Abstract
Purpose
Beneficial effects of white mulberry against diabetes mellitus have been reported. However, the molecular mechanisms of how white mulberry can attenuate diabetic retinopathy remain poorly understood. Here, the mechanism underlying the protective effect of Morus alba leaves ethanolic extract on oxidative stress, inflammation, apoptosis, and angiogenesis in diabetic retinopathy was investigated.
Methods
Diabetes was induced by injection of streptozotocin. One week after, M. alba (100 mg/kg) was administrated to the rats daily for 16 weeks.
Results
Morus alba extract showed high content of polyphenolics and free radical scavenging activity. Oral M. alba administration significantly attenuated hyperglycemia and weight loss, and decreased sorbitol, fructose, protein kinase C, pro-inflammatory cytokines, and oxidative stress markers in retinas of the diabetic rats. Moreover, M. alba produced marked down-regulation of caspase-3 and Bax, with concomitant up-regulation of Bcl-2 in the diabetic retinas. M. alba also reduced the expression of VEGF in the retina.
Conclusion
These results indicate that M. alba has protective effect on diabetic retinopathy with possible mechanisms of inhibiting hyperglycemia-induced oxidative stress, apoptosis, inflammation, polyol pathway activation, and VEGF expression in the retina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
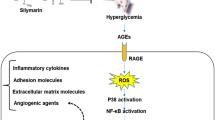
Diabetic retinopathy (DR) is the most common diabetic eye complication and a well-known cause of blindness in working-age population [1]. It has been estimated that roughly 34.6 % of all diabetic patients have some forms of DR [2]. DR represents a spectrum of disease, ranging from patients who have diabetes but no evidence of DR through mild, moderate, and severe stages of nonproliferative DR and progressing to proliferative DR (PDR) [3]. The pathophysiology of DR is a multifactorial process. It involves complex interactions between hyperglycemia and oxidative stress [4]. The retina is vulnerable to reactive oxygen species (ROS) and lipid peroxidation because of its rich content of polyunsaturated lipid membranes. The hyperglycemia-induced oxidative stress induces retinal basement membrane thickening, a hallmark of microangiopathy, and increased retinal vascular permeability, perhaps leading to macular edema which correlates with vision loss in diabetic patients [5, 6].
In addition to triggering oxidative stress, hyperglycemia is involved in the pathogenesis of DR via multiple mechanisms such as increased activation of protein kinase C (PKC) [7], aldose reductase (AR) [8], and elevated nonenzymatic glycoxidation and glycation of proteins [9]. The β-isoform of PKC is considered as a major mediator of vascular endothelial growth factor (VEGF)-induced blood–retinal barrier (BRB) disruption and retinal neovascularization [10]. Intracellular accumulation of sorbitol resulted from increased AR activity might contribute to the breakdown of BRB in DR patients [11]. Moreover, accumulated advanced glycation end products (AGEs) in the vascular wall have been reported to stimulate pro-inflammatory reaction and BRB breakdown in diabetes [12].
Many of the hyperglycemia-induced pathways merge to activate nuclear factor kappa B (NF-κB), with subsequent release of pro-inflammatory cytokines and oxidative stress, and finally lead to apoptosis [13]. Tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) are well-known representative inflammatory cytokines associated with the pathogenesis of DR. Through NF-κB activation, hyperglycemia-induced production of IL-1β and TNF-α results in apoptosis of endothelial cells and loss of retinal microvascular cells [14]. Thus, modulation of hyperglycemia-induced oxidative stress and inflammation might represent an important strategy for the treatment for DR.
Medicinal plants have been reported to be useful source of biologically active substances, including antioxidants and anticarcinogens [15]. White mulberry (Morus alba L., Moraceae) is a deciduous tree widely cultivated in subtropical, tropical, and moderate environments [16]. Mulberry is cultivated for fruit production [17], and its foliage is traditionally used as feed for silk worms [18]. Several recent studies have shown antioxidant, anti-inflammatory, antiviral, hypolipidemic, anti-hyperglycemic, neuroprotective, anti-hypotensive, and cytotoxic activities of different species of Morus [19–21]. Due to the presence of phenols, coumarins, and flavonoids, the leaves of M. alba possess pharmacological importance [22]. Since the leaves of M. alba have been recommended in the literature as a remedy for diabetes treatment, this study was carried out to explore its potential in management of experimentally induced DR in rats. This investigation could promote an understanding of its protective mechanism against diabetes-associated retinopathy, especially to the modulation of oxidative stress, inflammation, and apoptosis.
Materials and methods
Chemicals
Streptozotocin (STZ), reduced glutathione (GSH), pyrogallol, 1,1-diphenyl-2-picrylhydrazyl hydrate (DPPH), Folin–Ciocalteu reagent, gallic acid, rutin, thiobarbituric acid (TBA), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 1,1,3,3 tetramethoxypropane, sodium dodecyl sulfate (SDS), and 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) were purchased from Sigma (USA). All other chemicals were of analytical grade and supplied by standard commercial sources.
Preparation of M. alba leaves extract
M. alba leaves were collected from Beni-Suef governorate (Egypt), during the period from March to June 2013. The leaves were identified and authenticated by experts from Botany Department, Faculty of Science, Beni-Suef University (Egypt), and voucher samples were deposited at the Department of Botany, Faculty of Science, Beni-Suef University. The fourth and fifth leaves were plucked from the apex of healthy plants, washed thoroughly under running tap water, shade-dried for 5 days, and eventually ground to a fine powder in an electric grinder. The powdered plant material was extracted by maceration with 90 % ethanol for 72 h in ambient temperature. The extract was filtered with Whatman filter paper No 1, and filtrates were evaporated to dryness under reduced pressure in a rotary evaporator. The residual extract was used for the study.
Determination of total phenolics and flavonoids contents
Total phenolic content in the leaves extract of M. alba was determined according to the method of Waterman and Mole [23], using Folin–Ciocalteu reagent and gallic acid as a standard phenolic compound. Briefly, 200 µl of the extract solution was mixed with 1 ml of Folin–Ciocalteu reagent. After 5 min, 800 µl of sodium carbonate (75 g/L) was added and then incubated for 2 h at room temperature. The absorbance was measured at 760 nm.
Total flavonoids content was performed according to the method of Jia et al. [24] after slight modifications. The extract solution was mixed with 200 µl of 5 % sodium nitrite and incubated for 5 min at room temperature. 150 µl of 10 % aluminum chloride was added and finally mixed with 1 M sodium hydroxide. The absorbance was measured at 510 nm, and rutin was used as a standard flavonoid.
DPPH radical scavenging activity
A methanolic solution of DPPH was prepared and mixed with the extract solution with the ratio of 8:1. The mixture was shaken and incubated for 30 min at room temperature protected from light. The absorbance was measured at 517 nm [25]. Ascorbic acid was used as a standard antioxidant.
ABTS•+ radical scavenging activity
ABTS•+ radical scavenging capacity was determined according to the method of Re et al. [26]. ABTS•+ was prepared by reacting 2 mM ABTS in water with 2.45 mM potassium persulfate and was stored in the dark for 2 h at room temperature. The ABTS•+ was diluted in 0.1 M sodium phosphate buffer (pH 7.4) and mixed with the extract solution at the ratio of 1:3. The reaction mixture was incubated for 30 min at room temperature, and absorbance was measured at 730 nm. Ascorbic acid was used as a standard antioxidant.
Experimental animals
Adult male Wistar rats (Rattus norvegicus) weighing between 130 and 180 g were obtained from animal house of the National Institute of Opthalmology, El-Giza, Egypt. Rats were housed in standard cages at normal atmospheric temperature (25 ± 2 °C) and normal 12-h light/dark cycle. They were given access of water ad libitum and supplied daily with standard pellet diet of known composition (8.0 % moisture, 20.8 % crude protein, 4.8 % crude fat, 5.0 % crude ash, 37.2 % nonfiber carbohydrate, 3.2 % crude fiber, and vitamins and minerals adequate to meet the nutritional needs of rat). All animals were kept under observation before the onset of the experiment to exclude any intercurrent infection. All animal procedures were approved by the Institutional Ethics Committee of Beni-Suef University (Egypt).
Induction of experimental diabetes and animal grouping
Experimental diabetes mellitus was induced by a single intraperitoneal injection of freshly prepared solution of STZ (45 mg/kg body weight) in 0.1 M citrate buffer, pH 4.5 [27]. After 7 days of STZ administration, hyperglycemia was verified and rats having blood glucose levels ≥200 mg/dl were selected for the experiment.
Twenty-four rats were randomly divided into four equal groups, each consisting of six (N = 6) animals as follows:
-
Group 1: Normal control rats.
-
Group 2: Normal rats received 100 mg/kg/day M. alba extract dissolved in distilled water [28] by oral gavage for 16 weeks.
-
Group 3: Diabetic control rats.
-
Group 4: Diabetic rats received 100 mg/kg/day M. alba extract dissolved in distilled water [28] by oral gavage for 16 weeks.
Morus alba dose was balanced consistently as indicated by any change in the body weight to keep up comparable dosage over the entire period of study.
Samples collection and preparation
At the end of the experiment, overnight-fasted rats were euthanized by decapitation under mild ether anesthesia. Blood samples were collected to separate serum, and collected sera were stored at −20 °C until analyzed. The eye globes were quickly excised, and retinas were dissected and rinsed with ice-cold saline. Retina samples were homogenized in prechilled 0.2 M potassium phosphate buffer, pH 7.0, and used for assaying lipid peroxidation and antioxidant defenses. Some samples were kept frozen at −80 °C for Western blotting analysis. Other samples of the retina homogenized in 6 % (wt/vol) ice-cold perchloric acid, neutralized with potassium carbonate, were used to determine sorbitol and fructose concentrations.
Biochemical assays
Oral glucose tolerance test (OGTT)
On the day before killing, blood samples were obtained from lateral tail vein of control and diabetic rats deprived of food overnight. Successive blood samples were then collected at 30, 60, 90, and 120 min following the administration of 3 g/kg body weight glucose solution. Blood samples were left to coagulate and centrifuged for serum separation. Serum glucose concentration was determined according to the method of Trinder [29], using reagent kit purchased from bioMerieux chemicals (France).
Determination of insulin, fructosamine, and glycosylated hemoglobin (HBA1c)
Serum levels of insulin were determined using specific ELISA kits purchased from R&D systems (USA) following the manufacturer’s instructions. Serum fructosamine levels were determined according to the method of Baker et al. [30] using reagent kit purchased from Spinreact Company (Spain). A blood sample from each rat was collected on ethylenediaminetetraacetic acid solution and used for the estimation of HBA1c % according to the method of Abraham et al. [31] using reagent kits purchased from Stanbio Company (Texas, USA).
Determination of sorbitol and fructose
Sorbitol and fructose concentrations were determined in the retinal homogenates following the method of Clements et al. [32] and Foreman et al. [33], respectively.
Determination of oxidative stress and antioxidant status
Lipid peroxidation was determined in retinal homogenates by measuring malondialdehyde (MDA) following the method of Preuss et al. [34]. GSH content and the activity of the antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) were assayed according to the methods of Beutler et al. [35], Cohen et al. [36], Marklund and Marklund [37], and Matkovics et al. [38], respectively.
Determination of TNF-α, IL-1β, and PKCβ
The levels of TNF-α, IL-1β, and PKCβ1 were determined in retina homogenates using specific ELISA kits (R&D systems) following the manufacturer’s instructions. The concentrations of assayed parameters were measured spectrophotometrically at 450 nm. Standard curves were constructed by using standard TNF-α, IL-1β, and PKCβ1, and concentrations of the unknown samples were determined from the standard plots.
Western blotting analysis
The frozen retinas were homogenized in ice-cold lysis buffer. The samples were centrifuged at 10,000 g for 10 min to remove the insoluble material. Protein concentrations were determined according to the method of Bradford. Equal amounts of proteins were electrophoresed using 10 % SDS polyacrylamide gel electrophoresis and electro-transferred to nitrocellulose membrane. The membranes were blocked in 5 % w/v skimmed milk powder in phosphate-buffered saline (PBS)/Tween 20 (PBST) for 1 h at room temperature. The membranes were incubated with antibodies for VEGF, Bax, Bcl2, activated caspase-3, and β-actin (Santa Cruz Biotechnology, USA) diluted 1:1000 in blocking buffer. After washing, the membranes were incubated with the corresponding secondary antibodies for 1 h at room temperature, washed, and then developed. The optical densities were quantified with ImageJ analysis software, normalized to β-actin and presented as % of control.
Statistical analysis
Data were analyzed using GraphPad Prism 5 software, and all statistical comparisons were made by means of the one-way ANOVA test followed by Tukey’s test post hoc analysis. Results were articulated as mean ± standard error (SEM), and a P value <0.05 was considered significant.
Results
Total phenolic and flavonoid contents and antioxidant activity of M. alba
The amount of total phenolics in M. alba leaves 90 % (v/v) ethanol extract was 67.66 ± 2.92 mg gallic acid equivalent/g dry extract, and the recorded total flavonoids were 39.24 ± 1.18 mg rutin equivalent/g dry extract.
Results for the radical scavenging and antioxidant activity of M. alba leaves extract are represented in Fig. 1. The extract showed radical scavenging activity against DPPH and ABTS•+.
Morus alba represses body weight loss and hyperglycemia in diabetic rats
Data represented in Fig. 2a show the body weight changes after 16 weeks of treatment. STZ-induced diabetic rats exhibited significant (P < 0.001) body weight loss (−40.21 ± 7.91 g) when compared with the control rats (56.65 ± 10.02 g). Treatment of the diabetic rats with M. alba significantly (P < 0.001) prevented body weight loss and the rats recorded positively changed body weight (50.88 ± 6.03 g).
OGTT of STZ-induced diabetic rats showed significant elevation in blood glucose levels at fasting and at 30, 60, 90, and 120 min after oral glucose loading when compared with the control rats (Fig. 2b). Oral supplementation of M. alba extract to STZ-induced diabetic rats significantly ameliorated the blood glucose levels at all points of the OGTT. The OGTT areas under curve (AUCs) analysis showed a significant (P < 0.001) increase in STZ diabetic rats when compared with the control group. Treatment of the diabetic rats with M. alba potentially (P < 0.001) decreased OGTT AUC when compared with the diabetic control rats. Healthy rats received 100 mg/kg/day M. alba leaves extract for 16 weeks showed nonsignificant (P > 0.05) changes in body weight and glucose tolerance.
Morus alba ameliorates insulin release and attenuates protein glycation
Data summarized in Table 1 show the effect of M. alba on serum insulin and fructosamine levels, and HbA1c %. Serum insulin level was significantly (P < 0.001) decreased in STZ-induced diabetic rats compared to the control rats. Oral treatment of the STZ-induced diabetic rats with M. alba markedly ameliorated serum insulin levels. Conversely, diabetic rats exhibited significant (P < 0.001) increase in serum fructosamine levels and blood HbA1c % when compared with either the control or M. alba-treated diabetic rats. Oral supplementation of M. alba produced a significant (P < 0.001) decrease in serum fructosamine and blood HbA1c % in diabetic rats, with no recorded effect on normal rats.
Morus alba decreases the activity of the polyol pathway in retina of diabetic rats
To test the effect of M. alba on hyperglycemia-induced activation of the polyol pathway, sorbitol and fructose levels were determined in the retinal homogenates. Fructose levels showed a significant (P < 0.001) increase in retina of the STZ-induced diabetic rats (4746.86 ± 442.23 nmol/100 mg) when compared with the control group (591.23 ± 59.38 nmol/100 mg), as represented in Fig. 3a. Oral supplementation of M. alba leaf extract significantly (P < 0.01) decreased fructose level in the retina of diabetic rats (2979.40 ± 96.73 nmol/100 mg). Similarly, retinal content of sorbitol was significantly (P < 0.001) elevated in diabetic rats (90.61 ± 4.51 nmol/g) compared to the control group (29.97 ± 0.47 nmol/g). Treatment of the STZ-induced diabetic rats with M. alba produced a significant (P < 0.01) decrease in retinal sorbitol (69.22 ± 6.74 nmol/g) concentration (Fig. 3b). Fructose and sorbitol levels were nonsignificantly (P > 0.05) affected in M. alba-supplemented rats when compared with the control group.
Morus alba reduces inflammation and PKCβ in retina of diabetic rats
The levels of TNF-α (Fig. 4a) and IL-1β (Fig. 4b) in the retina of STZ-induced diabetic rats showed significant (P < 0.001) increase when compared with the corresponding normal control group. Oral supplementation of the diabetic rats with M. alba produced significant (P < 0.001) decrease in the levels of both TNF-α and IL-1β in the retina.
More or less similar, the levels of PKCβ were significantly (P < 0.001) increased in the retina of STZ-induced diabetic rats compared to the control group. Treatment of the diabetic rats with M. alba leaf extract significantly (P < 0.01) ameliorated retinal content of PKCβ, as represented in Fig. 4c. Of note, M. alba supplementation produced a nonsignificant (P > 0.05) effect on TNF-α, IL-1β, and PKCβ levels in retina of the normal rats.
Morus alba attenuates hyperglycemia-induced oxidative stress in retina of diabetic rats
Concerning lipid peroxidation, STZ-induced diabetic rats exhibited significantly (P < 0.001) increased MDA levels in retina (84.07 ± 1.49 nmol/100 mg) as compared to their respective normal controls (40.81 ± 1.01 nmol/100 mg), as shown in Fig. 5a. Treatment of the STZ-induced diabetic rats with M. alba extract markedly (P < 0.001) decreased retinal MDA content (64.08 ± 2.63 nmol/100 mg).
Effect of M. alba on oxidative stress and antioxidant defense system parameters in retina of control and STZ-induced diabetic rats. Results are mean ± SEM (N = 6). *P < 0.05; **P < 0.01; ***P < 0.001. MDA malondialdehyde, GSH glutathione, GPx glutathione peroxidase, SOD superoxide dismutase, CAT catalase
On the contrary, STZ administration produced a significant (P < 0.01) decrease in GSH content in retina of diabetic rats (1.95 ± 0.51 nmol/100 mg) when compared with the normal control group (6.09 ± 0.64 nmol/100 mg). Oral treatment of the diabetic rats with M. alba extract significantly (P < 0.05) ameliorated retinal GSH content (4.90 ± 0.58 nmol/100 mg), as depicted in Fig. 5b.
GPx activity showed a similar pattern where it was significantly (P < 0.01) declined in the retina of STZ-induced diabetic rats (15.17 ± 1.32 U/100 mg) compared to the control group (22.22 ± 0.97 U/100 mg), as represented in Fig. 5c. Similarly, the activities of retinal SOD and CAT showed a significant decrease in STZ-induced diabetic rats (Fig. 5d, e). On the other hand, treatment of the diabetic rats with M. alba markedly increased the activities of GPx (P < 0.001), SOD (P < 0.05), and CAT (P < 0.05) in the retina. Retina of M. alba-supplemented normal rats showed nonsignificant changes in lipid peroxidation and antioxidant defenses.
Morus alba prevents apoptosis and angiogenesis in retina of diabetic rats
Western blotting analysis of the apoptosis proteins showed significant (P < 0.001) increase in protein levels of activated caspase-3 (Fig. 6a) and Bax (Fig. 6b) in retinas of STZ-induced diabetic rats. M. alba oral supplementation produced significant (P < 0.001) decrease in protein levels of activated caspase-3 and Bax in retina of the diabetic rats. In opposite, protein levels of the anti-apoptotic protein Bcl-2 showed a significant (P < 0.001) decrease in retina of STZ-induced diabetic rats and markedly (P < 0.001) increased following treatment with M. alba leaf extract (Fig. 6c).
Data represented in Fig. 6d show the effect of STZ-induced diabetes and treatment with M. alba on the protein expression levels of the angiogenesis marker VEGF in retina of rats. Diabetic rats exhibited significant (P < 0.001) increase in retinal protein levels of VEGF. On the other hand, treatment with M. alba leaf extract significantly (P < 0.001) attenuated diabetes-induced VEGF expression in retina of rats. M. alba exerted no effect on retinal activated caspase-3, Bax, Bcl-2, and VEGF when supplemented to normal rats.
Discussion
DR is a common diabetes complication and is a leading cause of blindness in working-age population. Since clinical trials with pharmacologic agents that inhibited one of the specific pathways of DR showed disappointing results [39], inhibition of various pathways might represent an important strategy for the prevention of DR. Therefore, the current study was undertaken to evaluate the possible effectiveness of a polyphenol-rich M. alba leaves extract on hyperglycemia-induced oxidative stress, apoptosis, inflammation, and VEGF expression in retina of STZ-induced diabetic rats.
Under insulin deficiency and hyperglycemic conditions, the body provides itself energy by degrading proteins and lipids, which ultimately accounts for body weight loss [40]. Accordingly, STZ-induced diabetic rats in the present study showed significant hypoinsulinemia and weight loss. Treatment with M. alba leaves extract for 16 weeks attenuated hyperglycemia and its associated body weight loss, suggesting possible improvement in energy metabolism. These findings could be explained, at least in part, due to the insulinotropic effect of M. alba. In this context, Mohammadi and Naik [41] reported that M. alba increased serum insulin levels in diabetic rats through its ability to stimulate the spontaneous recovery of β-cells of the islets of Langerhans. In addition, the ameliorative effect of M. alba extract on blood glucose level seems to be mediated through other mechanisms. M. alba and some of its constituents have demonstrated an ability to inhibit hepatic gluconeogenesis by suppressing glucose 6-phosphatase activity [42], increase hexokinase, and glucose-6-phosphate dehydrogenase activity [43], and enhance hepatic glycogen synthesis secondary to β-glucosidase inhibitory activity [44]. The present findings are in agreement with several previous studies [28, 45, 46].
Oxidation of glucose is one of the mechanisms involved in the pathogenesis of diabetes complications [47]. It enhances glycation of hemoglobin and produces HbA1c [48]. The concentration of HbA1c is a good marker for diagnosis and prognosis of diabetes complications, and is strongly related to the risk of DR [49]. Hyperglycemia-triggered elevated HbA1c leads to red blood cell stiffening and decreased deformation capacity. As a result, both blood viscosity and shear stress at the endothelium of retinal vessel increase, leading to damage of the blood vessel integrity and pericytes loss [50]. Subsequently, the developed hypoxia results in compensatory expansion of retinal blood vessels to increase perfusion [51]. Reduction in HbA1c % in diabetic rats after 16-week M. alba supplementation indicates that M. alba has beneficial effects in attenuation of DR. In this context, the study conducted by Kowluru and Chan [52] reported that reduction of only one unit (~7 %) of HBA1c can reduce the risk of DR by over 30 %. Since insulin treatment of STZ diabetic rats significantly lowered the level of HbA1c [53], the insulinogenic effect of M. alba extract and the subsequently improved glycemic state account for its improved levels. The ability of M. alba to control the glycemic state was further evidenced by the lowered serum fructosamine level. Fructosamine is an early glycation end product results from the nonenzymatic reaction between glucose and amino acids [54]. It can be used to predict the concentration of AGEs and is an indicator of glycemic control over a 3 weeks or longer period [55]. In addition, the polyphenolic compounds, especially flavonoids, have the ability to interfere with protein glycation through scavenging free radicals. Here, we confirmed the presence of phenolics and flavonoids as well as the radical scavenging activity of M. alba leaves extract.
Many hyperglycemia-induced metabolic abnormalities such as increased oxidative stress are implicated in the pathogenesis of DR [52]. Chronic hyperglycemia has been reported to trigger oxidative stress either by direct generation of ROS or by altering the redox balance [48]. ROS is produced by multiple pathways including xanthine oxidase, the mitochondrial electron transport chain, and uncoupled nitric oxide synthases [56]. PKC activation, formation of AGEs, and polyol pathway can also contribute to oxidative stress by diminishing the activities of antioxidant enzymes [57]. Because of the highest uptake of oxygen and its high concentration of polyunsaturated fatty acids, retina is vulnerable to lipid peroxidative damage [6]. Several animal studies have demonstrated that hyperglycemia-induced oxidative stress is linked to the retinal capillary basement membrane thickening, which is an early abnormality of the microangiopathy seen in DR [6]. In addition, increased oxidative stress in diabetes mellitus is proposed to play central role in capillary cell apoptosis [58]. Thus, oxidative stress is a major contributor in the development of DR, and this makes it an important target for therapeutic strategies for this disease.
In the current study, retina of diabetic rats showed a significant increase in the lipid peroxidation marker, MDA, with concomitant declined GSH content and activity of SOD, CAT, and GPx. Our data are in agreement with the study of Soufi et al. [59] in which diabetic rats experienced chronic hyperglycemia with an increase in oxidative stress markers. M. alba supplementation markedly attenuated hyperglycemia-induced oxidative stress through preventing GSH depletion and enhancement of the enzymatic antioxidants. The antioxidant effects of M. alba were further confirmed by the in vitro DPPH and ABTS•+ radical scavenging assays. These effects could be directly linked to the rich polyphenolic constituents especially the flavonoids in M. alba. The leaves of mulberry contain high amounts of quercetin, quercetin 3-(6-malonylglucoside), rutin, oxyresveratrol, and 5,7-dihydroxycoumarin 7-methyl ether which are responsible for their antioxidant potential [60, 61].
Activation of PKC is another pathway implicated in the development of DR. In diabetes, elevated levels of diacylglycerol induced by hyperglycemia activate PKCβ [62]. PKC activation contributes to ROS production by increasing the activity of NADPH oxidase [63], increases the expression of VEGF [64], and decreases nitric oxide production in smooth muscle cells [65]. PKC is also implicated in NF-κB activation and thus connects hyperglycemia-induced oxidative stress to inflammation [66]. Because of the multiple effects of elevated PKC activation, it may be considered as a promising therapeutic target for DR. Increased activation of PKCβ occurs in retinas of diabetic animals and in endothelial cells exposed to high glucose (reviewed in Frank [67]). The elevated levels of PKCβ in retina of the diabetic rats in the present investigation provide additional evidence. Interestingly, retina of the diabetic rats received M. alba for 16 weeks showed decreased levels of PKCβ. Inhibition of PKC using general and specific inhibitors prevented retinal vascular permeability [62]. Therefore, attenuation of PKCβ seems to have a role in the protective mechanism of M. alba against DR.
Polyol pathway is one of the major pathways implicated in the development of DR. In this pathway, glucose is converted to sorbitol by the enzyme AR using NADPH as a cofactor. Sorbitol is further processed to fructose by the action of sorbitol dehydrogenase using NAD+ as a hydrogen donor [68]. Diabetic rats in the present investigation showed significant increase in retinal levels of sorbitol and fructose, indicating activated polyol pathway. Increased sorbitol level during hyperglycemia occurs due to the flux of glucose through the polyol pathway [69]. AR is the rate-limiting enzyme in the polyol pathway [70] and could be considered as an attractive therapeutic target for DR. Therefore, the protective effects of pharmacological inhibition and genetic deletion of AR have been examined in several studies. The specific AR inhibitor zoloperstat prevented ROS generation and retinal endothelial cell death [71]. Genetic deletion of AR protected diabetic mice against ROS production and retinal acellular capillaries [72]. The present data showed that treatment of the diabetic rats with M. alba attenuated hyperglycemia-induced sorbitol and fructose production, possibly through inhibition of AR. Recently, the AR inhibitory activity of M. alba and its flavonoid morusin was demonstrated by Rao et al. [73].
It is now increasingly appreciated that the pathogenesis of DR involves low-grade inflammation [74]. Several inflammatory cytokines are known to participate in the breakdown of BRB in diabetes. IL-1β and TNF-α are the representative inflammatory cytokines associated with the pathogenesis of DR. Their level is increased in both the vitreous humor and serum of patients with PDR [75]. In the present study, the levels of IL-1β and TNF-α showed a significant increase in retina of diabetic rats, reflecting the degree of inflammation. In agreement with our findings, the levels of IL-1β were found to be increased in retinas from diabetic rats [76]. Also, the activity of caspase-1, a proteolytic enzyme involved in the production of IL-1β, is up-regulated in the retinas of diabetic patients [77]. TNF-α as well is involved in the loss of retinal microvascular cells in diabetic retina [14]. Oral supplementation of the diabetic rats with M. alba for 16 weeks potentially attenuated the production of IL-1β and TNF-α in the retina, confirming a potent anti-inflammatory activity.
In addition, M. alba proved a potent anti-apoptotic activity as evident by down-regulation of Bax and caspase-3 and up-regulation of Bcl-2 protein expression in the retina of diabetic rats. The induced apoptosis in retina of diabetic rats could be directly connected to the hyperglycemia-induced inflammation. Endothelial IL-1β overexpression, stimulated by high concentration of glucose, induces apoptosis of endothelial cells through NF-κB activation in vitro. In addition, IL-1β has been reported to accelerate apoptosis in retinal pericytes under high glucose conditions through activation of NF-κB [78]. Likewise, TNF-α is involved in the loss of microvascular cells in diabetic retina [14]. It disturbs expression and subcellular localization of the tight junction proteins, claudin-5 and ZO-1, in bovine retinal endothelial cells [79]. The pro-inflammatory and pro-apoptotic effects of IL-1β and TNF-α were further confirmed through knockout studies and pharmacological inhibition. In the IL-1β receptor knockout mice, diabetes-induced retinopathy was markedly attenuated at 7-month duration of diabetes [80]. In addition, inhibition of caspase-1 using minocycline decreased the degeneration of retinal capillaries in the treated animals [80]. Similarly, TNF-α knockout protected rat against diabetes-associated retinal apoptosis, leukostasis, and breakdown of BRB [81].
The anti-inflammatory effect of M. alba in the present study is in agreement with several investigations. Choi and Hwang [82] reported the anti-inflammatory effects of M. alba leaf extract in RAW264.7 macrophages. Oxyresveratrol, an active ingredient of M. alba, has been previously demonstrated to exert anti-inflammatory activity through inhibition of NF-κB activation, iNOS/NO production, and PGE2 synthesis [83]. More recently, Chen et al. [84] reported the anti-inflammatory effects of both M. alba and the active compound oxyresveratrol. Moreover, prenylated flavonoids from M. alba prevented the lipopolysaccharide-induced inflammatory response in macrophages [85]. Quercetin and rutin, a flavonol and its glycoside present in M. alba [60], have been reported to exert anti-inflammatory, antioxidant, and anti-apoptotic effects in STZ-induced diabetic rat retina [86, 87].
Oxidative stress and pro-inflammatory cytokine are implicated in VEGF up-regulation in the diabetic retina [57]. In addition to induction of apoptosis, IL-1β is known to increase the expression of VEGF in retinal endothelial cells [77]. VEGF is a potent vascular permeability factor, and studies demonstrated its up-regulation in neovascular eye diseases including DR [88]. In addition, increased levels of VEGF have been identified in ocular fluids of patients with PDR [89]. Accordingly, diabetic rats in the present investigation showed significant up-regulation of retinal VEGF protein levels. M. alba supplementation for 16 weeks alleviated VEGF expression levels in retina of the diabetic rats. These findings could be attributed to the anti-inflammatory and anti-angiogenic effects of M. alba leaves extract. A recent study conducted by Hong et al. [90] reported the anti-angiogenic effect of a herbal composition containing M. alba. More or less similar, administration of anti-VEGF antibodies to experimental animals attenuated high glucose-induced vascular hyperpermeability [91]. In addition, clinical trials using anti-VEGF therapy are displaying promising results against stages of DR [92]. Therefore, down-regulation of VEGF seems to participate in the protective mechanism of M. alba against hyperglycemia-induced DR. For achieving better results, additional research is required to elucidate the effect of M. alba extract on histopathological alterations of retina in diabetic animals.
In conclusion, the present study depicts that M. alba administration proved a potent anti-hyperglycemic effect. Since DR is triggered by a persistent increase in blood glucose levels, good glycemic control can reduce its development. M. alba has protective effect on DR with possible mechanisms of inhibiting hyperglycemia-induced oxidative stress, apoptosis, inflammation, polyol pathway activation, and VEGF expression in the retina of diabetic rats (summarized mechanistic pathways are presented in Fig. 7). Given the key role of oxidative stress in the progression of DR, the observed antioxidant and anti-diabetic properties of M. alba make it candidate as a therapeutic supplement to reduce DR.
References
Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd (1998) Diabetic retinopathy. Diabetes Care 21:143–156
Yau JW, Rogers SL, Kawasaki R et al (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564
Aiello PL (2006) The molecular biology of diabetic retinopathy: opportunities for therapeutic intervention. Adv Stud Ophthalmol 3:8–12
Mohamed Q, Gillies MC, Wong TY (2007) Management of diabetic retinopathy: a systematic review. JAMA 298:902–916
Santos JM, Tewari S, Kowluru RA (2012) A compensatory mechanism protects retinal mitochondria from initial insult in diabetic retinopathy. Free Radic Biol Med 53:1729–1737
Mitsuhashi J, Morikawa S, Shimizu K, Ezaki T, Yasuda Y, Hori S (2013) Intravitreal injection of erythropoietin protects against retinal vascular regression at the early stage of diabetic retinopathy in streptozotocin-induced diabetic rats. Exp Eye Res 106:64–73
Chung SS, Chung SK (2005) Aldose reductase in diabetic microvascular complications. Curr Drug Targets 6:475–486
Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH (2005) Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets 6:511–524
Curtis TM, Scholfield CN (2004) The role of lipids and protein kinase Cs in the pathogenesis of diabetic retinopathy. Diabetes Metab Res Rev 20:28–43
Aiello LP, Bursell SE, Clermont A et al (1997) Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes 46:1473–1480
Vinores SA, Van Niel E, Swerdloff JL, Campochiaro PA (1993) Electron microscopic immunocytochemical demonstration of blood-retinal barrier breakdown in human diabetics and its association with aldose reductase in retinal vascular endothelium and retinal pigment epithelium. Histochem J 25:648–663
Yan SF, Ramasamy R, Naka Y, Schmidt AM (2003) Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res 93:1159–1169
Singh CK, Kumar A, Hitchcock DB, Fan D, Goodwin R, LaVoie HA (2011) Resveratrol prevents embryonic oxidative stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol Nutr Food Res 55:1186–1196
Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT (2008) Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol 172:1411–1418
Dillard CJ, German JB (2000) Phytochemicals: nutraceuticals and human health. J Sci Food Agric 80:1744–1756
Amarowicz R, Pegg RB, Bautista DA (2000) Antibacterial activity of green tea polyphenols against Escherichia coli K 12. Nahrung 44:60–62
Ercisli S (2004) A short review of the fruit germplasm resources of Turkey. Genet Resour Crop Evol 51:419–435
Arabshahi-D S, Vishalakshi Devi D, Urooj A (2007) Evaluation of antioxidant activity of some plant extracts and their heat, pH and storage stability. Food Chem 100:1100–1105
Du J, He ZD, Jiang RW, Ye WC, Xu HX, But PP (2003) Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 62:1235–1238
Pan G, Lou C (2008) Isolation of an 1-aminocyclopropane-1-carboxylate oxidase gene from mulberry (Morus alba L.) and analysis of the function of this gene in plant development and stresses response. J Plant Physiol 165:1204–1213
Sun F, Shen L, Ma Z (2011) Screening for ligands of human aromatase from mulberry (Mori alba L.) leaf by using high-performance liquid chromatography/tandem mass spectrometry. Food Chem 126:1337–1343
Zhang M, Chen M, Zhang HQ, Sun S, Xia B, Wu FH (2009) In vivo hypoglycemic effects of phenolics from the root bark of Morus alba. Fitoterapia 80:475–477
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. Blackwell Scientific Publication, Oxford
Jia Z, Tang M, Wu Ju (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Brandwilliams W, Cuvelier M, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Food Sci Technol 28:25–30
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
El-Seifi SA-MA, Badir N (1993) The effect of Ambrosia maritima and Cleome droserifolia on insulin release in vitro. J Egypy Ger Soc Zool 12:347–363
El-Sayyad HI, El-Sherbiny MA, Sobh MA, Abou-El-Naga AM, Ibrahim MA, Mousa SA (2011) Protective effects of Morus alba leaves extract on ocular functions of pups from diabetic and hypercholesterolemic mother rats. Int J Biol Sci 7:715–728
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–27
Baker JR, Metcalf PA, Holdaway IM, Johnson RN (1985) Serum fructosamine concentration as measure of blood glucose control in type I (insulin dependent) diabetes mellitus. Br Med J (Clin Res Ed) 290:352–355
Abraham EC, Huff TA, Cope ND, Wilson JB Jr, Bransome ED Jr, Huisman TH (1978) Determination of the glycosylated hemoglobins (HB AI) with a new microcolumn procedure. Suitability of the technique for assessing the clinical management of diabetes mellitus. Diabetes 27:931–937
Clements RS Jr, Morrison AD, Winegrad AI (1969) Polyol pathway in aorta: regulation by hormones. Science 166:1007–1008
Foreman D, Gaylor L, Evans E, Trella C (1973) A modification of the Roe procedure for determination of fructose in tissues with increased specificity. Anal Biochem 56:584–590
Preuss HG, Jarrell ST, Scheckenbach R, Lieberman S, Anderson RA (1998) Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr 17:116–123
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. FEBS Eur J Biochem 47:469–474
Matkovics B, Szabo L, Varga IS (1998) Determination of enzyme activities in lipid peroxidation and glutathione pathways (in Hungarian). Lab Diagn 15:248–249
PKC-DRS Study Group (2005) The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes 54:2188–2197
Palsamy P, Subramanian S (2010) Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol 224:423–432
Mohammadi J, Naik PR (2012) The histopathologic effects of Morus alba leaf extract on the pancreas of diabetic rats. Turk J Biol 36:211–216
van Dijk TH, van der Sluijs FH, Wiegman CH, Baller JF, Gustafson LA, Burger HJ (2001) Acute inhibition of hepatic glucose-6-phosphatase does not affect gluconeogenesis but directs gluconeogenic flux toward glycogen in fasted rats. A pharmacological study with the chlorogenic acid derivative S4048. J Biol Chem 276:25727–25735
Hamdy SM (2012) Effect of Morus alba Linn extract on enzymatic activities in diabetic rats. J Appl Sci Res 8:10–16
Asano N, Yamashita T, Yasuda K, Ikeda K, Kizu H, Kameda Y (2001) Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J Agric Food Chem 49:4208–4213
Laddha GPBS, Mahale V, Baile SB (2012) Antidiabetic effect of Morus alba on rabbit as animal model. Int Res J Pharm 3:334–336
Naowaboot J, Pannangpetch P, Kukongviriyapan V, Kukongviriyapan U, Nakmareong S, Itharat A (2009) Mulberry leaf extract restores arterial pressure in streptozotocin-induced chronic diabetic rats. Nutr Res 29:602–608
Maritim AC, Sanders RA, Watkins JB 3rd (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38
Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50:567–575
Howlett J, Ashwell M (2008) Glycemic response and health: summary of a workshop. Am J Clin Nutr 87:212S–216S
Lakshminarayanan S, Gardner TW, Tarbell JM (2000) Effect of shear stress on the hydraulic conductivity of cultured bovine retinal microvascular endothelial cell monolayers. Curr Eye Res 21:944–951
Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T (2004) Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53:1104–1110
Kowluru RA, Chan PS (2007) Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007:43603
Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M (2003) A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes 52:506–511
Liu CT, Chen KM, Lee SH, Tsai LJ (2000) Effect of supplemental l-arginine on the function of T lymphocytes and the formation of advanced glycosylated end products in rats with streptozotocin-induced diabetes. Nutrition 21:615–623
Li K, Yang HX (2006) Value of fructosamine measurement in pregnant women with abnormal glucose tolerance. Chin Med J (Engl) 119:1861–1865
Dikalov S (2011) Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51:1289–1301
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070
Kowluru RA, Kowluru V, Xiong Y, Ho YS (2006) Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med 41:1191–1196
Soufi FG, Mohammad-Nejad D, Ahmadieh H (2012) Resveratrol improves diabetic retinopathy possibly through oxidative stress—nuclear factor kappaB—apoptosis pathway. Pharmacol Rep 64:1505–1514
Oh H, Ko EK, Jun JY, Oh MH, Park SU, Kang KH (2002) Hepatoprotective and free radical scavenging activities of prenylflavonoids, coumarin, and stilbene from Morus alba. Planta Med 68:932–934
Iqbal S, Younas U, Sirajuddin Chan KW, Sarfraz RA, Uddin K (2012) Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): a comparative study. Int J Mol Sci 13:6651–6664
Das Evcimen N, King GL (2007) The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res 55:498–510
Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M (2000) High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49:1939–1945
Williams B, Gallacher B, Patel H, Orme C (1997) Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes 46:1497–1503
Ganz MB, Seftel A (2000) Glucose-induced changes in protein kinase C and nitric oxide are prevented by vitamin E. Am J Physiol Endocrinol Metab 278:E146–E152
Ha H, Yu MR, Choi YJ, Kitamura M, Lee HB (2002) Role of high glucose-induced nuclear factor-kappaB activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am Soc Nephrol 13:894–902
Frank RN (2002) Potential new medical therapies for diabetic retinopathy: protein kinase C inhibitors. Am J Ophthalmol 133:693–698
Miwa K, Nakamura J, Hamada Y, Naruse K, Nakashima E, Kato K (2003) The role of polyol pathway in glucose-induced apoptosis of cultured retinal pericytes. Diabetes Res Clin Pract 60:1–9
Safi SZ, Qvist R, Kumar S, Batumalaie K, Ismail IS (2014) Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res Int 2014:801269
Oates PJ (2002) Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol 50:325–392
El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB (2003) High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci 44:3135–3143
Tang J, Du Y, Petrash JM, Sheibani N, Kern TS (2013) Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PLoS ONE 8:e62081
Rao ARSP, Veeresham C, Asres K (2015) Aldose reductase inhibitory and antiglycation activities of four medicinal plant standardized extracts and their main constituents for the prevention of diabetic complications. Ethiop Pharm J 31:1–14
van Hecke MV, Dekker JM, Nijpels G, Moll AC, Heine RJ, Bouter LM (2005) Inflammation and endothelial dysfunction are associated with retinopathy: the Hoorn Study. Diabetologia 48:1300–1306
Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S (2006) Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 20:1366–1369
Vincent JA, Mohr S (2007) Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes 56:224–230
Mohr S, Xi X, Tang J, Kern TS (2002) Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes 51:1172–1179
Fan F, Stoeltzing O, Liu W, McCarty MF, Jung YD, Reinmuth N (2004) Interleukin-1beta regulates angiopoietin-1 expression in human endothelial cells. Cancer Res 64:3186–3190
Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA (2010) TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 59:2872–2882
Grant MB, Mames RN, Fitzgerald C, Hazariwala KM, Cooper-DeHoff R, Caballero S (2000) The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy: a randomized controlled study. Diabetes Care 23:504–509
Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ (2011) TNF alpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci 52:1336–1344
Choi EM, Hwang JK (2005) Effects of Morus alba leaf extract on the production of nitric oxide, prostaglandin E2 and cytokines in RAW264.7 macrophages. Fitoterapia 76:608–613
Chung KO, Kim BY, Lee MH, Kim YR, Chung HY, Park JH (2003) In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol 55:1695–1700
Chen YC, Tien YJ, Chen CH, Beltran FN, Amor EC, Wang RJ (2013) Morus alba and active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signaling. BMC Complement Altern Med 13:45
Kollar P, Barta T, Hosek J, Soucek K, Zavalova VM, Artinian S (2013) Prenylated flavonoids from Morus alba L. cause inhibition of G1/S transition in THP-1 human leukemia cells and prevent the lipopolysaccharide-induced inflammatory response. Evid Based Complement Altern Med 2013:350519
Kumar B, Gupta SK, Nag TC, Srivastava S, Saxena R, Jha KA, Srinivasan BP (2014) Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp Eye Res 125:193–202
Ola MS, Ahmed MM, Ahmad R, Abuohashish HM, Al-Rejaie SS, Alhomida AS (2015) Neuroprotective effects of rutin in streptozotocin-induced diabetic rat retina. J Mol Neurosci 56:440–448
Yang Y, Andresen BT, Yang K, Zhang Y, Li X, Wang H (2010) Association of vascular endothelial growth factor -634C/G polymorphism and diabetic retinopathy in type 2 diabetic Han Chinese. Exp Biol Med (Maywood) 235:1204–1211
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Hong Y, Kim MY, Yoon M (2011) The anti-angiogenic herbal extracts Ob-X from Morus alba, Melissa officinalis, and Artemisia capillaris suppresses adipogenesis in 3T3-L1 adipocytes. Pharm Biol 49:775–783
Tilton RG, Kawamura T, Chang KC, Ido Y, Bjercke RJ, Stephan CC (1997) Vascular dysfunction induced by elevated glucose levels in rats is mediated by vascular endothelial growth factor. J Clin Invest 99:2192–2202
Montero JA, Ruiz-Moreno JM, Correa ME (2011) Intravitreal anti-VEGF drugs as adjuvant therapy in diabetic retinopathy surgery. Curr Diabetes Rev 7:176–184
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Rights and permissions
About this article
Cite this article
Mahmoud, A.M., Abd El-Twab, S.M. & Abdel-Reheim, E.S. Consumption of polyphenol-rich Morus alba leaves extract attenuates early diabetic retinopathy: the underlying mechanism. Eur J Nutr 56, 1671–1684 (2017). https://doi.org/10.1007/s00394-016-1214-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1214-0