Abstract
Purpose
The aim of this study was to assess the association between physical fitness (PF) and energy and nutrient intake in Spanish older adults.
Methods
Three hundred and twenty-four participants (59.9% females, aged over 55 years) performed a battery of four validated PF tests and participants were divided into three: low, medium, and high PF. Dietary intake was assessed by two non-consecutive 24 h dietary recalls. Energy and nutrient intake was calculated using the ALIMENTA software. Energy expenditure (EE) was calculated using a validated questionnaire.
Results
Median energy intake (EI) was 2135, 1999, and 2111 kcal/day in the low, medium, and high PF in males, respectively. In females, the median EI was 1576, 1564, and 1625 kcal/day in the low, medium, and high PF groups. There were significant and positive associations between participants in the high PF group and intake of phosphorous, selenium, vitamin B6, C, D, E, niacin, and folates (all p < 0.05). However, subjects in the high PF group presented negative associations with thiamine and riboflavin intake (all p < 0.05). A total of 8.3% of participants presented inadequate intake of 11 micronutrients. PF seems to affect total nutrient intake.
Conclusions
Higher protein and fat intake was observed in the high PF group compared to the other PF groups in males, although participants in the high PF group had also higher EE. However, females presented different patterns. In both sexes participants in the high PF group showed a better micronutrient intake profile than the other PF groups. There is a need to develop combined nutritional and fitness programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of physical fitness (PF) and diet in reducing the progression of chronic disease is becoming increasingly crucial [1]. Diet has been described as the main modifiable behaviour and PF has also been defined as an important marker related to health [2]. Some authors suggest that improvements in these two factors could prevent the functional limitations that are strongly associated with advanced age, and could lead to a healthier, more active, and more independent aging process [3, 4].
Older adults are particularly at risk of deficient or suboptimal micronutrient status and they are also at risk of overconsumption of macronutrients which leads to overweight and non-communicable diseases [5, 6]. As energy expenditure (EE) decreases in the older populations [7], mainly because of less daily activity [8], a reduction of food intake leading to a proportional reduction of the intake of some nutrients is often the consequence [9]. Likewise, another reason is the role of drug intake on micronutrient intake availability or high intake based on drug intake consumption. Intake deficiency of several vitamins and minerals had already been observed previously in older adults [10]. Meanwhile, the prevalence of malnutrition is increasing in this kind of population [11], which is associated with a decline in functional status [12], impaired muscle function and sarcopenia [13], anemia [14], immune dysfunction [15], decreased bone mass, and reduced cognitive function [11].
PF is accepted as a health marker and is also considered an independent factor of all-cause diseases and mortality [16, 17]. A 50% of reduction in mortality has been observed among highly fit people compared to low fit people [18]. In addition, PF is dependent on several factors like nutritional habits, smoking habits, genetics, and socio-economic status [19].
Because the number of older adults is increasing in developed countries, it is essential to understand the effects of modifiable risk factors such as PF and diet [19]. There are few data analysing the association between PF level and diet in Spanish older adults [20]. Nevertheless, both PF and diet are a key for the development of public health policies to promote healthy lifestyles based on the evidence [21]. PF and diet can keep adults and older adults healthy, fit, and independent [18]. Therefore, the aim of this study was to investigate the relationship between energy and nutrient intake with PF level in Spanish older adults.
Methods
Study design, sample, and ethics
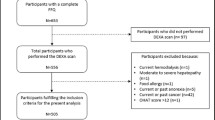
The present study was based on a cross-sectional multi-centre study aiming at identifying cardiovascular risk factors in active and sedentary older adult participants (The PHYSMED Study). Data collection took place from April 2013 to May 2014 in Madrid and Mallorca (Spain). Volunteers were recruited through health centres, sport federations, sport facilities, and municipal clubs located at Madrid and Mallorca. The study population consisted of 433 participants, 184 male (43%), and 244 females (57%) aged 55–88 years. The exclusion criteria were age under 55 years, being institutionalized, suffering from a physical or mental illness which would have limited their participation in the PF tests or their ability to respond to the questionnaires or drug intake for clinical research. For the purpose of this study, participants who provided data on two non-consecutive 24 h dietary recalls were included in the analysis, resulting in 428 older adults. Likewise, underreporting was considered when the individual ratio of energy intake (EI) divided by the estimated basal metabolic rate was lower than 0.96 (EI/basal metabolic rate < 0.96) [22]. Excluding the underreporting, valid reporters resulted in a final sample of 324 participants (59.9% females) for statistical analysis. The group of underreporting consisted of slightly higher percentage of females (57.0% compared to 59.9%, p = 0.035) and had higher median body mass index (BMI) values (28.2 kg/m2 compared to 26.5 kg/m2), weight (77.0 kg compared to 68.4 kg), and fat mass (24.6 kg compared to 21.4 kg) (all p < 0.001). No difference in age was observed. The number of participants in each group of PF and data comparing the whole sample vs. only underreporting is shown in Online Appendix (Table 1).
The study was performed according to the principles established in the Declaration of Helsinki and the final protocol was approved by the Ethical Committee of the Technical University of Madrid. All subjects were informed of the protocol and older adults signed a written informed consent prior to participation.
Physical activity assessment
The Minnesota Leisure-Time Physical Activity Questionnaire was carried out by trained interviewers. It has been validated for Spanish males [23] and females [24]. To calculate the total EE of physical activity (PA), an intensity value was assigned to all the items in the PA questionnaire, according to the metabolic equivalent (METs) classification proposed by Ainsworth [25]. Total PA was calculated by multiplying the total number of hours and their correspondent intensity performed for each activity.
Physical fitness tests
Each participant completed a multi-component battery of PF test [26] and validated reference ranges for Spanish older adults proposed by Pedrero-Chamizo et al. [17]. Lower body strength was measured by the chair stand test, agility/dynamic balance by the 8-foot up-and-go test, aerobic endurance by the 6-min walk test and handgrip strength with a dynamometer (Takei TKK-5401, Tokyo, Japan, range 5–100 kg, precision 0.1 kg) [27]. The handgrip strength was assessed for both hands in a standing position. All tests were performed twice, except the 6-min walk test and the chair stand test, and the best score was retained.
The results of each PF test were stratified by sex and age groups (distributed by five years periods without truncating the last group) as previously proposed by Pedrero-Chamizo et al. [17]. Furthermore, the result of each PF test was divided into quartiles considering also the variables mentioned above (sex and age groups). The score for each test ranged from 0 (worst) to 3 (best) points. Thus, the maximum score was 12 points. Scores of PF tests were added together in a cluster and participants were divided into different levels of PF: low (0–3 points), medium (4–8 points), and high (9–12 points).
Anthropometric measurements
Height was measured to the nearest millimetre using a mobile stadiometer (SECA 213, Germany), with the participant’s head in the Frankfurt plane. Weight and body composition (fat mass and fat free mass) was assessed by means of bioimpedance analysis (TANITA Corp, BC-418MA, Tokyo, Japan) in standardized conditions. In addition, BMI was calculated as weight (kg/height2) (m2). Waist and hip circumference were performed by a trained ISAK level II anthropometrist to minimize the inter-observer coefficients of variation according to ISAK Society [28].
Dietary intake assessment
Dietary intake was obtained by means of two 24 h dietary recalls by well-trained dieticians. The 24 h dietary recalls were collected on two non-consecutive days within a period of 2 weeks. Volumes and portion sizes were reported with the aid of a book of photographs [29]. Energy and nutrient intake were calculated using a computer program (ALIMENTA; NUCOX, Palma, Spain) based on Spanish [30, 31] and European [32] food composition tables, and complemented with food composition data available for Majorcan food items [33].
Furthermore, the estimate average requirements (EAR) cut-off point was applied to estimate the prevalence of nutrient intake adequacy [34]. When EAR was not defined for a specific micronutrient, such as pantothenic acid, potassium, and sodium, the adequate intake (AI) was chosen [34].
Statistical analyses
Statistical analyses were run on SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp). Descriptive characteristics were summarized by calculating median and 5th–95th percentiles unless otherwise stated. All PF tests and dietary intakes were checked for normality of distribution with the Kolmogorov–Smirnov test. Macro- and micronutrient results were transformed (log-transformation) except for energy, water, fat, and mono-unsaturated fatty acids. Comparisons between PF groups and sexes with nutrient intake were analyzed by One-Way Anova or Kruskal–Wallis test, according to the normality of the variables.
To determine the adequacy or inadequacies of nutrient intake, subjects were split into two groups taking into account EAR or AI: below EAR or AI (inadequate; coded as 0) and above EAR or AI (adequate; coded as 1).
Multilevel linear regression analysis was used to examine the relationship between the macro and micronutrient intake, energy expenditure, and PF groups. Confounders (age and sex) were entered as covariates. To adjust for multiple testing, a Bonferroni correction was applied to lower the significance level (α) taking into account the number of tests (0.05/number of tests). A p value of 0.0013 was used as threshold of significance for the association between macro- and micronutrient intake, energy expenditure, and PF groups.
Results
Table 1 shows descriptive characteristics of the sample divided by sex. Table 2 displays energy expenditure, macronutrients and micronutrients intake split by sex and PF groups. In Table 2 (Appendix) energy expenditure, macro-, and micronutrient for underreporting is shown.
Figure 1 presents the percentage of micronutrients below EAR or AI according to sex. More than 85% of males and females showed inadequate intake of potassium, vitamin D and vitamin E. Around 50% of males and females showed inadequate intake of folate and a total of 82% of females presented inadequate intake of calcium.
Figure 2a shows the percentage of micronutrients below EAR or AI split by PF groups in each sex. There were more participants within the low PF group compared to the high PF one which showed inadequate intake for potassium, vitamin D, and vitamin E; however, males in the high PF group presented higher inadequate calcium intake compared to low PF group. Females in the low PF group showed lower inadequate intake for calcium, iodine, and phosphorous compared to the high PF group.
a Percentage of micronutrients below estimate average requirements or adequate intake according to physical fitness groups in males. b. Percentage of micronutrients below estimate average requirements or adequate intake according to physical fitness groups in females. Data are presented as percentage (%)
In addition, percentage of participants by number of micronutrients intake below EAR or AI is shown in Table 3. A total of 13.0 and 8.3% of the participants presented intakes below EAR or AI for 6 and 11 micronutrients, respectively. Only two participants were above EAR or AI for all micronutrients.
Multilevel regression analysis of the PF groups with the usual intake of macro- and micronutrients and energy expenditure is presented in Table 4. A positive association was observed for vegetable proteins and high PF group (all p < 0.05). Regarding minerals, there were significant and positive associations between subjects in the high PF group and phosphorous and selenium (all p < 0.05). Furthermore, positive and significant associations were found between high PF group and vitamin B6, vitamin C, vitamin D, niacin, and folates. However, subjects in the high PF group presented significant negative associations with thiamine and riboflavin (both p < 0.05).
Discussion
The results from this cross-sectional study showed a remarkable inadequate intake of potassium, vitamin D, and vitamin E. It is important to highlight that participants in the low PF group showed inadequate intake only for potassium, vitamin D, and vitamin E; nevertheless, males in the high PF group presented higher inadequate intake compared to low PF group of calcium. Furthermore, higher macronutrient intakes were observed in the high PF group compared to the other PF groups in both sexes, but participants in the high PF group had also higher EE. Although the relationship between dietary patterns and PA has been studied [2, 35], there is a paucity of data analysing the association of diet and PF in older adults, concretely in Spain.
In our study, males in the high PF group and in the low PF group showed similar EI (2111 vs. 2135 kcal/day, respectively). In females, the median EI was 1576 kcal/day in the low PF group and 1625 kcal/day in the high PF group. EE was higher in the high PF group compared to low PF group in males (4901 METs min/week vs. 3485 METs min/week, p < 0.05); however, females in the low PF group presented higher EE than females in the high PF group (4142 METs min/week vs. 3966 METs min/week, respectively). Camoes et al. found that active males had significantly higher mean intake of energy than sedentary males (2570.7 vs. 2336.9 kcal/day), respectively [2]. Cao et al. observed in a cross-sectional study that compliance with dietary recommendations for macronutrients is significantly associated with higher cardiovascular fitness levels in Japanese male adults [36]. Our findings showed that males in the high PF group presented higher relative intake (% of energy) of proteins, fat, mono-unsaturated fatty acids and polyunsaturated fatty acids than in the low PF group, while females in the high PF group did not displayed higher relative intake of all macronutrients than in the low PF group. On the other hand, Brodney et al. observed that subjects in the high fit category consumed a lower percentage of energy from total fat, saturated fat, mono-unsaturated fat, and cholesterol and a higher percentage of energy from carbohydrate, and fiber in both sexes [35]. Lee et al. observed that no significant differences between groups were obtained for energy and fat intake between PA groups split by METs hours/week [37]. Camoes et al. observed a significant lower level of protein consumption (16.9 vs. 17.6% of energy) when comparing active and sedentary males [2]. In an earlier study, Rolland et al. observed that active healthy older adult women did not have a better nutritional profile than their inactive peers [9].
The EAR cut-off point method has been accepted as the best method to estimate nutrient intake inadequacy [34]. For those nutrients for which an EAR could not be stated, AI was used. In our study, more than 85% of males and females showed inadequate intake of potassium, vitamin D, and vitamin E. Román-Viñas et al. observed that vitamin C, folic acid, calcium, selenium, and iodine showed a higher prevalence of inadequate intakes considering EAR cut-off in European older adults [38]. Cao et al. observed 25–35% of inadequate intakes for magnesium, calcium and zinc, and also a relatively high low intake for vitamin A (61.1%) and thiamine (81.0%) in the low PF group [36]. Fisberg et al. observed high prevalence of inadequate intake (> 50%) of vitamins D, E, B6, and A, calcium, and magnesium pyridoxine [39]. It is important to highlight that in our study participants in the low PF group showed inadequate intake for potassium, vitamin D, and vitamin E. Nevertheless, more males in the high PF group presented inadequate intake of calcium compared to the low PF group. In addition, around 8% of the participants presented inadequate intakes for 11 micronutrients in our study. Likewise, participants from the high PF groups showed greater mean intake of potassium, magnesium, phosphorous, selenium and vitamins (B3, B5, B6, B9, C, D, and E) and retinol (only females) than low PF groups. Cao et al. showed that males who had a poor overall micronutrient intake status have a significantly higher risk of being unfit compared to males with a good micronutrient intake status [36]. In this sense, Brodney et al. observed that subjects in the high fit category had higher intakes of calcium, folate, vitamin B6, vitamin B12 (only female), vitamin A, vitamin C, and vitamin E than the low and moderate fit in both sexes [35]. Because micronutrients are essential for humans’ health [40], it is important to take into account the inadequate intake of vitamins and minerals to prevent deficiencies and considering the influence in the different biological pathways of micronutrients over PF. Long periods of inadequate intake of one or some micronutrients may display clinical manifestations of deficiency [39].
The findings of our study also showed that a positive and significant association was observed for vegetable protein intake and the high PF group. There were significant and positive associations between high PF group with phosphorous and selenium. Furthermore, positive and significant associations were found between high PF group and vitamin B6, vitamin C, vitamin D, niacin, and folates. However, high PF group presented negative associations with thiamine and riboflavin. Camoes et al. observed a significant and positive association between PA and intake of vitamin C. In addition, leisure-time activity was positively associated with intakes of vitamin E, folate, calcium, and magnesium in females [2]. Other authors who studied the association between PF and diet quality observed that diet-quality score was positively associated with cardiorespiratory fitness [41] and more physical exercise was associated with improvement in the Mediterranean Diet Adherence [42].
As shown above, data on micro- and macronutrient intake, obtained by different authors, were discrepant. This could be mainly due to different approaches when assessing the quantity of a particular micro and macronutrient in a particular kind of food, or differences in the dietary questionnaires used. A considerable heterogeneity exists in dietary patterns and nutritional status in older adults [19]. In addition, dietary intake tends to decline over time [43]. It should be highlighted that there are some nutrients with an adequate intake (iron, copper). Overall, diet and PF modifications seem to be strong promoters of healthy aging [19].
Limitations and strength
This study has several limitations. First, one limitation of the study was the use of 24 h dietary recalls. The 24 h dietary recall method is one of the most widely used tools and validated methods [44]; however, the 24 h dietary recall method does not allow quantifying proportions of non-consumers for particular items, especially for infrequently consumed foods. Second, this study has a cross-sectional design, thus preventing the determination of cause–effect relationships. Moreover, only intake but no status data were measured. Nevertheless, according to this study, it seems reasonable to think that macro- and micronutrient status can be associated to PF, whereas the mechanism by which PF could determine higher or lower intake status is not so clear.
Despite the aforementioned, this study also has several strengths which included the use of objective, precise and validated methods for assessing PF. Furthermore, the use of clustering of activities produces an alternative approach to summarizing PF participation. Another strength is the sampling procedure and the strict standardization of the field work among the cities involved in the study, thus avoiding to a great extent the kind of confounding bias.
Conclusions
Participants in the high PF group show a better micronutrient intake profile than the other PF groups; nonetheless, there are a considerable number of participants who present inadequate intake of micronutrients below the EAR. The relationship between PF and nutrient present different patterns depending on the PF level showing higher relative protein and fat intake males in the high PF group than males in the low PF group. Nonetheless, females in the high PF group display lower relative macronutrient intake than the other PF groups. There is a requirement to develop combined nutritional and fitness programs among older adults and to consider supplementation for micronutrients at risk.
References
Gil A, Martinez de Victoria E, Olza J (2015) Indicators for the evaluation of diet quality. Nutr Hosp 31(Suppl 3):128–144
Camoes M, Lopes C (2008) Dietary intake and different types of physical activity: full-day energy expenditure, occupational and leisure-time. Public Health Nutr 11(8):841–848
Strandberg TE, Levälahti E, Ngandu T, Solomon A, Kivipelto M, FINGER Study Group (2017) Health-related quality of life in a multidomain intervention trial to prevent cognitive decline. Eur Geriatr Med 8:164–167
Robinson F (2003) Nutrition for healthy ageing. British Nutrition Foundation, London
Montgomery SC, Streit SM, Beebe ML, Maxwell PJ (2014) Micronutrient needs of the elderly. Nutr Clin Pract 29(4):435–444
Gonzalez-Gross M, Sola R, Albers U et al (2007) B-vitamins and homocysteine in Spanish institutionalized elderly. Int J Vitam Nutr Res 77(1):22–33
Manini TM (2010) Energy expenditure and aging. Ageing Res Rev 9(1):1–11
Westerterp KR (2015) Daily physical activity as determined by age, body mass and energy balance. Eur J Appl Physiol 115(6):1177–1184
Rolland Y, Pillard F, Garrigue E, Amouyal K, Riviere D, Vellas B (2005) Nutritional intake and recreational physical activity in healthy elderly women living in the community. J Nutr Health Aging 9(6):397–402
Schroder H, Marrugat J, Covas M et al (2004) Population dietary habits and physical activity modification with age. Eur J Clin Nutr 58(2):302–311
Ahmed T, Haboubi N (2010) Assessment and management of nutrition in older people and its importance to health. Clin Interv Aging 5:207–216
Favaro-Moreira NC, Krausch-Hofmann S, Matthys C et al (2016) Risk factors for malnutrition in older adults: a systematic review of the literature based on longitudinal data. Adv Nutr 7(3):507–522
Deer RR, Volpi E (2015) Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care 18(3):248–253
Rohrig G, Rucker Y, Becker I et al (2016) Association of anemia with functional and nutritional status in the German multicenter study “GeriAnaemie2013”. Z Gerontol Geriatr 50(6):532–537
França TGD, Ishikawa LLW, Zorzella-Pezavento SFG, Chiuso-Minicucci F, da Cunha MLRS., Sartori A (2009) Impact of malnutrition on immunity and infection. J Venom Anim Toxins Incl Trop Dis 15(3):374–390
Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW (1989) Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262(17):2395–2401
Pedrero-Chamizo R, Gomez-Cabello A, Delgado S et al (2012) Physical fitness levels among independent non-institutionalized Spanish elderly: the elderly EXERNET multi-center study. Arch Gerontol Geriatr 55(2):406–416
Mertens E, Clarys P, Mullie P et al. (2016) Stability of physical activity, fitness components and diet quality indices. Eur J Clin Nutr 71(4):519–524
Hammar M, Ostgren CJ (2013) Healthy aging and age-adjusted nutrition and physical fitness. Best Pract Res Clin Obstet Gynaecol 27(5):741–752
Martinez Roldan C, Veiga Herreros P, Cobo Sanz JM, Carbajal Azcona A (2011) Assessment of the nutritional status of a group of people older than 50 years by means of dietary and body composition parameters. Nutr Hosp 26(5):1081–1090
Serra-Majem L, Ribas-Barba L, Salvador G et al (2007) Trends in energy and nutrient intake and risk of inadequate intakes in Catalonia, Spain (1992–2003). Public Health Nutr 10(11A):1354–1367
Black AE (2000) Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord 24(9):1119–1130
Elosua R, Marrugat J, Molina L, Pons S, Pujol E (1994) Validation of the minnesota leisure time physical activity questionnaire in Spanish men. The MARATHOM investigators. Am J Epidemiol 15(12):1197–1209 139(
Elosua R, Garcia M, Aguilar A, Molina L, Covas MI, Marrugat J (2000) Validation of the minnesota leisure time physical activity questionnaire in Spanish women. Investigators of the MARATDON Group. Med Sci Sports Exerc 32(8):1431–1437
Ainsworth BE, Haskell WL, Whitt MC et al (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32(9 Suppl):S498–S504
Rikli R, Jones CJ (2001) Senior fitness test manual. Human Kinetics, Illinois
VV.AA (1998) Eurofit para adultos: evaluación de la aptitud física en relación con la salud. Primera ed. CSD, Madrid
Marfell-Jones M, Olds T, Stewart A et al (2006) International standards for anthropometric assessment. ISAK. South Africa
Gómez-Candela C, Loria-Kohen V, Lourenço-nogueira T (2007) Guía visual de alimentos y raciones. Editores Médicos, Madrid
Mataix J, Mañas M, Llopis J, Martínez de Victoria E, Juan J, Borregón A (2004) Tablas de composición de alimentos españoles, 4th edn. INTA-Universidad de Granada: Granada, ed. Granada
Ortega RM, López AM, Requejo AM, Carvajales PA (2004) La composición de los alimentos. Herramienta básica para lavaloración nutricional. Editorial Complutense, Madrid
Feinberg M, Favier JC, Ireland-Ripert J (1995) Répertoire general des aliments. Tec&Doc Lavoisier, Paris
Ripoll L (1992) La cocina de las Islas Baleares, 5th edn. Ripoll Pub, Palma de Mallorca
Institute of Medicine. Dietary Reference intakes. Washington, National Academies Press, update November 2010. https://www.nal.usda.gov/fnic/dri-tables-and-application-reports. Accessed 10 Oct 2016
Brodney S, Mcpherson RS, Carpenter RS, Welten D, Blair SN (2001) Nutrient intake of physically fit and unfit men and women. Med Sci Sports Exerc 33(3):459–467
Cao ZB, Sasaki A, Oh T et al (2012) Association between dietary intake of micronutrients and cardiorespiratory fitness in Japanese men. J Nutr Sci 1:e12
Lee IM, Djousse L, Sesso HD, Wang L, Buring JE (2010) Physical activity and weight gain prevention. JAMA 303(12):1173–1179
Roman Vinas B, Ribas Barba L, Ngo J et al (2011) Projected prevalence of inadequate nutrient intakes in Europe. Ann Nutr Metab 59(2–4):84–95
Fisberg RM, Marchioni DM, Castro MA et al (2013) Inadequate nutrient intake among the Brazilian elderly: National Dietary Survey 2008–2009. Rev Saude Publica 47(Suppl 1):222S–230S
Lal A, Ames BN (2011) Association of chromosome damage detected as micronuclei with hematological diseases and micronutrient status. Mutagenesis 26(1):57–62
Shikany JM, Jacobs DR, Lewis CE et al (2013) Associations between food groups, dietary patterns, and cardiorespiratory fitness in the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr 98(6):1402–1409
Patino-Alonso MC, Recio-Rodriguez JI, Belio JF et al (2014) Factors associated with adherence to the Mediterranean diet in the adult population. J Acad Nutr Diet 114(4):583–589
Ruiz E, Avila JM, Valero T et al (2016) Macronutrient distribution and dietary sources in the Spanish population: findings from the ANIBES study. Nutrients 8(3):177
Salvador Castell G, Serra-Majem L, Ribas-Barba L (2015) What and how much do we eat? 24-hour dietary recall method. Nutr Hosp 26(31 Suppl 3):46–48
Acknowledgements
The authors are grateful to all of the participants who took part in the PHYSMED study and to all the researchers who set up the data for further analysis. Special thanks to Ms. Raquel Seco for technical assistance and Laura Barrios for her statistical assistance. ImFINE and NUCOX are members of the EXERNET research network (http://www.spanishexernet.com/). Raquel Aparicio-Ugarriza was supported with a predoctoral grant from the Technical University of Madrid.
Funding
The PHYSMED study took place with the financial support of the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III (Projects 11/01791 and 14/00636, Red Predimed-RETIC RD06/0045/1004, and CIBEROBN CB12/03/30038). Grant of support to research groups no. 35/2011 (Balearic Islands Gov.), and European Union FEDER funds. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
RAU, GP, and MGG analyzed the data, and drafted and wrote the manuscript. RLS, MB, EA, and JAT contributed to the analysis. MGG and JAT designed the study and wrote the protocol. All read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not declare any conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aparicio-Ugarriza, R., Luzardo-Socorro, R., Palacios, G. et al. What is the relationship between physical fitness level and macro- and micronutrient intake in Spanish older adults?. Eur J Nutr 58, 1579–1590 (2019). https://doi.org/10.1007/s00394-018-1696-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1696-z