreadsheet estimates sample size requirements for magnitude-based inferences when the typical error and the smallest worthwhile change for the cycling performance measure are entered. The data used for these values were obtained from the systematic review of a previous study (Astorino and Roberson 2010) that focused on the acute effects of caffeine on variation in power output during short-term, high-intensity exercise. These values were 5.5 and 6.5 % for the typical error and the smallest worthwhile change, respectively. A sample size calculation using these values indicated that this study required 13 participants.
SPSS for Windows software (version 17.0, SPSS, Inc., Chicago, IL, USA) was used to conduct statistical analysis. The results are expressed as mean ± standard deviation (SD). The Shapiro–Wilk normality test was performed to determine the homogeneity of the sample. The test of normality verified that all of the data produced were normally distributed (P > 0.05). The dependent-measures t test was used to evaluate the performance data (EP, WEP, peak power, mean power, total work, power output averaged every 30 s, FR), \(\dot{V}{\text{O}}_{2}\)peak, and HRpeak. A 2 (condition) × 3 (time) within-participants repeated measures ANOVA was used to investigate differences in blood variables, including the blood lactate levels, pH values, sodium concentration [Na+], and [K+]. If a significant interaction was found, it was followed up by a one-way ANOVA with Bonferroni post hoc comparisons to examine differences over time and between conditions. The effect size (ES) and 95 % confidence intervals (95 % CI) were calculated to evaluate differences between conditions for mean values of performance data and blood variables. The ES was calculated by dividing the difference between mean values of the conditions by the pooled SD (Cohen 1988). ES of <0.5, 0.5–0.79 and ≥0.8 were considered as small, moderate and large effects, respectively (Cohen 1988). Statistical significance was denoted by a P value ≤0.05.
Results
Exercise performance
The results of the performance data for the trial in CAF and PL conditions are represented in Table 1. A significantly higher WEP was observed in the CAF condition (+10.7 %, P < 0.05, ES = 0.85, 95 % CI = −0.7 to 2.2) than in the PL condition; however, no significant difference in EP was determined between the conditions. No significant differences in peak power, mean power, or total work were determined between the conditions during the 3MT. Regarding mean cumulative power output, the PO60 (+2.0 %, P < 0.05, ES = 1.02, 95 % CI −17.6 to 20.3), PO90 (+2.5 %, P < 0.05, ES = 1.20, 95 % CI −16.3 to −19.7), and PO120 (+2.2 %, P < 0.05, ES = 0.94, 95 % CI −16.5 to 20.0) were all significantly higher for the CAF condition than for the PL condition. The PO150 in the CAF condition was slightly higher than that in PL with non-significant difference; however, PO150 exhibited a moderate magnitude of effect size on the CAF condition (+1.9 %, P = 0.09, ES = 0.68, 95 % CI −16.9 to 20.1). As shown in Fig. 1a, the power output between approximately 30 and 150 s was higher for the CAF condition than for the PL condition. In addition, the FR for the CAF condition was significantly smaller than that for the PL condition (P = 0.006, ES = −1.20, 95 % CI −1.20 to −1.19). Figure 1b demonstrates that the \(\dot{V}{\text{O}}_{2}\) reached a plateau during the 3MT. No significant difference was found in \(\dot{V}{\text{O}}_{2}\)peak between the conditions (CAF vs. PL, 48.0 ± 7.5 vs. 49.2 ± 6.3 mL kg−1 min−1, P > 0.05, ES = −0.35, 95 % CI −4.2 to 2.8). However, the HRpeak was significantly higher for the CAF condition than for the PL condition (CAF vs. PL, 172 ± 7 vs. 165 ± 8 bpm, P < 0.05, ES = 1.35, 95 % CI −2.3 to 5.2). No significant difference in RPE was determined between the conditions after the 3MT (CAF vs. PL, 18.7 ± 1.5 vs. 18.7 ± 1.0, P > 0.05).
Effects of caffeine ingestion on group mean power output (a) and oxygen uptake (\(\dot{V}{\text{O}}_{2}\)) (b) during 3-min all-out cycling test. a The dashed lines represent the standard deviation. The power outputs between 30 s and 150 s in the CAF (caffeine) condition were higher than those in the PL (placebo) condition, and the rate at which the power output decreased was slower in the CAF condition than in the PL condition. b The \(\dot{V}{\text{O}}_{2}\) plateau was reached at approximately 60 s
Blood parameters
Figure 2 demonstrates the effect of caffeine ingestion on the blood parameters before and after the 3MT. A significant condition × time interaction effect was found for the blood lactate concentrations (F = 10.131, P < 0.05). Regardless of whether the participant ingested caffeine or the placebo, the blood lactate concentrations were substantially higher after the 3MT than at baseline or at 1 h after ingestion (Fig. 2a). In addition, the blood lactate concentrations after the 3MT were substantially higher with the caffeine than with the placebo (CAF vs. PL, 11.25 ± 2.58 vs. 9.80 ± 1.76 mmol L−1, P < 0.05, ES = 1.28, 95 % CI −0.03 to 2.17). No significant interaction effect was determined for blood pH (F = 0.658, P > 0.05,), but a significant main effect was found for time (baseline vs. 1 h after ingestion vs. after 3MT, 7.40 ± 0.02 vs. 7.39 ± 0.04 vs. 7.17 ± 0.07, F = 193.368, P < 0.05). Regardless of whether the participant ingested caffeine or the placebo, blood pH was substantially lower after the 3MT than at baseline or 1 h after ingestion (Fig. 2b).
In both conditions, plasma [K+] was significantly higher after the 3MT than at baseline or 1 h after ingestion and significantly higher 1 h after ingestion than at baseline (Fig. 2c). At 1 h after ingestion, plasma [K+] was significantly lower in the caffeine condition than in the placebo condition (CAF vs. PL, 3.87 ± 0.22 vs. 3.99 ± 0.30 mmol L−1, P < 0.05, ES = −1.03, 95 % CI −1.14 to −0.88); no significant differences between the conditions were found at other time points. No significant interaction effects were determined for plasma [Na+]. A significant main effect was determined for time in plasma [Na+] (F = 69.100, P < 0.05). The main effect for conditions in plasma [Na+] was not significant (F = 0.047, P > 0.05). Regardless of whether the participant ingested caffeine or a placebo, plasma [Na+] was substantially higher after the 3MT than at baseline or 1 h after ingestion (Fig. 2d).
Discussion
The primary aim of this study was to examine the effect of acute caffeine ingestion on 3MT performance and plasma electrolytes in athletes. The key findings were that (1) caffeine increased the WEP of well-trained athletes but had no effect on EP and that (2) caffeine ingestion raised the fatigue resistance of the participants by delaying the rapid decline of power output between the 60th and 120th seconds during short-term, high-intensity exercise, and lowered plasma [K+] prior to exercising. In addition, caffeine ingestion increased blood lactate concentrations after exercising but had no effect on blood pH.
Our results are consistent with those of Fukuda et al. (2010), who found that the CP values derived using the traditional CP model were not affected by low dosages of caffeine ingestion but that the W′ was improved by the caffeine. Particularly, the 3MT is considered an extremely high-intensity exercise. A possible explanation for the unchanged EP and increased WEP is that the caffeine stimulation experienced by the central nervous system (CNS) decreased the perceived pain levels during the late period of the 3MT. Decreased pain sensitivity could likely postpone the fatigue, thereby postponing the time at which the exercise would be terminated (Davis and Green 2009). Therefore, caffeine ingestion may not affect an athlete’s EP as estimated from the 3MT, and this finding holds true for amounts of caffeine ≤6 mg kg−1.
Although the study results suggested that caffeine ingestion had no effect on EP, it substantially increased WEP. Traditionally, W′ has been associated with anaerobic capacity (Moritani et al. 1981) and believed to be anaerobic in nature (Dekerle et al. 2008). In addition, previous studies (Doherty 1998; Bell et al. 2001; Simmonds et al. 2010) have reported that caffeine ingestion can improve the maximal accumulated oxygen deficit, which correlates well with W′ (Dekerle et al. 2008), in short-term, high-intensity exercises (at an exercise intensity with a \(\dot{V}{\text{O}}_{2}\)max of 120 or 125 % for approximately 2–3 min). Thus, a moderate to high dose of caffeine ingestion might improve anaerobic capacity in well-trained athletes.
The present study was the first to determine that caffeine ingestion increased mean cumulative power output between the 60th and 120th seconds of the 3MT and improved FR. The results also suggested that caffeine ingestion increased HRpeak and lactate concentrations postexercise but had no effect on RPE, which indicates that, at the same RPE, caffeine can improve power output performance and delay the rapid decline of power output during high-intensity exercises. This finding suggests that caffeine ingestion can increase an athlete’s tolerance for short-term, high-intensity exercises. The antagonism of adenosine receptors is commonly considered to be the primary ergogenic mechanism of caffeine (Davis and Green 2009; Goldstein et al. 2010). Caffeine acts as an adenosine antagonist on the CNS and changes the perception of pain during exercise. Previous studies reported that caffeine ingestion reduces leg muscle pain (Motl et al. 2003, 2006; Gliottoni and Motl 2008; Gliottoni et al. 2009) during moderate and heavy cycling exercise, but this hypoalgesic effect disappeared with increases in performance during heavy to severe-intensity exercise (Jenkins et al. 2008; Black et al. 2015; Gonglach et al. 2016). Astorino et al. (2011) similarly determined that caffeine improved exercise performance during two sets of 40 repetitions of isokinetic knee extensions and flexions but did not change the RPE or pain perceptions in the leg. It has been proposed that during high-intensity exercise, the nociceptive inputs required to produce severe muscle pain may be too greater for antagonism of adenosine receptors alone to induce hypoalgesia (Black et al. 2015; Gonglach et al. 2016). Another possible explanation for improving FR and increasing power output in the first 90 s during the 3MT might be the reduction in force sensation. Plaskett and Cafarelli (2001) found that caffeine diminished force sensation at the onset of sustained submaximal contractions, which indicates that the participants were either more willing or motivated to tolerate discomfort or felt less discomfort during the early period of exercise. Therefore, caffeine ingestion may improve performance in the absence of altering EP by allowing individuals to pace their work rate at a value closer to EP while keeping muscle pain and RPE at a manageable level.
Another explanation for the improvements in WEP and FR might be related to the alterations in muscle strength and voluntary muscle activation after caffeine administration. Results from a meta-analysis study of Warren et al. (2010) suggested that caffeine ingestion can enhance motor unit recruitment during a maximal voluntary isometric contraction, thus increasing the muscular strength. Previous studies (Kalmar and Cafarelli 1999; Plaskett and Cafarelli 2001; Bazzucchi et al. 2011) found that 6 mg kg−1 of caffeine significantly increased muscle activation and force generation during maximal voluntary contractions. A recent study (Black et al. 2015) also indicated that the augmented muscular strength and motor unit recruitment, rather than reductions in pain and effort, might contribute to the effects of caffeine on heavy and severe cycling exercise performance. Although the present study did not measure the muscle strength and motor unit recruitment, both mechanisms may explain the ergogenic effects of caffeine on WEP and FR during high-intensity anaerobic exercise.
This study found that caffeine ingestion slightly increased mean power output and total work with non-significant differences between the conditions. Although WEP was improved by caffeine intake, two participants decreased their total work under the CAF condition (nonresponders). However, there were also no significant differences in total work (n = 13, +0.9 %, P > 0.05) and mean power output (n = 13, +1.0 %, P > 0.05) between the conditions when excluding nonresponder data. These findings were similar to those of Santos et al. (2013), which revealed that caffeine ingestion improved the power output performance in the middle of 4000-m cycling time trial without changes in total work (+3.8 %, P > 0.05). Our previous study (Lee et al. 2012) reported that both mean power output and total work at second set were significantly lower than those at first set during the intermittent cycling sprint exercise. As mentioned previously, the changes in force sensation caused by caffeine intake might have altered the pacing strategy at the early period of 3MT, thus influencing the total work performed. Nevertheless, further studies are warranted to investigate the effects of caffeine ingestion on total work performance during high-intensity exercise lasting more than 3 min.
The results suggested that, 1 h after caffeine ingestion, the resting values of plasma [K+] decreased significantly. However, caffeine had no effect on plasma [K+] after the 3MT. This decrease in plasma [K+] may be related to increased activity of the Na+/K+ ATPase pumps (Davis and Green 2009; Astorino and Roberson 2010). Therefore, caffeine may enhance performance by a decrease in plasma [K+] and maintenance of the resting membrane potential of muscle cells (Lindinger et al. 1993; Crowe et al. 2006; Simmonds et al. 2010; Mohr et al. 2011). Previous studies have exhibited similar results: caffeine ingestion substantially decreased plasma [K+] at rest (Lindinger et al. 1993; Crowe et al. 2006) and after low-intensity warm-ups (Simmonds et al. 2010; Mohr et al. 2011). Although Lindinger et al. (1993) determined that caffeine clearly lowered plasma [K+] both during and after prolonged endurance exercises, more studies have found that caffeine has no effect on plasma [K+] after exercise, including short-term supramaximal cycling (Simmonds et al. 2010), the repeated Wingate exercise (Greer et al. 1998; Crowe et al. 2006), and the Yo–Yo intermittent recovery test (Mohr et al. 2011); our findings are consistent with these studies. Therefore, caffeine ingestion may not prevent an increase in plasma [K+] during short-term, high-intensity exercises. Mohr et al. (2011) asked participants to perform three sets of intense one-legged knee-extensor exercises with 5-min rest intervals after caffeine ingestion and determined that caffeine substantially lowered plasma [K+] after the first set of exercises but had no effect on plasma [K+] after the subsequent two sets of exercises. However, caffeine did substantially decrease the muscle interstitial [K+] after each set of exercises, which indicates that, when examining the effect of caffeine on [K+] in short-term, high-intensity exercises, the plasma [K+] may not reflect the accumulation of muscle interstitial K+. Therefore, additional studies are required to examine the effect of caffeine on muscle interstitial K+ during short-term, high-intensity exercises to clarify the effect of caffeine on the activity of Na+/K+ ATPase pumps.
Conclusions
The study results suggested that caffeine ingestion in well-trained athletes did not alter EP as determined by the 3MT but increased WEP. In addition, caffeine could prevent a rise in the values of resting plasma [K+], thereby improving anaerobic capacity and delaying the decline in power output during short-term, high-intensity exercises. These findings can be of considerable practical value to exercise physiologists, sports scientists, and clinicians who wish to estimate an athlete’s CP using the 3MT. The ingestion of 6 mg kg−1 of caffeine will not affect the estimation of EP but may interfere with the estimation of WEP. Furthermore, caffeine ingestion can improve an athlete’s performance during severe-intensity exercises, which may be related to the ability of caffeine to increase exercise tolerance, muscle strength, and/or motor unit recruitment. However, the actual mechanism underlying caffeine’s ergogenic benefits during short-term, high-intensity exercises (i.e., increased Na+/K+ ATPase pump activity leading to changes in [K+] in plasma and muscle) requires further study.
Abbreviations
- 3MT:
-
3-Minute all-out test
- CAF:
-
Caffeine treatment
- CI:
-
Confidence intervals
- CNS:
-
Central nervous system
- CP:
-
Critical power
- EP:
-
End-test power
- ES:
-
Effect size
- FR:
-
Fatigue rate
- GET:
-
Gas exchange threshold
- HR:
-
Heart rate
- HRpeak:
-
Peak heart rate
- K+ :
-
Potassium
- Na+ :
-
Sodium
- PL:
-
Placebo treatment
- RPE:
-
Rating of perceived exertion
- SD:
-
Standard deviation
- \(\dot{V}{\text{O}}_{2}\) :
-
Oxygen uptake
- \(\dot{V}{\text{O}}_{2}\)max:
-
Maximal oxygen uptake
- \(\dot{V}{\text{O}}_{2}\)peak:
-
Peak oxygen uptake
- Wʹ:
-
Curvature constant
- WEP:
-
Work done above EP
References
Astorino TA, Roberson DW (2010) Efficacy of acute caffeine ingestion for short-term high-intensity exercise performance: a systematic review. J Strength Cond Res 24(1):257–265. doi:10.1519/JSC.0b013e3181c1f88a
Astorino TA, Terzi MN, Roberson DW, Burnett TR (2011) Effect of caffeine intake on pain perception during high-intensity exercise. Int J Sport Nutr Exerc Metab 21(1):27–32
Bazzucchi I, Felici F, Montini M, Figura F, Sacchetti M (2011) Caffeine improves neuromuscular function during maximal dynamic exercise. Muscle Nerve 43(6):839–844. doi:10.1002/mus.21995
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60(6):2020–2027
Bell DG, Jacobs I, Ellerington K (2001) Effect of caffeine and ephedrine ingestion on anaerobic exercise performance. Med Sci Sports Exerc 33(8):1399–1403
Bergstrom HC, Housh TJ, Zuniga JM, Traylor DA, Camic CL, Lewis RW Jr, Schmidt RJ, Johnson GO (2013) The relationships among critical power determined from a 3-min all-out test, respiratory compensation point, gas exchange threshold, and ventilatory threshold. Res Q Exerc Sport 84(2):232–238
Black CD, Waddell DE, Gonglach AR (2015) Caffeine’s ergogenic effects on cycling: neuromuscular and perceptual factors. Med Sci Sports Exerc 47(6):1145–1158. doi:10.1249/MSS.0000000000000513
Borg G (1970) Perceived exertion as an indicator of somatic stress. Scand J Rehab Med 2:92–98
Burnley M, Doust JH, Vanhatalo A (2006) A 3-min all-out test to determine peak oxygen uptake and the maximal steady state. Med Sci Sports Exerc 38(11):1995–2003
Cheng CF, Yang YS, Lin HM, Lee CL, Wang CY (2012) Determination of critical power in trained rowers using a three-minute all-out rowing test. Eur J Appl Physiol 112(4):1251–1260. doi:10.1007/s00421-011-2081-2
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Laurence Erlbaum Associates, New Jersey
Collomp K, Ahmaidi S, Audran M, Chanal JL, Prefaut C (1991) Effects of caffeine ingestion on performance and anaerobic metabolism during the Wingate Test. Int J Sports Med 12(5):439–443
Collomp K, Ahmaidi S, Chatard JC, Audran M, Prefaut C (1992) Benefits of caffeine ingestion on sprint performance in trained and untrained swimmers. Eur J Appl Physiol 64(4):377–380
Crowe MJ, Leicht AS, Spinks WL (2006) Physiological and cognitive responses to caffeine during repeated, high-intensity exercise. Int J Sport Nutr Exerc Metab 16(5):528–544
Davis JK, Green JM (2009) Caffeine and anaerobic performance: ergogenic value and mechanisms of action. Sports Med 39(10):813–832. doi:10.2165/11317770-000000000-00000
Dekerle J, Vanhatalo A, Burnley M (2008) Determination of critical power from a single test. Sci Sports 23:231–238. doi:10.1016/j.scispo.2007.06.015
Desbrow B, Biddulph C, Devlin B, Grant GD, Anoopkumar-Dukie S, Leveritt MD (2012) The effects of different doses of caffeine on endurance cycling time trial performance. J Sports Sci 30(2):115–120. doi:10.1080/02640414.2011.632431
Dodd SL, Brooks E, Powers SK, Tulley R (1991) The effects of caffeine on graded exercise performance in caffeine naive versus habituated subjects. Eur J Appl Physiol 62(6):424–429
Doherty M (1998) The effects of caffeine on the maximal accumulated oxygen deficit and short-term running performance. Int J Sport Nutr Exerc Metab 8(2):95–104
Doherty M, Smith PM (2005) Effects of caffeine ingestion on rating of perceived exertion during and after exercise: a meta-analysis. Scand J Med Sci Sports 15(2):69–78
Fitts RH (1994) Cellular mechanisms of muscle fatigue. Physiol Rev 74(1):49–94
Forbes SC, Candow DG, Little JP, Magnus C, Chilibeck PD (2007) Effect of Red Bull energy drink on repeated Wingate cycle performance and bench-press muscle endurance. Int J Sport Nutr Exerc Metab 17(5):433–444
Fukuda DH, Smith AE, Kendall KL, Stout JR (2010) The possible combinatory effects of acute consumption of caffeine, creatine, and amino acids on the improvement of anaerobic running performance in humans. Nutr Res 30(9):607–614. doi:10.1016/j.nutres.2010.09.004
Gaesser GA, Rich RG (1985) Influence of caffeine on blood lactate response during incremental exercise. Int J Sports Med 6(4):207–211
Ganio MS, Klau JF, Casa DJ, Armstrong LE, Maresh CM (2009) Effect of caffeine on sport-specific endurance performance: a systematic review. J Strength Cond Res 23(1):315–324. doi:10.1519/JSC.0b013e31818b979a
Gliottoni RC, Motl RW (2008) Effect of caffeine on leg-muscle pain during intense cycling exercise: possible role of anxiety sensitivity. Int J Sport Nutr Exerc Metab 18(2):103–115
Gliottoni RC, Meyers JR, Arngrimsson SA, Broglio SP, Motl RW (2009) Effect of caffeine on quadriceps muscle pain during acute cycling exercise in low versus high caffeine consumers. Int J Sport Nutr Exerc Metab 19(2):150–161
Goldstein ER, Ziegenfuss T, Kalman D, Kreider R, Campbell B, Wilborn C, Taylor L, Willoughby D, Stout J, Graves BS, Wildman R, Ivy JL, Spano M, Smith AE, Antonio J (2010) International society of sports nutrition position stand: caffeine and performance. J Int Soc Sports Nutr 7(1):5. doi:10.1186/1550-2783-7-5
Gonglach AR, Ade CJ, Bemben MG, Larson RD, Black CD (2016) Muscle pain as a regulator of cycling intensity: effect of caffeine ingestion. Med Sci Sports Exerc 48(2):287–296. doi:10.1249/MSS.0000000000000767
Graham TE, Spriet LL (1995) Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. J Appl Physiol 78(3):867–874
Greer F, McLean C, Graham TE (1998) Caffeine, performance, and metabolism during repeated Wingate exercise tests. J Appl Physiol 85(4):1502–1508
Hopkins W (2006) Estimating sample size for magnitude-based inferences. Sportscience 10:63–70
Jenkins NT, Trilk JL, Singhal A, O’Connor PJ, Cureton KJ (2008) Ergogenic effects of low doses of caffeine on cycling performance. Int J Sport Nutr Exerc Metab 18(3):328–342
Johnson TM, Sexton PJ, Placek AM, Murray SR, Pettitt RW (2011) Reliability analysis of the 3-min all-out exercise test for cycle ergometry. Med Sci Sports Exerc 43(12):2375–2380. doi:10.1249/MSS.0b013e318224cb0f
Kalmar JM (2005) The influence of caffeine on voluntary muscle activation. Med Sci Sports Exerc 37(12):2113–2119
Kalmar JM, Cafarelli E (1999) Effects of caffeine on neuromuscular function. J Appl Physiol 87(2):801–808
Kolbe T, Dennis SC, Selley E, Noakes TD, Lambert MI (1995) The relationship between critical power and running performance. J Sports Sci 13(3):265–269
Lee CL, Cheng CF, Lin JC, Huang HW (2012) Caffeine’s effect on intermittent sprint cycling performance with different rest intervals. Eur J Appl Physiol 112(6):2107–2116. doi:10.1007/s00421-011-2181-z
Lindinger MI, Graham TE, Spriet LL (1993) Caffeine attenuates the exercise-induced increase in plasma [K+] in humans. J Appl Physiol 74(3):1149–1155
McKenna MJ, Bangsbo J, Renaud JM (2008) Muscle K+, Na+, and Cl disturbances and Na+–K+ pump inactivation: implications for fatigue. J Appl Physiol 104(1):288–295
Mohr M, Nielsen JJ, Bangsbo J (2011) Caffeine intake improves intense intermittent exercise performance and reduces muscle interstitial potassium accumulation. J Appl Physiol 111(5):1372–1379. doi:10.1152/japplphysiol.01028.2010
Monod H, Scherrer J (1965) The work capacity of a synergic muscular group. Ergonomics 8:329–338
Moritani T, Nagata A, deVries HA, Muro M (1981) Critical power as a measure of physical work capacity and anaerobic threshold. Ergonomics 24(5):339–350
Motl RW, O’Connor PJ, Dishman RK (2003) Effect of caffeine on perceptions of leg muscle pain during moderate intensity cycling exercise. J Pain 4(6):316–321
Motl RW, O’Connor PJ, Tubandt L, Puetz T, Ely MR (2006) Effect of caffeine on leg muscle pain during cycling exercise among females. Med Sci Sports Exerc 38(3):598–604
Naharudin MN, Yusof A (2013) Fatigue index and fatigue rate during an anaerobic performance under hypohydrations. PLoS One 8(10):e77290. doi:10.1371/journal.pone.0077290
Plaskett CJ, Cafarelli E (2001) Caffeine increases endurance and attenuates force sensation during submaximal isometric contractions. J Appl Physiol 91(4):1535–1544
Poole DC, Ward SA, Gardner GW, Whipp BJ (1988) Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics 31:1265–1279
Santos Rde A, Kiss MA, Silva-Cavalcante MD, Correia-Oliveira CR, Bertuzzi R, Bishop DJ, Lima-Silva AE (2013) Caffeine alters anaerobic distribution and pacing during a 4000-m cycling time trial. PLoS One 8(9):e75399. doi:10.1371/journal.pone.0075399
Shimoda M, Kawakami Y (2005) Critical power determination with ergometry rowing: relation to rowing performance. Int J Sport Health Sci 3:21–26
Simmonds MJ, Minahan CL, Sabapathy S (2010) Caffeine improves supramaximal cycling but not the rate of anaerobic energy release. Eur J Appl Physiol 109(2):287–295. doi:10.1007/s00421-009-1351-8
Smith JC, Dangelmaier BS, Hill DW (1999) Critical power is related to cycling time trial performance. Int J Sports Med 20(6):374–378
Vanhatalo A, Doust JH, Burnley M (2007) Determination of critical power using a 3-min all-out cycling test. Med Sci Sports Exerc 39(3):548–555
Warren GL, Park ND, Maresca RD, McKibans KI, Millard-Stafford ML (2010) Effect of caffeine ingestion on muscular strength and endurance: a meta-analysis. Med Sci Sports Exerc 42(7):1375–1387. doi:10.1249/MSS.0b013e3181cabbd8
Wiles JD, Coleman D, Tegerdine M, Swaine IL (2006) The effects of caffeine ingestion on performance time, speed and power during a laboratory-based 1 km cycling time-trial. J Sports Sci 24(11):1165–1171
Woolf K, Bidwell WK, Carlson AG (2008) The effect of caffeine as an ergogenic aid in anaerobic exercise. Int J Sport Nutr Exerc Metab 18(4):412–429
Acknowledgments
The authors wish to thank the participants who contributed their time and effort to undertake this study and to Polypact International Co., Ltd., who sponsored the consumable materials of Cortex metabolic analysis system. This work is particularly supported by “Aim for the Top University Plan” of National Taiwan Normal University and the Ministry of Education, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Communicated by Anni Vanhatalo.
Rights and permissions
About this article
Cite this article
Cheng, CF., Hsu, WC., Kuo, YH. et al. Caffeine ingestion improves power output decrement during 3-min all-out exercise. Eur J Appl Physiol 116, 1693–1702 (2016). https://doi.org/10.1007/s00421-016-3423-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3423-x