Abstract
Purpose
Maternal diet with a high glycemic index (GI) is associated with fetal overgrowth and higher infant body adiposity. Effects of low-GI diet on maternal and newborn outcomes have been assessed in both healthy pregnancy and gestational diabetes mellitus, but the results remain inconclusive. This meta-analysis aimed to examine the effects of low-GI diets on maternal and newborn outcomes.
Methods
PubMed, Clinical Trials, and Cochrane Library databases were searched for relevant randomized trials up to January 2016. Random- or fixed-effects models were used to calculate combined treatment effects.
Results
A total of 11 trials involving 1985 women were eligible for analysis. This meta-analysis assessed 7 maternal and 11 newborn outcomes. Of these, gestational weight gain (GWG), fasting blood glucose (FBG), newborn birth weight, ponderal index (PI), proportion of macrosomia, and large for gestational age (LGA) were investigated in more than 8 trials. Compared with control diets, low-GI diets significantly reduced FBG (weight mean differences (WMD) = −0.18 mmol/L, 95 % CI: −0.33, −0.02), 2-h postprandial glucose level (WMD = −0.33 mmol/L, 95 % CI: −0.54, −0.12), and the proportion of LGA (RR = 0.52, 95 % CI: 0.31, 0.89). A lower GWG (WMD = −0.69 kg, 95 % CI: −1.74, 0.36) and birth weight (WMD = −0.10 kg, 95 % CI: −0.23, 0.03) were also observed without significant differences. Heterogeneity was observed in the GWG, FBG, and birth weight analyses. Low-GI diets did not affect other maternal and newborn outcomes. In subgroup and sensitivity analyses, the intervention effects of low GI on GWG and FBG varied.
Conclusions
Low-GI diets may have beneficial effects on maternal outcomes for those at risk of developing high glucose levels, without causing adverse effects on newborn outcomes. However, results should be interpreted with caution because of the evidence of heterogeneity and limited number of studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nutritional status of the mother during pregnancy plays a vital role in fetal growth and development, with glucose as the main energy substrate [1]. However, different carbohydrate foods produce varied glycemic responses, which influence maternal blood glucose concentrations [2]. In 1981, Jenkins [3] proposed the use of glycemic index (GI) to rank postprandial glycemic responses to the equivalent portions of carbohydrates in different foods. Carbohydrates are then classified according to their induced glycemic responses as either high or low GI. Among these two types of carbohydrates, those with low GI produce low glycemic response, e.g., whole grain breads, cereals, and nuts, whereas high-GI foods produce a high glycemic response, e.g., refined grains, desserts, and soft drinks. Alterations in maternal metabolism provide nutrients in excess of those required for normal fetal growth and for maternal and fetal energy requirements. In this context, the presence of any degree of abnormal glucose tolerance represents an altered environment for the growth of the fetus [4]. A dietary intake of carbohydrates with low GI induces individuals to obtain normal gestational weight gain (GWG) and normal infant birth weight, whereas carbohydrates with high GI results in feto-placental overgrowth and predisposition to fetal macrosomia [5].
Previous systematic reviews and meta-analyses demonstrated that low-GI diets may reduce insulin requirements and birth weight without adverse effects on pregnant women with gestational diabetes mellitus (GDM), suggesting that low-GI diets are an appropriate dietary intervention for GDM when glucose load is controlled [6, 7]. As the research in this field is active and fast-moving, a number of recent randomized controlled trials (RCTs) have been published to assess the effects of dietary GI on maternal and newborn outcomes in pregnant women with or without GDM. However, these trials yielded varied results because of the differences in the study design and participant characteristics [8–18]. Thus, the feasibility of using low-GI diets to replace current recommended pregnant diets remains inconclusive. The present study aims to analyze the overall effects of low-GI diets on maternal and newborn outcomes in pregnant women regardless of their health status by conducting a meta-analysis of RCTs.
Materials and methods
Literature search
This study was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [19]. A systematic literature search for English publications was conducted in the databases of PubMed, Clinical Trials, and the Cochrane Central Register of controlled Trials up to January 2016. Search terms included glycemic index, glycemic load, carbohydrates combining with pregnancy, and gravidas. The search was limited to human clinical trials. A manual search was also performed using the reference lists of original articles and recent reviews. Authors of the original studies were not contacted for additional information.
Study selection
Studies were selected based on the following criteria: (1) The study design was a RCT; (2) the study was conducted in pregnant women (≥18 years old, with a singleton pregnancy); (3) the study comprised a control or a comparison group, and the intervention was low-GI diet with dietary GI level; (4) the dietary intervention was more than 4 weeks; and (5) pregnancy outcomes included maternal or newborn outcomes providing data for statistical analysis. Maternal outcomes included the following: GWG, fasting blood glucose (FBG), 2-h postprandial glucose (2-h PG), glycated Hb A1c (HbA1c), gestational age at delivery, proportion of pregnant women who use insulin, and proportion of cesarean delivery. Newborn outcomes included the following: birth weight, ponderal index (PI), head circumference, body length, abdominal circumference, proportion of large for gestational age (LGA; birth weight >90th centile), small for gestational age (SGA; birth weight <10th centile), macrosomia (birth weight >4 kg), prematurity, birth centile, and birthweight centile.
Data extraction and quality assessment
The following characteristics of each study were recorded: the first author’s name, publication year, country of origin, sample size, study design details, participant characteristics (mean age, body weight, body mass index (BMI), health status, and gestation age at recruitment), dietary GI level, and maternal or newborn outcomes mentioned above. If more than one time point for the follow-up was reported, data from the longest period were used. The Jadad scale was used to assess the methodological quality of each included trial by assigning scores ranging from 0 to 5 for reported randomization, blinding, and withdrawal [20]. Two authors (R-Z and LQ-Q) independently conducted the literature search, study selection, and data extraction. Any divergence was resolved by discussion.
Statistical analysis
For binary data, combined relative risk (RR) with 95 % confidence interval (CI) was evaluated. For continuous data, weighted mean difference (WMD) with 95 % CIs was calculated. Standard deviations (SDs) for net changes were obtained from the baseline in each group. If not reported, they were derived from standard errors, median and interquartile ranges by using a standard formula [21]. If SDs for the baseline and final values were only provided, SDs were imputed according to the method of Follmann et al. [22] with an assumed correlation coefficient of 0.5.
The heterogeneity of the effect size among studies was tested using the Cochran’s Q test at the P < 0.10 level of significance. We calculated I 2 values, a quantitative measure of inconsistency across studies [23]. A random-effects model was used when P < 0.10 at the Q test; otherwise, a fixed-effects model was applied [24]. To explore the possible influences of study designs and participant characteristics on the combined effect sizes, we further conducted pre-specified subgroup analyses using stratified outcomes from ≥8 trials. In addition, we investigated the influence of a single study on the overall risk estimate by omitting one study in each turn. Potential publication bias was assessed using Begg’s funnel plots and the Egger’s regression test [25]. All analyses were performed using STATA version 12.0 (StataCorp, College Station, TX, USA). P < 0.05 was considered statistically significant, except otherwise specified.
Results
Literature search
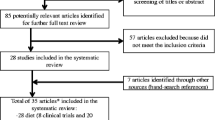
We initially identified 339 potentially eligible publications, most of which were excluded after browsing titles and abstracts because they were animal studies and reviews, and did not follow a randomized design. After reviewing the full text of the remaining 27 articles, 16 were excluded. The main reasons for which were as follows: Diet was not the primary intervention, the dietary GI levels were not reported, and pregnancy outcomes were not of our interest. Walsh et al. [12] and Macgowan et al. [26] reported the results from the same trial (the ROLO study), and the study of Walsh et al. was included because of the larger population size. Clapp et al. [5, 27] provided additional information for the previous article [18]. To obtain sufficient data, we used supplementary data in one systematic review [7], which were not reported in the original study [17], instead of contacting authors for additional information. Eleven RCTs were selected for the final analysis [8–18]. The flowchart of literature search is presented in Fig. 1.
Study characteristics
The characteristics of the selected trials are presented in Table 1. Of the 11 trials published from 1997 to 2016, 5 were conducted in Australia, 2 in the USA, and 1 each in Ireland, Mexico, Canada, and China. Only 1 trial followed a double-blind design and 2 applied a single-blind design. Sample sizes varied from 20 to 576, with a total of 1985 pregnant women. The mean age of pregnant women ranged from 29.9 to 34.7 years, and mean BMI from 21.5 to 32.4 kg/m2. Five trials were conducted in pregnant women with GDM, pregestational type 2 diabetes mellitus (T2DM), or impaired glucose tolerance during pregnancy. The other trials studied healthy pregnant women (n = 3), overweight/obese pregnant women (n = 1), women who previously delivered a macrosomic infant (n = 1), and women who were at high risk of GDM (n = 1). Dietary intervention was initially conducted from 12.9 to 30.1 weeks of gestation, and five trials continued the intervention until delivery. The dietary GI ranged from 47 to 71 (median 50.4) in the intervention groups and from 48.6 to 84 (median 57.7) in the control groups. Nine trials measured the dietary intake by using 3-day diet records, and other two used 24-h dietary recalls. Most participants in the control group received healthy eating diet advice. The Jadad scale of these trials ranged from 1 to 4. The characteristics of maternal and newborn outcomes in the included trials are given in Table 2.
Effects of low-GI diets on maternal outcomes
Low-GI diet significantly reduced FBG (WMD = −0.18 mmol/L, 95 % CI: −0.33, −0.02, n = 8) (Fig. 2) and 2-h PG (n = −0.33 mmol/L, 95 % CI: −0.54, −0.12, n = 4), but did not affect HbA1c levels (WMD = 0.02 %, 95 % CI: −0.03, 0.08, n = 3). Low-GI diets produced somewhat lower GWG (WMD = −0.69 kg, 95 % CI: −1.74, 0.36, n = 9) than control diets without significant difference (Fig. 3). In contrast, no significant change was observed in gestational age at delivery (MWD = 0.03, 95 % CI: −0.14, 0.20, n = 5), proportion of cesarean delivery (RR = 1.07, 95 % CI: 0.75, 1.53, n = 5), and insulin use (RR = 1.01, 95 % CI: 0.77, 1.33, n = 3) (Supplementary Fig. 1). GWG (P = 0.027, I 2 = 53.8 %) and FBG analyses (P < 0.001, I 2 = 73.7 %) showed heterogeneity across studies.
Effects of low-GI diets on newborn outcomes
The meta-analysis results showed a borderline significant reduction in birth weight (WMD = −0.10 kg, 95 % CI: −0.23, 0.03, n = 11) (Fig. 4) and a significant reduction in the proportion of LGA (RR = 0.52, 95 % CI: 0.31, 0.89, n = 8) (Fig. 5). No differences were observed in other newborn outcomes, including PI (MWD = −0.07 kg/m3, 95 % CI: −0.71, 0.57, n = 8), body length (MWD = −0.05 cm, 95 % CI: −0.66, 0.55, n = 6), head circumference (MWD = −0.13 cm, 95 % CI: −0.68, 0.41, n = 5), abdominal circumference (MWD = −0.65 cm, 95 % CI: −2.23, 0.92, n = 3), SGA(RR = 1.33, 95 % CI: 0.71, 2.50, n = 6), macrosomia (RR = 0.95, 95 % CI: 0.83, 1.09, n = 8), prematurity (RR = 0.70, 95 % CI: 0.39, 1.28, n = 5), birth centile (MWD = −7.87, 95 % CI: −21.92, 6.19, n = 3), and birthweight centile (MWD = −1.22, 95 % CI: −4.46, 2.02, n = 3) (Supplementary Fig. 2). Significant heterogeneity was observed for the analysis of birth weight (P < 0.001, I 2 = 80.7 %), PI (P = 0.009, I 2 = 62.6 %), body length (P = 0.002, I 2 = 73.5 %), head circumference (P = 0.005, I 2 = 73.3 %), abdominal circumference (P < 0.001, I 2 = 91.9 %), and birth centile (P = 0.005, I 2 = 81.1 %).
Subgroup and sensitivity analyses
GWG, FBG, birth weight, LGA, macrosomia, and PI were investigated in 9, 8, 11, 8, 8, and 8 trials, respectively. Subgroup analysis was performed on these six outcomes. The significant decrease in maternal FBG was diminished when trials were limited to blind design, BMI ≥25 kg/m2, and GI difference <7. Low-GI diets did not affect FBG when trials were stratified by GDM condition. The significant decrease in GWG by low-GI diets was observed when the analysis was limited to trials with a GI difference ≥7. On the other hand, the results of birth weight, LGA, macrosomia, and PI by any stratification were consistent with the overall estimates (Table 2).
Sensitivity analyses were performed to examine the effect of a single trial on the overall results by omitting one trial in each turn. When the study of Walsh et al. [12] was excluded, maternal FBG reduction became more pronounced (WMD = −0.28 mmol/L, 95 % CI: −0.36, −0.20) without heterogeneity across the studies (P = 0.190, I 2 = 31.2 %). In addition, omitting the trial by Ma et al. [9] resulted in less reduction in FBG by 0.13 mmol/L (95 % CI: −0.29, 0.02) and in 2-h PG by 0.21 mmol/L (95 % CI: −0.49, 0.07). When the study of Moses et al. [17] was excluded, reduced GWG became significant (WMD = −0.99 kg, 95 % CI: −1.95, −0.03) without heterogeneity across the studies (P = 0.122, I 2 = 38.6 %). When the trial by Perichart-Perera et al. [11] was excluded, low-GI diet significantly decreased prematurity (RR = 0.45, 95 % CI: 0.20, 0.99).
Publication bias
No publication bias was found as assessed by Begg’s funnel plot and Egger’s test, except a possible publication bias for analyses 2-h PG (Egger’s test, P = 0.004), LGA (Egger’s test, P = 0.014), SGA (Egger’s test, P = 0.044), and macrosomia (Egger’s test, P = 0.033).
Discussion
Maternal dietary macronutrient intake throughout pregnancy affects maternal metabolism and in utero environment. As glucose is a major energy substrate for fetal growth, varying degrees of glucose intolerance, even if less than that conventionally required for the diagnosis of gestational diabetes, will result in alterations in utero environment and thus will influence fetal growth and adiposity [28]. Oostdam et al. [29] evaluated six types of intervention during pregnancy in the prevention of GDM using meta-analysis; the results showed that dietary intervention, such as low-GI diet advice, may reduce GDM incidence compared with the standard care. A previous systemic review also reported that low-GI diets may reduce the need for insulin in pregnant women with GDM [6]; this finding was confirmed by a recent meta-analysis [7].
So far, both reviews excluded trials conducted among healthy women and the number of selected trials was less than five. In fact, women with even a mildly elevated blood glucose level have a higher risk of giving birth to a LGA infant [29]. Beneficial effects on birth weight were already observed in pregnant women without GDM after low-GI diets [18]. Thus, in the present updated meta-analysis, we have included participants of healthy pregnant women, women with gestational hyperglycemia, and women with pregestational T2DM. Moreover, the pregnancy outcomes in the present meta-analysis included 7 maternal and 11 newborn outcomes, which were more comprehensive than the previous relevant studies.
Maternal hyperglycemia and hyperglycemic excursions (during fasting, after a glucose load, and postprandial) could lead to adverse pregnancy and offspring outcomes [30–32]. By definition, a low-GI diet is expected to lower postprandial glucose when glucose load is controlled. A recent trial found that glucose levels after breakfast, lunch, and dinner in pregnant women with GDM were significantly lower after administering low-GI staple diets than those after providing normal diabetic control diet. This trial was not included in the present study because it is a short-term intervention with no other outcomes evaluated [33]. The present meta-analysis showed a significant reduction in FBG by 0.18 mmol/L and 2 h PG by 0.33 mmol/L, which suggested the beneficial effects of low-GI diets beyond postprandial glucose. The current results updated the previous meta-analysis including only 3 trials, in which the lowering effect of low-GI diet on maternal FBG was not observed [29]. However, the subgroup analysis revealed that the effects of low-GI diets on FBG diminished when trials were stratified by GDM condition and gestation week at recruitment. This observation may be explained by a low statistical power resulting from the limited number of trials in each subgroup (n = 4). It is important to note that the intervention in GDM group usually started after GDM diagnosis around 24–28 weeks, which leaves a very short time window for any intervention. Nevertheless, the minor improvement in circulating blood glucose is effective to reduce excessive newborn weight.
Excessive GWG is considered a risk factor not only for GDM but also for excessive fetal growth [34, 35]. High GWG was related to long-term adverse health outcomes for the mother–newborn pair [36]. Mourtakos et al. [37] found that excessive GWG was associated with a higher risk of greater infant size at birth and a higher BMI status at the ages of 2 and 8 years. Sridhar et al. [38] also demonstrated that GWG outside the recommendations increased the odds of childhood overweight and obesity, independent of several potential confounders, such as birth weight. Thus, gestational weight must be controlled in a rational range. A recent meta-analysis has shown that a low-GI diet promoted weight loss in overweight or obese people [39]. Therefore, a low-GI diet may also provide benefits to weight management during pregnancy. Dietary interventions have shown the greatest reduction in gestational weight gain compared to other methods. Although reduced GWG did not reach significant in the present meta-analysis, the low-GI diets significantly decreased GWG by 0.99 kg compared with the control group when the study of Moses et al. [17] was excluded. Moses study was the source of heterogeneity in GWG analysis, and low-GI diet caused higher GWG compared with the control group. However, that result may be wrested by the significant lower BMI in the low-GI diet group than in the control group (24.4 vs. 26.6 kg/m2, P = 0.04). A recent 6-month randomized trial conducted on women with previous GDM found that subjects in the low-GI group lost an average of 1.3 kg compared with the 0.1 kg in the conventional healthy dietary recommendation [40]. The present analysis revealed inconsistent results on GWG and FBG, suggesting that the effects of low-GI diets on GWG and FBG appeared to be related to study design and characteristics of participants. This result was consistent with previous meta-analysis on patients with GDM [7].
In theory, reduced FBG by low-GI diet in the present meta-analysis could decrease newborn birth weight. Horan et al. [28] found that low-GI dietary intervention in pregnancy had a beneficial effect on neonatal central adiposity as determined using the ratio of waist to length. The maternal dietary GI even affects childhood health. Okubo et al. [41] found that maternal dietary GI in early pregnancy was positively associated with fat mass at 4 and 6 years of age. In the present meta-analysis, a borderline significant reduction in birth weight of 0.12 kg was observed. In a previous meta-analysis on 4 trials, low-GI diets reduced the newborn birth weight (WMD −0.16 kg, 95 % CI: −0.25, −0.08) compared with control diets in pregnant women with GDM [7]. However, in the present subgroup for women with GDM with recent trials added, the reduction in newborn weight was still retained in borderline significance. In fact, the disadvantage of birth weight reduction should be considered, particularly in pregnant women who are underweight, at nutritional risk, or from a low-income country. Participants in the included trials did not suffer from apparent malnutrition. Thus, the issue of low birth weight did not fall within the scope of this study. Besides, women exhibited reduced risk of having an LGA infant after administering with low-GI diets in the present meta-analysis, which is consistent with Oostdam et al. [29]. In addition to the borderline significant reduction in birth weight and LGA, low-GI diets minimally affected the other 9 newborn outcomes. The current results were generally consistent with these of previous studies, in which no effects were observed in the majority of outcomes. However, Viana et al. [7] found that pregnant women with GDM used insulin less frequently. The partial discrepancy among these meta-analysis results could be related to a relative small number of trials and low quality of evidence.
Some observational epidemiological studies also supported the present findings. A prospective cohort study on 13,110 eligible women in the Nurses’ Health Study II found that women whose dietary GI was higher than 57 units had a 30 % increased risk of developing GDM compared with participants whose dietary GI was lower than 51 units [42]. A cohort including 1082 gravidas in the Camden Study found that GI was positively and significantly related to maternal plasma glucose, HbA1c, and infant birth weight [43]. A recent prospective cohort study during the 10 years of follow-up has shown that higher intake of nuts, which are typical low-GI food sources [44], was associated with a significantly lower risk of GDM by 27 % as a result of improved insulin sensitivity [45]. These results highlighted the clinical importance of dietary sources in assessing the health effects of low-GI diets.
The current meta-analysis was primarily limited by considerable heterogeneity across studies, such as the main outcomes of GWG and FBG and birth weight, which complicated the interpretation of the findings. The heterogeneity can be attributed to study design and characteristics of participants. Most included RCTs used an open-label design. As such, blinding of the treatment to the participants or investigator is difficult, perhaps impossible, because of the nature of dietary intervention/advice design. More importantly, the methods of intervention and control may be the source of heterogeneity. Regarding intervention, these RCTs differed in values of dietary GI and sometimes in co-interventions. For example, all participants in the Clapp study participated in an exercise program before and after pregnancy, which may reinforce the effect of the dietary intervention [18]. On the other hand, three studies declared that low-GI diets for intervention were supported or supplied by relevant companies [13, 15, 16]. Regarding control, high-GI dietary advice [14, 16–18] or low-fat dietary advice [15] was used in some trials. In general, high-GI diets, rather than normal diets, are the dominant diet in individuals living in the Western industrialized societies [27]. Other limitations, which also resulted in heterogeneity, included different criteria for screening and diagnosis of GDM, start of counseling in first or second trimester, and frequency of counseling that varied from twice only to weekly during pregnancy. Finally, the SDs of the net changes were not available in some trials. SDs were derived from the standard errors, median and interquartile ranges, or standard deviations for the initial and final values. These methods employed may not be ideal and result in some inaccuracies.
Based on the current available evidence, we concluded that low-GI diets may have beneficial effects on maternal outcomes without causing adverse effects on newborn outcomes in general pregnant women. The risk of adverse pregnancy outcomes can be reduced by low-GI dietary intervention because of the controlled maternal blood glucose level. However, the results should be interpreted with caution because of the evidence of heterogeneity across studies, possible publication bias, and limited number of studies. Hence, large, well-designed, intervention RCTs must be conducted on pregnant women to address the effects of low-GI diets on pregnancy outcomes.
References
Butte NF (2000) Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 71:1256s–1261s
Jovanovicpeterson L, Peterson CM, Reed GF, Metzger BE, Mills JL, Knopp RH, Aarons JH (1991) Maternal postprandial glucose-levels and infant birth-weight: the diabetes in early-pregnancy study. Am J Obstet Gynecol 164:103–111
Jenkins DJA, Wolever TMS, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV (1981) Glycemic index of foods: a physiological-basis for carbohydrate exchange. Am J Clin Nutr 34:362–366
Kaufmann RC, Mcbride P, Amankwah KS, Huffman DG (1992) The effect of minor degrees of glucose-intolerance on the incidence of neonatal macrosomia. Obstet Gynecol 80:97–101
Clapp JF (1998) Effect of dietary carbohydrate on the glucose and insulin response to mixed caloric intake and exercise in both nonpregnant and pregnant women. Diabetes Care 21:B107–B112
Louie JC, Brand-Miller JC, Markovic TP, Ross GP, Moses RG (2010) Glycemic index and pregnancy: a systematic literature review. J Nutr Metab 2010:282464
Viana LV, Gross JL, Azevedo MJ (2014) Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care 37:3345–3355
Markovic TP, Muirhead R, Overs S, Ross GP, Louie JCY, Kizirian N, Denyer G, Petocz P, Hyett J, Brand-Miller JC (2016) Randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in women at high risk of gestational diabetes mellitus: the GI Baby 3 study. Diabetes Care 39:31–38
Ma WJ, Huang ZH, Huang BX, Qi BH, Zhang YJ, Xiao BX, Li YH, Chen L, Zhu HL (2015) Intensive low-glycaemic-load dietary intervention for the management of glycaemia and serum lipids among women with gestational diabetes: a randomized control trial. Public Health Nutr 18:1506–1513
Moses RG, Casey SA, Quinn EG, Cleary JM, Tapsell LC, Milosavljevic M, Petocz P, Brand-Miller JC (2014) Pregnancy and glycemic index outcomes study: effects of low glycemic index compared with conventional dietary advice on selected pregnancy outcomes(1–3). Am J Clin Nutr 99:517–523
Perichart-Perera O, Balas-Nakash M, Rodriguez-Cano A, Legorreta-Legorreta J, Parra-Covarrubias A, Vadillo-Ortega F (2012) Low glycemic index carbohydrates versus all types of carbohydrates for treating diabetes in pregnancy: a randomized clinical trial to evaluate the effect of glycemic control. Int J Endocrinol 2012:296017
Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM (2012) Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ 345:e5605–e5613
Grant SM, Wolever TMS, O’Connor DL, Nisenbaum R, Josse RG (2011) Effect of a low glycaemic index diet on blood glucose in women with gestational hyperglycaemia. Diabetes Res Clin Pract 91:15–22
Louie JCY, Markovic TP, Perera N, Foote D, Petocz P, Ross GP, Brand-Miller JC (2011) A randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in gestational diabetes mellitus. Diabetes Care 34:2341–2346
Rhodes ET, Pawlak DB, Takoudes TC, Ebbeling CB, Feldman HA, Lovesky MM, Cooke EA, Leidig MM, Ludwig DS (2010) Effects of a low-glycemic load diet in overweight and obese pregnant women a pilot randomized controlled trial. Am J Clin Nutr 92:1306–1315
Moses RG, Barker M, Winter M, Petocz P, Brand-Miller JC (2009) Can a low-glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care 32:996–1000
Moses RG, Luebcke M, Davis WS, Coleman KJ, Tapsell LC, Petocz P, Brand-Miller JC (2006) Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. Am J Clin Nutr 84:807–812
Clapp JF (1997) Diet, exercise, and feto-placental growth. Arch Gynecol Obstet 260:101–108
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. [Updated March 2011]. The Cochrane Collaboration
Follmann D, Elliott P, Suh I, Cutler J (1992) Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 45:769–773
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
McGowan CA, Walsh JM, Byrne J, Curran S, McAuliffe FM (2013) The influence of a low glycemic index dietary intervention on maternal dietary intake, glycemic index and gestational weight gain during pregnancy: a randomized controlled trial. Nutr J 12:140–148
Clapp JF (2002) Maternal carbohydrate intake and pregnancy outcome. Proc Nutr Soc 61:45–50
Horan MK, McGowan CA, Gibney ER, Donnelly JM, McAuliffe FM (2014) Maternal low glycaemic index diet, fat intake and postprandial glucose influences neonatal adiposity: secondary analysis from the ROLO study. Nutr J 13:78
Oostdam N, van Poppel MNM, Wouters MGAJ, van Mechelen W (2011) Interventions for preventing gestational diabetes mellitus: a systematic review and meta-analysis. J Womens Health 20:1551–1563
Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PM, Damm P, Dyer AR, de Leiva A, Hod M, Kitzmiller JL, Lowe LP, McIntyre HD, Oats JJN, Omori Y, Schmidt MI, Balaji V, Callaghan WM, Chen R, Conway D, Corcoy R, Coustan DR, Dabelea D, Fagen C, Feig DS, Ferrara A, Geil P, Hadden DR, Hillier TA, Hiramatsu Y, Houde G, Inturissi M, Jang HC, Jovanovic L, Kautsky-Willer A, Kirkman MS, Kjos SL, Landon MB, Lapolla A, Lowe J, Mathiesen HER, Mello G, Meltzer SJ, Moore TR, Nolan CJ, Ovesen P, Pettitt P, Reader DM, Rowan JA, Sacks DA, Schaefer-Graf U, Seshiah V, Simmons D, Sugiyama T, Trimble ER, Varma S, Yang HX, Yasuhi I, Pregnancy IAD (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33:676–682
Sung JF, Kogut EA, Lee HC, Mannan JL, Navabi K, Taslimi MM, El-Sayed YY (2015) Correlation of continuous glucose monitoring profiles with pregnancy outcomes in nondiabetic women. Am J Perinatol 32:461–467
Yu F, Lv LJ, Liang ZJ, Wang Y, Wen JY, Lin XH, Zhou YH, Mai CY, Niu JM (2014) Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocr Metab 99:4674–4682
Hu ZG, Tan RS, Jin D, Li W, Zhou XY (2014) A low glycemic index staple diet reduces postprandial glucose values in asian women with gestational diabetes mellitus. J Invest Med 62:975–979
Brunner S, Stecher L, Ziebarth S, Nehring I, Rifas-Shiman S, Sommer C, Hauner H, von Kries R (2015) Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: a meta-analysis. Diabetologia 58:2229–2237
Lee JM, Kim MJ, Kim MY, Han JY, Ahn HK, Choi JS, Chung JH, Lee SW, Han YJ, Kwak DW, Ryu HM, Kim MH (2014) Gestational weight gain is an important risk factor for excessive fetal growth. Obstet Gynecol Sci 57:442–447
Zilko CEM, Rehkopf D, Abrams B (2010) Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol 202:574 e1–574 e8
Mourtakos SP, Tambalis KD, Panagiotakos DB, Antonogeorgos G, Alexi CD, Georgoulis M, Saade G, Sidossis LS (2016) Association between gestational weight gain and risk of obesity in preadolescence: a longitudinal study (1997–2007) of 5125 children in Greece. J Hum Nutr Diet. doi:10.1111/jhn.12398
Sridhar SB, Darbinian J, Ehrlich SF, Markman MA, Gunderson EP, Ferrara A, Hedderson MM (2014) Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol 211:259e1–259e8
Thomas DE, Elliott EJ, Baur L (2007) Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev 18:CD005105
Shyam S, Arshad F, Ghani RA, Wahab NA, Safii NS, Nisak MYB, Chinna K, Kamaruddin NA (2013) Low glycaemic index diets improve glucose tolerance and body weight in women with previous history of gestational diabetes: a 6 months randomized trial. Nutr J 12:68–79
Okubo H, Crozier SR, Harvey NC, Godfrey KM, Inskip HM, Cooper C, Robinson SM (2014) Maternal dietary glycemic index and glycemic load in early pregnancy are associated with offspring adiposity in childhood: the Southampton Women’s survey. Am J Clin Nutr 100:676–683
Zhang C, Liu S, Solomon CG, Hu FB (2006) Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 29:2223–2230
Scholl TO, Chen X, Khoo CS, Lenders C (2004) The dietary glycemic index during pregnancy: influence on infant birth weight, fetal growth, and biomarkers of carbohydrate metabolism. Am J Epidemiol 159:467–474
Viguiliouk E, Kendall CW, Blanco Mejia S, Cozma AI, Ha V, Mirrahimi A, Jayalath VH, Augustin LS, Chiavaroli L, Leiter LA, de Souza RJ, Jenkins DJ, Sievenpiper JL (2014) Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS ONE 9:e103376
Bao W, Bowers K, Tobias DK, Hu FB, Zhang C (2013) Prepregnancy dietary protein intake, major dietary protein sources, and the risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care 36:2001–2008
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Irma Silva-Zolezzi is employee of Nestlé Research Center, Lausanne. Gerard Vinyes Parés and Yi Wang are employees of Nestlé Research Center Beijing. The authors state that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, R., Han, S., Chen, GC. et al. Effects of low-glycemic-index diets in pregnancy on maternal and newborn outcomes in pregnant women: a meta-analysis of randomized controlled trials. Eur J Nutr 57, 167–177 (2018). https://doi.org/10.1007/s00394-016-1306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1306-x








