Abstract
Purpose
The present study was undertaken to explore the possible anti-diabetic mechanism(s) of Emblica officinalis (EO) and its active constituent, ellagic acid (EA), in vitro and in vivo.
Method
Neonatal streptozotocin-induced non-obese type 2 diabetic rats were treated with a methanolic extract of EO (250 or 500 mg/kg) for 28 days, and blood glucose, serum insulin, and plasma antioxidant status were measured. Insulin and glucagon immunostaining and morphometry were performed in pancreatic section, and liver TBARS and GSH levels were measured. Additionally, EA was tested for glucose-stimulated insulin secretion and glucose tolerance test.
Results
Treatment with EO extract resulted in a significant decrease in the fasting blood glucose in a dose- and time-dependent manner in the diabetic rats. It significantly increased serum insulin in the diabetic rats in a dose-dependent manner. Insulin-to-glucose ratio was also increased by EO treatment. Immunostaining of pancreas showed that EO250 increased β-cell size, but EO500 increased β-cells number in diabetic rats. EO significantly increased plasma total antioxidants and liver GSH and decreased liver TBARS. EA stimulated glucose-stimulated insulin secretion from isolated islets and decreased glucose intolerance in diabetic rats.
Conclusion
Ellagic acid in EO exerts anti-diabetic activity through the action on β-cells of pancreas that stimulates insulin secretion and decreases glucose intolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diet and nutrition are part of our everyday life and play important roles in preventing, delaying or even treating many diseases including diabetes. Management of diabetes by dietary agents (DAs) or supplements is more applicable and practical due to safety and availability issues. Diabetes often referred as a lifestyle disease, and changes and/or modification in lifestyle through DAs is one of the best and easiest approaches to combating the disease [1]. Indeed, DAs are being used more and more in the developing countries for the management of diabetes and many other diseases [2, 3]. In treating diabetes, lowering of the abnormally high blood glucose level is the ultimate goal. Therefore, treatments that reduce blood glucose level including insulinotropic effects of any treatment are ideal approaches for the management of this disease. Emblica officinalis (EO), belonging to the Euphorbiaceae family, is widely used in the ayurveda and is believed to be useful for many diseases including diabetes. The EO fruit is native to India and also grows in tropical and subtropical regions including Pakistan, Uzbekistan, Sri Lanka, South East Asia, China, Bangladesh, and Malaysia [4]. The EO fruit contains amino acids and minerals and is rich in vitamin C [5].
Studies have shown that the fruit of EO exerts anti-diabetic effects via their free radical scavenging and antioxidant properties [6]. Akhtar et al. [7] reported anti-hyperglycemic and lipid-lowering properties of EO fruit in normal as well as diabetic subjects. Tiwari et al. [8] demonstrated that EO attenuated diabetic condition as well as neuropathic pain through decreasing oxidative stress in experimental diabetic rats. Patel et al. [9] reported the effect of EO fruits on glucose homeostasis and metabolic parameters. It is reported that EO contains tannins which are well known for strong antioxidant activity and can improve glycemic status and oxidative stress in type 2 diabetic rats. These scientific articles also described the anti-diabetic property of EO [10, 11]. However, we note that antioxidants by themselves do not lower blood glucose level, and so, the precise cellular mechanism(s) of blood glucose-lowering activity of EO, such as β-cell hypertrophy or hyperplasia, or other mechanisms are not yet clear.
EO extract is rich in emblicanin A, emblicanin B, gallic acid, ellagic acid (EA), and vitamin C [11]. Gallic acid is a potent antioxidant and used as a standard compound in radical scavenging experiments. EA, a derivative of gallic acid [12], is one of the predominant compounds present in EO, has antioxidant property and was studied for its anti-diabetic potential [13]. Researches have pondered only over the anti-diabetic effect of EA, and plausible conclusions are reported without a handle on the precise mechanism of action [14, 15].
Type 2 diabetes is manifested in β-cell dysfunction and/or insulin resistance. However, controversies exist regarding pathophysiology of type 2 diabetes as it differs from Asians to Western [16–18]. Most of the Asian subjects are non-obese rather than obese as mostly seen in Western population [19]. In the non-obese diabetic subjects, mostly found in Asia, β-cell dysfunction and insulin secretion impairment are predominant [19, 20]. Therefore, a suitable model that mimics the pathophysiology of non-obese type 2 diabetes is required to study the role of DAs and their active constituents to explore their anti-diabetic mechanism(s). Many animal models have been reported for type 2 diabetes; however, the neonatal streptozotocin-induced type 2 diabetic rat model is considered to be one of the suitable experimental animal models of non-obese type 2 diabetes for the study of β-cell dysfunction [21–23]. It is reported [21–23] and we also found in our previous studies that [24, 25] neonatal STZ model develops partial β-cell damage and insulin secretion impairment that is found in Asian non-obese type 2 diabetic subjects. For the management of diabetes through DAs, we used this neonatal STZ non-obese rat model to address Asian diabetic type.
The present study was undertaken to explore the possible cellular mechanism(s) of the anti-diabetic activity of EO in a neonatal streptozotocin-induced non-obese type 2 diabetic rat model. EO treatment to diabetic rats decreased blood glucose, increased serum insulin, and enhanced the antioxidant status. Additionally, EO modulated pancreatic β-cell size and number. EA, a major compound in EO, induced glucose-stimulated insulin secretion in vitro and decreased glucose intolerance in vivo.
Materials and methods
Materials
Streptozotocin (STZ), glibenclamide, tolbutamide, collagenase-V, bovine serum albumin (BSA), EDTA, hydroxylated toluene, sodium lauryl sulfate, acetic acid, thiobarbituric acid, butanol, meta phosphoric acid, and 5,5-dithiobis (2-nitrobenzoic acid) were obtained from Sigma (St. Louis, MO, USA). Rat and mouse ultra-sensitive insulin ELISA kits were from Crystal Chem Inc. (IL, USA), and the total antioxidant status kit was from Randox (Antrim, UK). Ellagic acid (>98 % pure) was purchased from Alfa Aesar (Karlsruhe, Germany). Insulin antibody (guinea pig anti-insulin) was purchased from Abcam (Cambridge, UK), and glucagon antibody (mouse anti-glucagon) was from Sigma. The fluorescent-labeled secondary antibodies, Texas red-donkey anti-guinea-pig IgG and Cy2-donkey anti-mouse IgG, were obtained from Jackson ImmunoResearch (Baltimore, PA, USA). 4′,6-Diamidino-2-phenylindole (DAPI) was obtained from Wako (Osaka, Japan).
Extract preparation
Emblica officinalis (2 kg) was purchased from the local market. Fruit authentication was carried out from Botany Department of Karachi University, Pakistan (Plant authentication # 86468). The fruit pulp was separated and blended with aqueous (80 %) methanol. The extract was prepared according to standard procedure and used for experimental feeding. The extract was dissolved in water to make suspension and given to experimental rats.
HPLC analysis of EO extract
Standardization of methanolic extract of EO was performed by RP-HPLC (Shimadzu LC 10A) system comprising Shimadzu binary pump LC (LC-10 ATvp) with degasser (DGU-14A), high-performance auto-sampler (SIL-10 ADvp) with Shimadzu column oven (CTO-10 ASvp), and UV detector (SPD 10Avp). Data acquisition and integration were controlled by LC Solution software. A Phenonenex C-18(2) Luna 5 µ 100 A column (150 mm × 4.60 mm I.D., 5 µ) was used. The mobile phase was composed of mixture of 550 ml 0.2 % H3PO4: 250 ml methanol in 800 ml with isocratic mode of run. Flow rate was 1.2 ml/min throughout the analysis. The samples and standard were injected through an auto-sampler; the standard EA and methanolic extract of EO were injected 10 µl. Column oven temperature was maintained at 40 ◦C. The detection wavelength of UV was 254 nm. A solution of EA standard was prepared by weighing 2.8 mg in a 25-ml volumetric flask with 1 M NaOH in the concentration of 1 ml/5 ml, and volume was made up to 26 ml with mobile phase. The solution of methanolic extract of EO was prepared by dissolving 10.9 mg in a 25-ml volumetric flask, and volume was made up to 26.5 ml with mobile phase. All samples were filtered with 0.45 µm Millex-HN syringe-driven filter unit (Millipore, Bedford, USA) and preserved at 4 °C prior to analysis.
Animals and development of non-obese type 2 diabetic rats
Streptozotocin solution (90 mg/kg) prepared in citrate buffer was injected to pups for developing non-obese type 2 model diabetic rats. After 12 weeks of STZ induction, an oral glucose tolerance test (OGTT) was performed. The rats that showed fasting blood glucose (FBG) levels between 7.6 and 11.0 mmol/l at 0 min and showed the maximum rise at 30 min after glucose load (12.7–22.2 mmol/l) were divided into four groups (6–8 rats per group). Group I, diabetic rats (Db); Group II, diabetic rats + EO 250 mg/kg dose (EO250); Group III, diabetic rats + EO 500 mg/kg dose (EO500); Group IV, diabetic rats + 5 mg/kg glibenclamide (Gb). The oral doses of extract, Gb, and equivalent volume of water were given once a day for 28 days to diabetic rats and control rats, respectively. All animals were treated according to the guidelines for care and use of laboratory animals with the approval of the Institutional Ethics Committee of the ICCBS, University of Karachi, Pakistan.
Collection of blood and tissue for biochemical parameters and immunohistochemistry and morphometry
Rats were killed after 28 days of treatment; serum was collected from venous blood, aliquoted and kept at −80 °C for biochemical assays. After collection of blood samples, liver was processed for homogenization, and pancreata were processed for immunohistochemistry. Serum insulin was measured using rat ELISA kit, and total antioxidant status, serum creatinine, ALT and AST were measured as described previously [25]. TBARS and GSH from liver homogenate were measured spectrophotometrically by using the method described by Noyan et al. [26] and Ohkawa et al. [27] for TBARS estimation and Chandra et al. [28] for GSH. Insulin and glucagon immunostaining and morphometry were performed as described previously [24, 25].
Oral glucose tolerance test with EA in diabetic rats
Type 2 diabetic rats (n = 30) were divided into five groups (6 rats each group). Group I received only distilled water (Db); Group II received 5 mg/kg glibenclamide (GB); Group III received EA at a dose of 25 mg/kg (EA-25); Group IV received EA at a dose of 50 mg/kg (EA-50); Group V received EA at a dose of 100 mg/kg (EA-100). EA was given orally in 1 ml of water suspension to the diabetic rats.
All diabetic rats were fasted overnight (14-h) prior to OGTT. Sixty minutes following EA administration, 3 g/kg glucose load was given orally to each rat. Blood glucose levels of all rats were measured at -60, 0, 45, 60, and 120 min after feeding.
Isolation of islets from mouse and insulin secretion assay
Isolation of islets and insulin secretion assay was performed as described previously [29, 30]. In brief, batches of islets (n = 5) were incubated for 60 min in KRB buffer solution with 3 mmol/l (basal) or 16.7 mmol/l (stimulatory) glucose supplemented with EA in different concentrations. After incubation, 100 μl aliquots were taken from each tube and insulin secretion was measured by mouse insulin ELISA kit. Insulin concentrations were normalized for the number of islets.
Toxicity evaluation
The acute toxicity of EO extract (if any) was tested in rats by oral administration of EO extract at different doses (0.5, 1.0, 1.5, and 2.0 g/kg body weight) to different groups of Wistar rats (n = 3) of either sex as a single treatment. Following extract administration, animals’ mortality and general behaviors were observed for 72 h, intermittently. No mortality was found up to a dose of 2.0 g/kg body weight (data not shown). After 28 days of extract treatment serum creatinine, ALT and AST were also assayed to check the toxicity to kidney and liver, respectively. EA was evaluated for its cytotoxicity in pancreatic MIN6 cells, and no toxic effects were observed (IC50 > 200 μmol).
Statistical analysis
SPSS 19.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used to perform statistical analyses. Values were expressed as mean ± standard error. The significance of the differences among the mean values was calculated using one-way ANOVA and repeated ANOVA as appropriate. p < 0.05 was considered statistically significant.
Results
Standardization of methanolic extract of E. officinalis
First we standardized the methanolic extract of EO by RP-HPLC using EA as a standard. Figure 1 shows chromatograms of EO extract. EO extract showed the presence of several peaks eluted at various retention times of 4.3, 5.1, 6.2, 8.1, 10.5, 12.3, and 13.6 min. The peak of EA appeared as a major peak at retention time of 13.6 min in the EO chromatogram. The amount of EA present in EO extract was ~1.3 % (w/w).
Emblica officinalis extract lowers fasting blood glucose in non-obese type 2 diabetic rats
We analyzed the effect of EO supplementation on FBG in model diabetic rats (Table 1). In diabetic rats, FBG was increased moderately after 3 months of STZ induction when compared to control rats (8.3 ± 0.22 vs. 4.2 ± 0.23 mmol/l). Very little changes in FBG were observed in control or untreated diabetic rats during the study periods (1–29 days). Interestingly, EO extract treatment resulted in a significant decrease in the FBG in dose- and time-dependent manner. At 250 mg/kg dose, the blood glucose level was significantly (p < 0.001) decreased when compared to untreated diabetic rats at the end of the experiment on day 29 (6.5 ± 0.17 vs. 9.2 ± 0.30 mmol/l). At the 500 mg/kg dose, blood glucose level decreased even more efficiently compared to the 250 mg/kg dose. This 500 mg/kg dose significantly (p < 0.001) decreased FBG levels compared with untreated diabetic rats (5.4 ± 0.16 vs. 9.2 ± 0.30 mmol/l). After treatment with Gb, blood glucose level was 5.5 ± 0.31 mmol/l on day 29. Therefore, EO extract showed dose-dependent anti-diabetic activity in the non-obese type 2 diabetic rats, and the 500 mg/kg dose was found to be comparable to the standard drug Gb.
When we compared the values of the blood glucose of EO-treated rats with their initial values (day 1), we found that both the doses of EO extract resulted in significant decrease in the FBG in a time-dependent manner. The blood glucose of EO250 rats significantly (p < 0.01) decreased on day 15 (7.7 ± 0.11 mmol/l) as well as on day 29 (6.5 ± 0.17 mmol/l) with respect to their value of day 1 (8.5 ± 0.21 mmol/l). The blood glucose of EO500 rats also significantly (p < 0.001) decreased on day 15 (6.50 ± 0.22 mmol/l) as well as on day 29 (5.4 ± 0.16 mmol/l) with respect to their value on day 1 (8.3 ± 0.22 mmol/l). Treatment with Gb also significantly (p < 0.001) lowered blood glucose level in a time-dependent manner.
Emblica officinalis extract increases fasting serum insulin and β-cell function in type 2 diabetic rats
We analyzed the effect of EO supplementation on serum insulin in model diabetic rats and found that serum insulin decreased significantly (p < 0.001) in diabetic rats as compared to non-diabetic control (41.2 ± 6.8 pmol/l v. 132.4 ± 13.7 pmol/l, Table 1). A significant (p < 0.05) increase in serum insulin (61.9 ± 8.6 pmol/l) was found in diabetic rats when they were treated with EO250 extract for 29 days. EO500 showed more significant (p < 0.01) increase in serum insulin (89.4 ± 8.6 pmol/l) which was comparable to Gb (91.1 ± 12.7 pmol/l).
Insulin-to-glucose ratio, an index of β-cell function [31], was dramatically decreased (p < 0.001) in non-obese diabetic rats compared with control (4.4 ± 1.2 vs. 30.1 ± 2.7 pmol/mmol, Table 1). However, EO treatment increased insulin-to-glucose ratio in a dose-dependent manner. Insulin-to-glucose ratio was increased to (9.1 ± 1.2 pmol/mmol, p < 0.05) and (16.4 ± 1.8 pmol/mmol, p < 0.01) in EO250- and EO500-treated rats, respectively. The increased insulin-to-glucose ratio caused by EO might be due to its modulation of pancreatic β-cells.
Emblica officinalis modulates pancreatic β-cells in type 2 diabetic rats
We performed immunohistochemistry in order to analyze the effects of EO administration on β-cell morphology and morphometry. In the control rats, α- and β-cells were evenly distributed throughout the islets; β-cells were abundant and mostly in the core of islets, whereas α-cells were found around the periphery of β-cells (Fig. 2a). In diabetic rats, both qualitative and quantitative deterioration of pancreatic β-cells was observed (Fig. 2b). β-cells were reduced to about one-fourth in the diabetic rats compared with the control. In EO250-treated rats a marginal increase in β-cell area was observed (Fig. 2c). However, in the EO500-treated rats, β-cell area was increased to almost double as compared to the untreated diabetic β-cell area (Fig. 2d). Moreover, the distribution patterns of α- and β-cells were very similar to that of the control. As expected, the Gb-treated rats also showed increased total β-cell area and number (Fig. 2e). The distribution pattern of α-cells in diabetic rats was mostly similar to that of control (Fig. 2a, b); however, in some cases α-cells were also seen scattered within the center of the islets (data not shown). Interestingly, there was a relative increase in the number of α-cells relatively increased in the diabetic rats. The EO extract had no effect on the distribution of α-cells in the diabetic rats (Fig. 2c, d).
With high-resolution immunohistochemistry, we observed widely dispersed, bright and distinctly granulated insulin granules inside the control non-diabetic β-cells (Fig. 3a). In the diabetic rats, however, the distinction and brightness of the insulin granules were markedly reduced and the β-cells appeared cloudy (Fig. 3b). In the EO250 group, insulin granulation was more distinct and brighter than that of diabetic β-cells but still less than that of control non-diabetic β-cells (Fig. 3c). In the EO500 group, insulin granules were even more distinct and brighter than the EO250 group and appeared almost similar to that of the control non-diabetic β-cells (Fig. 3d). Similar distinct and bright insulin granules were also seen in the case of Gb-treated β-cells (Fig. 3e).
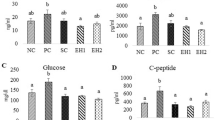
Morphometric studies showed that in control rats the total β-cells area per islet was 19,506 ± 1634 µm2, whereas in diabetic rats it was decreased (6522 ± 712 µm2) significantly (p < 0.001, Fig. 4a). The β-cells area was 8485 ± 469 µm2 and 10,525 ± 989 µm2 in EO250- and EO500-treated rats, respectively. In control rats, the number of β-cells per islet was 103.5 ± 9.2 (Fig. 4b). However, their number decreased significantly (p < 0.001) in the diabetic rats (29.9 ± 4.0). As compared to the diabetic rats, there was a significant (p < 0.05) increase in the number of β-cell in the EO500-treated rats (49.2 ± 5.8), but not in the EO250-treated rats (37.3 ± 4.1) (Fig. 4b). Although β-cells area and number were decreased significantly in the untreated diabetic rats, very interestingly the individual β-cells size was increased significantly (p < 0.05) in the diabetic rats (290.2 ± 29.1 µm2) compared with control rats (199.3 ± 15.7 µm2, Fig. 4c). This data suggest that hypertrophy of β-cells may occur in this non-obese type 2 diabetic rat model as a compensatory mechanism. The individual β-cell size was also increased significantly (p < 0.05) in EO250-treated rats (265.3 ± 14.7 µm2) compared with control rats (199.3 ± 15.7 µm2). There was no significant change of individual β-cell size in the EO500- or Gb-treated rats. These immunohistochemical and morphometric analyses suggest that EO may modulate β-cell morphology in non-obese diabetic rats by qualitative and quantitative improvement of pancreatic β-cells.
Morphometric analysis of β-cell area (a); β-cell number (b); and β-cell size (c) in pancreatic islets of the experimental rats. All values are expressed as mean ± SEM. Results represent the average from 6 to 8 rats with an average of 60–100 fields per rat. Bars that do not share a common notation are significantly different at p < 0.05
Emblica officinalis extract increased plasma antioxidant status and liver GSH level and decreased liver TBARS level in the type 2 diabetic rats
In vitro DPPH assay showed 77 % radical scavenging activity of the EO extract (data not shown). In diabetic rats, plasma total antioxidant (0.99 ± 0.04 mmol/l) was decreased significantly (p < 0.01) when compared to control rats (1.79 ± 0.15 mmol/l, Table 2). Only a moderate increment was found with EO250-treated rats (1.42 ± 0.08 mmol/l); however, more significant increment (p < 0.001) was found with EO500-treated rats (1.89 ± 0.10 mmol/l). These data suggest that EO can enhance antioxidant status in non-obese type 2 diabetic rats.
Among the antioxidant markers, GSH and TBARS levels from the liver tissue was determined. GSH levels were decreased significantly (p < 0.001) in diabetic rats compared to control rats (60.6 ± 2.9 vs. 100.0 ± 7.1 %). EO250-treated rats showed moderately increased GSH levels (70.7 ± 5.8 %), whereas significant (p < 0.001) increase was found with EO500-treated rats (81.4 ± 8.8 %). These data suggest that EO can increase antioxidant defense status in type 2 diabetic rats.
Liver TBARS levels were increased significantly (p < 0.01) in diabetic rats (310.2 ± 21.2 %) compared with control rats (100.0 ± 5.1 %, Table 2). A moderate decrease was found in EO250-treated rats (242.5 ± 17.9 %); however, more decrease was found in EO500-treated rats (225.7 ± 14.1 mmol/l). These data suggest that EO can decrease lipid peroxidation in type 2 diabetic rats.
EA decreases glucose intolerance in non-obese type 2 diabetic rats
EA was found as a major compound present in EO extract. Therefore, we performed OGTT with EA in non-obese type 2 diabetic rats. OGTT revealed that blood glucose attained peak level at 45 min after glucose load in all rats (Fig. 5). EA, at 25 mg/kg dose, had little to no effects on glucose tolerance. Interestingly, 50 and 100 mg/kg doses of EA decreased glucose intolerance significantly (p < 0.05) at 45 min as compared to the vehicle. A maximum decrease of 23.2 % was observed with 100 mg/kg of EA at 45 min which is similar to that of standard drug Gb.
Effect of ellagic acid on glucose tolerance in type 2 diabetic rats. All values are expressed as mean ± SEM (n = 6). * p < 0.05 versus Db. Con age-matched non-diabetic control, Db diabetic; EA-25 EA-50, and EA-100 diabetic rats treated with 25, 50, and 100 mg/kg of ellagic acid, respectively, Gb glibenclamide
EA stimulates glucose-induced insulin secretion in isolated islets
The insulin secretion activity of EA was assessed on isolated mice islets. At basal glucose (3 mmol/l), EA with 50–200 μmol/l concentrations had little to no effects on insulin secretion (Table 3). At stimulatory glucose (16.7 mmol/l), 50 μmol/l EA slightly enhanced insulin secretion which was not to the level of significance. Interestingly, 100 μmol/l EA significantly (p < 0.01) increased insulin secretion (5.78 ± 0.56 ng/islet/hour) compared with insulin secretion by 16.7 mmol/l glucose alone (2.08 ± 0.08 ng/islet/hour). No further significant change was observed above the 100 μmol/l dose of EA.
Discussion
Inappropriate and/or disproportionate food intake is related to metabolic disorders such as diabetes, where carbohydrate, protein, and fat metabolism become impaired. Lack of vitamins and minerals intake also exaggerates diabetes. Accumulating evidences of clinical trials demonstrate the effectiveness of diet and lifestyle modification for the management of type 2 diabetes [1]. EO is rich in vitamin C and polyphenolic compounds and is being taken as a fruit in many parts of the world and may be a suitable candidate as DAs for the management of type 2 diabetes.
Our neonatal STZ-induced diabetic rats developed mild hyperglycemia and moderate hypoinsulinemia (Table 1) being similar in features resembling non-obese type 2 diabetes in Asian subjects [19]. It is increasingly recognized that type 2 diabetes in Asian patients has a different pattern to non-Asian patients [19, 20] which is substantially impaired β-cell function and insulin secretion deficiency. ‘Asian Diabetes’ term has been coined in ‘Kyoto Declaration’ which states that diabetes in Asia is mostly non-obese type with a dysfunction of insulin secretion [19]. The rationale for neonatal STZ-induced non-obese type 2 diabetic rat model is that DAs can only be effective in type 2 diabetes where pancreatic β-cells have been partially damaged and mild to moderate hypoinsulinemia exist.
In EO-treated diabetic rats, blood glucose decreased significantly in a dose- and time-dependent manner (Table 1). The effects of EO500 dose were comparable with that of the standard drug, Gb. Therefore, it may be inferred that EO500 dose is working by increasing insulin secretion from pancreatic β-cells. These hypotheses were tested experimentally.
Serum insulin level was decreased significantly in the diabetic rats (Table 1). The decreased insulin levels may be due to the impairment of β-cell function as decreased β-cell function (Table 1) was found in our diabetic model rats. Interestingly, EO treatment significantly increased the serum insulin level as well as β-cell function (Table 1). The increase in serum insulin levels may be related to the up-regulation of insulin synthesis or enhanced insulin secretion from pancreatic β-cells. In vitro insulin secretory assay showed that EA has insulin secretory activity (Table 3), suggesting that increased serum insulin levels are most probably due to insulin secretion from pancreatic β-cells.
Immunohistochemical data from diabetic rats suggest that EO increased both number and size of the pancreatic β-cells (Figs. 2, 3, 4). In EO250-treated rats β-cells hypertrophy (Figs. 2c, 4c) may be due to the compensatory mechanism for increased insulin demand and/or existing β-cell maturation. In EO500-treated rats, hyperplasia of β-cell was found (Figs. 2d, 4b) which may be due to β-cell regeneration, neogenesis, or decreased apoptosis. The results demonstrated that two different doses of EO work by two different mechanisms, i.e., EO250 by increasing the β-cell size and EO500 by increasing the β-cell number. The reason for the discrepancies between these two doses will need to be studied further in the future.
In diabetic rats, decreased insulin granules inside β-cell reflect lowered intracellular glucose (Fig. 3). Though in diabetic rats high blood glucose (extracellular) was observed, less glucose is actually available inside the cells resulting in decreased ratio of intracellular-to-extracellular glucose which inhibits insulin release. The treatment with EO extract showed increased insulin granules which may be due to increased ratio of intracellular-to-extracellular glucose. These findings correlate well with our serum (extracellular) insulin-to-glucose ratio data (Table 1; Fig. 3).
Diabetes is also associated with free radical formation [32]. Free radicals can lead to hyperglycemia, increased tissue peroxidation, and inadequate antioxidant defense mechanism [33]. Knowing the importance of free radical scavenging, the antioxidant potential of EO involved in its anti-diabetic effect was further investigated. Using an in vitro DPPH radical scavenging assay, we found 77 % radical scavenging activity (data not shown) with EO extract. In diabetic rats, plasma total antioxidant status was enhanced after EO treatment. EO is well known for its rich polyphenolic compounds which may be responsible for its antioxidant activity. The enhanced antioxidant status offered by EO in the diabetic rats may be providing additional antioxidant defense for the β-cells or protecting from oxidative damage and apoptosis to the β-cells which ultimately will help in β-cell’s functional improvement. In diabetes, lipid peroxidation increases that result in the loss of cell membrane integrity, resulting in impairment of function. GSH is one of the players of body defense function. Loss of GSH levels in diabetes makes the body susceptible to be damaged and distorted by the free radicals that are formed in the body which eventually raised in the diseased state.
Our methanolic extract of EO HPLC profiling (Fig. 1) revealed that EA is one of the major components of the EO extract. Luo et al. [34] also found similar results. EA (the dilactone of hexahydroxydiphenic acid) is phenol in nature, and its phenolic nature enabled it to be eluted in methanol phase like other phenols and flavonoids [35–38]. Other flavonoids, phenols, and tannins like emblicanins, vitamin C are yet in small fractions (Fig. 1) that are present in the methanolic extract of EO may contribute to improve the activity of this extract. Thus EO extract containing EA and other phenols and flavonoids may be synergistically acting as antioxidant and exerting its protective effects on pancreas. Further studies are needed to test this hypothesis.
In this study, elevation in glucose tolerance by EA was found which suggests that EA probably works by stimulating insulin secretion from pancreatic β-cells. In our in vitro insulin secretory assay, we found that EA-stimulated insulin secretion from isolated mice islets is in good agreement with our in vivo glucose tolerance data. EA significantly stimulated insulin secretion from isolated mice islets, suggesting a possible blood glucose-lowering mechanism. In in vitro DPPH radical scavenging assay, EA showed 92.0 % radical scavenging activity comparable to radical scavenging activity by gallic acid (93.1 %, data not shown). This strong antioxidant activity of EA may be effective in preventing or delaying diabetes by providing antioxidant defense to the pancreatic β-cells, as β-cells are especially vulnerable to oxidative stress.
In the above context, it can be said that EA present in EO exerts anti-diabetic activity through the action on β-cells of pancreas resulting in an increase in β-cell size and number, increasing antioxidant status, decreasing blood glucose, increasing serum insulin, and β-cell morphology and morphometry. Its low cost, availability, affordability, and safety profile make it a relatively low-risk alternative to traditional glucose-lowering medication and a strong candidate for diabetic drug research.
References
Hu FB (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34(6):1249–1257. doi:10.2337/dc11-0442
Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71:1397–1421. doi:10.1016/j.bcp.2006.02.009
Ayyanar M, Sankarasivaraman K, Ignacimuthu S (2008) Traditional herbal medicines used for the treatment of diabetes among two major tribal groups in south Tamil Nadu, India. Ethnobot Leafl 12:276–280. doi:10.1016/j.fitote.2008.06.003
Khan KH (2009) Roles of Emblica officinalis in medicine—a review. Bot Res Int 2(4):218–228
Mirunalini S, Krishnaveni M (2010) Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J Basic Clin Physiol Pharmacol 21(1):93–105. doi:10.1515/JBCPP.2010.21.1.93
D’souza JJ, D’souza PP, Fazal F, Kumar A, Bhat HP, Baliga MS (2014) Anti-diabetic effects of the Indian indigenous fruit Emblica officinalis Gaertn: active constituents and modes of action. Food Funct 5:635–644. doi:10.1039/C3FO60366K
Akhtar MS, Ramzan A, Ali A, Ahmad M (2011) Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int J Food Sci Nutr 62:609–616. doi:10.3109/09637486.2011.560565
Tiwari V, Kuhad A, Chopra K (2011) Emblica officinalis corrects functional, biochemical and molecular deficits in experimental diabetic neuropathy by targeting the oxido-nitrosative stress mediated inflammatory cascade. Phytother Res 25:1527–1536. doi:10.1002/ptr.3440
Patel SS, Goyal RK, Shah RS, Tirgar PR, Jadav PD (2013) Experimental study on effect of hydroalcoholic extract of Emblica officinalis fruits on glucose homeostasis and metabolic parameters. AYU 34:440. doi:10.4103/0974-8520.127731
Ansari A, Shahriar MSZ, Hassan MM, Das SR, Rokeya B, Haque MA, Sarkar T (2014) Emblica officinalis improves glycemic status and oxidative stress in STZ induced type 2 diabetic model rats. Asian Pac J Trop Med 7:21–25. doi:10.1016/S1995-7645(13)60185-6
Pozharitskaya ON, Ivanova SA, Shikov AN, Makarov VG (2007) Separation and evaluation of free radical-scavenging activity of phenol components of Emblica officinalis extract by using an HPTLC–DPPH method. J Sep Sci 30:1250–1254. doi:10.1002/jssc.200600532
Amakura Y, Okada M, Tsuji S, Tonogai Y (2000) High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. J Chromatogr A 896(1):87–93. doi:10.1016/S0021-9673(00)00414-3
Malini P, Kanchana G, Rajadurai M (2011) Antidiabetic efficacy of ellagic acid in streptozotocin induced diabetes mellitus in albino Wistar rats. Asian J Pharm Clin Res 4:124–128
Vattem DA, Shetty K (2005) Biological functionality of ellagic acid: a review. J Food Biochem 29(3):234–266. doi:10.1111/j.1745-4514.2005.00031.x
Farzaneh V, Carvalho IS (2015) A review of the health benefit potentials of herbal plant infusions and their mechanism of actions. Ind Crop Prod 65:247–258. doi:10.1016/j.indcrop.2014.10.057
Kahn SE (2003) The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46:3–19
Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V (2013) Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci 1281(1):51–63. doi:10.1111/j.1749-6632.2012.06838.x
Ma RC, Chan JC (2013) Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 1281(1):64–91. doi:10.1111/nyas.12098
Asian Association for the Study of Diabetes (2013) Promoting research for better diabetes care in Asia: Kyoto declaration on diabetes. J Diabetes Investig 4:222–224. doi:10.1111/jdi.12073
Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L (2014) The many faces of diabetes: a disease with increasing heterogeneity. Lancet 383:1084–1094
Portha B, Blondel O, Serradas P et al (1989) The rat models of non-insulin dependent diabetes induced by neonatal streptozotocin. Diabete Metab 15:61–75
Arulmozhi DK, Veeranjaneyulu A, Bodhankar SL (2004) Neonatal streptozotocin-induced rat model of type 2 diabetes mellitus: A glance. Indian J Pharmacol 36:217–221
Portha B, Movassat J, Cuzin-Tourrel C et al (2007) Neonatally streptozotocin-induced (n-STZ) diabetic rats: a family of type 2 diabetes models. In: Shafrir E (ed) Animal models of diabetes, 2nd edn. CRC Press, Boca Raton, pp 223–250
Hafizur RM, Kabir N, Chishti S (2011) Modulation of pancreatic β-cells in neonatally streptozotocin-induced type 2 diabetic rats by the ethanolic extract of Momordica charantia fruit pulp. Nat Prod Res 25:353–367. doi:10.1080/14786411003766904
Hafizur RM, Kabir N, Chishti S (2012) Asparagus officinalis extract controls blood glucose by improving insulin secretion and β-cell function in streptozotocin-induced type 2 diabetic rats. Br J Nutr 108:1586–1595. doi:10.1017/S0007114511007148
Noyan T, Balaharoğlu R, Kömüroğlu U (2005) The oxidant and antioxidant effects of 25-hydroxyvitamin D3 in liver, kidney and heart tissues of diabetic rats. Clin Exp Med 5:31–36
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Chandra D, Ramana KV, Wang L, Christensen BN, Bhatnagar A, Srivastava SK (2002) Inhibition of fiber cell globulization and hyperglycemia-induced lens opacification by aminopeptidase inhibitor bestatin. Invest Ophthalmol Vis Sci 43:2285–2292
Siddiqui BS, Hasan M, Mairaj F, Mehmood I, Hafizur RM, Hameed A, Khan Shinwari Z (2014) Two new compounds from the aerial parts of Bergenia himalaica Boriss and their anti-hyperglycemic effect in streptozotocin-nicotinamide induced diabetic rats. J Ethnopharmacol 152(3):561–567. doi:10.1016/j.jep.2014.02.002
Hafizur RM, Hameed A, Shukrana M, Raza SA, Chishti S, Kabir N, Siddiqui RA (2015) Cinnamic acid exerts anti-diabetic activity by improving glucose tolerance in vivo and by stimulating insulin secretion in vitro. Phytomedicine 22(2):297–300. doi:10.1016/j.phymed.2015.01.003
Hanson RL, Pratley RE, Bogardus C, Narayan KV, Roumain JM, Imperatore G, Knowler WC (2000) Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemioiogic studies. Am J Epidemiol 151:190–198
Fujita K, Nishizawa H, Funahashi T, Shimomura I, Shimabukuro M (2006) Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ J 70:1437–1442. doi:10.1253/circj.70.1437
Vincent HK, Taylor AG (2005) Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 30:400–418. doi:10.1038/sj.ijo.0803177
Luo W, Zhao M, Yang B, Shen G, Rao G (2009) Identification of bioactive compounds in Phyllanthus emblica L. fruit and their free radical scavenging activities. Food Chem 114(2):499–504. doi:10.1016/j.foodchem.2008.09.077
Hincapié CA, Monsalve Z, Seigler DS, Alarcón J, Cespedes CL (2011) Antioxidant activity of Blechnum chilense (Kaulf.) Mett., Curcuma domestica Valeton and Tagetes verticillata Lag. & Rodriguez. Boletín latinoamericano y del Caribe de plantas medicinales y aromáticas 10:315–324
Saeed MK, Deng Y, Dai R, Li W, Yu Y, Iqbal Z (2010) Appraisal of antinociceptive and anti-inflammatory potential of extract and fractions from the leaves of Torreya grandis Fort Ex. Lindl. J Ethnopharmacol 127:414–418
Prasad KN, Hao J, Yi C, Zhang D, Qiu S, Jiang Y et al (2009) Antioxidant and anticancer activities of wampee [Clausena lansium (Lour.) Skeels] peel. BioMed Res Int 2009. doi:10.1155/2009/612805
Roome T, Dar A, Ali S, Naqvi S, Choudhary MI (2008) A study on antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective actions of Aegiceras corniculatum (stem) extracts. J Ethnopharmacol 118:514–521
Acknowledgments
This work was supported by a HEC Grant (No. 20-1963/R&D/10/9164) to Md. Hafizur Rahman from the Higher Education Commission (HEC), Pakistan. We are thankful to the Asian Network of Research on Antidiabetic Plants (ANRAP) for financial support to Abdul Hameed for a visit to BIRDEM, Dhaka, Bangladesh. We highly acknowledge Sidra Chisti for skillful technical assistance in animal handling, feeding extract and blood glucose estimation. We would like to show our gratitude to Prof. Dr. Rafat Ali Siddiqui (Department of Medicine, Indiana University School of Medicine, Indianapolis, Indiana, USA) and Dr. Shahidul Islam (Department of Molecular, Cellular and Developmental Biology, Yale University, USA) for critical reading of the manuscript and their comments.
Authors’ contributions
R M. H. and N. F. designed the research. R. M. H. and N. F. conducted the research, analyzed the data and also wrote the manuscript. R. M. H. analyzed the immunohistochemical data. S. A., M. N., and N. F. were involved in HPLC analysis. A. H. was involved in islet isolation and insulin secretion assay. N. K. was involved in technical support for microscopic analysis and morphometry and writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fatima, N., Hafizur, R.M., Hameed, A. et al. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur J Nutr 56, 591–601 (2017). https://doi.org/10.1007/s00394-015-1103-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1103-y