Abstract
Purpose
Glutathione (GSH), the most abundant endogenous antioxidant, is a critical regulator of oxidative stress and immune function. While oral GSH has been shown to be bioavailable in laboratory animal models, its efficacy in humans has not been established. Our objective was to determine the long-term effectiveness of oral GSH supplementation on body stores of GSH in healthy adults.
Methods
A 6-month randomized, double-blinded, placebo-controlled trial of oral GSH (250 or 1,000 mg/day) on GSH levels in blood, erythrocytes, plasma, lymphocytes and exfoliated buccal mucosal cells was conducted in 54 non-smoking adults. Secondary outcomes on a subset of subjects included a battery of immune markers.
Results
GSH levels in blood increased after 1, 3 and 6 months versus baseline at both doses. At 6 months, mean GSH levels increased 30–35 % in erythrocytes, plasma and lymphocytes and 260 % in buccal cells in the high-dose group (P < 0.05). GSH levels increased 17 and 29 % in blood and erythrocytes, respectively, in the low-dose group (P < 0.05). In most cases, the increases were dose and time dependent, and levels returned to baseline after a 1-month washout period. A reduction in oxidative stress in both GSH dose groups was indicated by decreases in the oxidized to reduced glutathione ratio in whole blood after 6 months. Natural killer cytotoxicity increased >twofold in the high-dose group versus placebo (P < 0.05) at 3 months.
Conclusions
These findings show, for the first time, that daily consumption of GSH supplements was effective at increasing body compartment stores of GSH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutathione (GSH) is the major endogenous intracellular antioxidant. It has numerous functions including protecting cells against oxidative stress, detoxification of toxins and carcinogens, posttranslational regulation of protein function and maintenance of immune function [1–5]. Nearly, all tissues in the body synthesize GSH by sequential addition of the precursor amino acids, cysteine (Cys), glutamic acid (Glu) and glycine (Gly) through enzymatic catalysis by two ATP-dependent enzymes, glutamine cysteine ligase (GCL) and GSH synthetase (GS) [6, 7]. The maintenance of tissue levels of GSH is critical for maintaining health, preventing diseases and age-related biological insults. Even partial GSH depletion impairs immune function [8] and increases susceptibility to a wide range of xenobiotics [9] and oxidative damage [10]. Low GSH levels are associated with increased risks of numerous diseases including cancer [11], cardiovascular diseases, arthritis and diabetes [12, 13].
There is a wide range of inter-individual variability in blood and tissue GSH levels, and low levels can be associated with exposure to oxidants/drugs/toxins, poor nutrition and other factors. GSH levels are also dependent on the availability of its precursor amino acids, Cys, Glu and Gly, with Cys most often considered as rate limiting. Consequently, intracellular GSH levels can be depleted in certain tissues including liver by short periods of fasting such as that which occurs overnight [14, 15].
Increasing GSH represents a potentially important approach to counteract disorders associated with GSH depletion, enhance detoxification capacity and protect against disease. Oral GSH supplementation represents one such strategy for enhancing tissue GSH levels. The use of oral GSH is supported by studies linking high dietary GSH intake with high blood levels and reduced risk of cancer [16, 17]. Studies in animal models have shown that oral GSH, administered either in the diet or by gavage, increases plasma and tissue GSH levels [18–23] and protects against aging-related impairments in immune function [24, 25], influenza infections and cancer [26–29]. These effects of oral GSH have been accounted for, in part, by the direct absorption and transport of intact GSH in the small intestine [19, 21].
There is less data on the bioavailability of oral GSH in humans. While GSH was found to be absorbed and transported in human intestinal epithelial cells in vitro [30] and in buccal mucosal cells in vivo [23], results from a clinical study of oral GSH, administered as a single dose (150 μmol/kg) to 7 healthy adults, showed no significant effect on plasma GSH levels during a 4.5 h period [31]. However, the rapid turnover of GSH in human plasma would likely make it difficult to detect an increase in plasma after a single oral dose. Thus, our current objectives were to determine the long-term effects of daily oral GSH supplementation on GSH levels in different body stores. We conducted a randomized, double-blinded, placebo-controlled trial of oral GSH at two doses, 250 and 1,000 mg/day, administered for 6 months in healthy adults on the levels of GSH in different blood compartments and exfoliated buccal mucosal cells. GSH oxidation products, GSH disulfide (GSSG) and GSH protein mixed disulfides (GSSP), are commonly used biomarkers of oxidative stress [32]; thus, we also examined the effects of oral GSH on GSSG/GSH and GSSP/GSH ratios in blood. Since intracellular GSH plays a key role in the maintenance and regulation of certain immunological functions [33, 34] including the activation of lymphocytes and functional activity of NK cells [35–37], secondary endpoint analysis included the assessment of hematologic measurements of immune function including neutrophil phagocytosis, neutrophil respiratory burst, lymphocyte proliferation and natural killer (NK) cell cytotoxicity in a subset of subjects.
Materials and methods
Study protocol
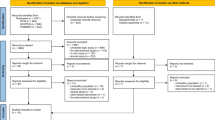
The study (ClinicalTrials.gov identifier: NCT01044277) was approved by the Institutional Review Board of the Penn State College of Medicine in accordance with the Helsinki Declaration of 1975 as revised in 1983. The study design is summarized in the CONSORT form (Fig. 1). All clinical activities were conducted at the Penn State Hershey Cancer Institute, Hershey, PA. Healthy subjects were recruited by the study coordinator from the local Hershey/Harrisburg, PA area using fliers, newspaper and radio advertisements, online announcements and word of mouth. Interested individuals were prescreened by telephone to assess potential eligibility. Individuals who passed the initial screening were asked to visit the clinic where, after signing the informed consent, they were screened for eligibility based upon the following criteria: Healthy male and female non-smokers, 30–79 years of age, not taking antioxidant supplements for at least 1 month. Eligible subjects were randomly assigned to one of three treatment groups with equal probability: 250 mg GSH per day orally (provided as two 125 mg capsules); 1,000 mg GSH per day orally (provided as two 500 mg capsules); and placebo (provided as two capsules). Glutathione (Setria®) was provided by Kyowa Hakko USA, Inc., and capsules were formulated as follows: GSH 125 mg (125 mg GSH, 360 mg cellulose); GSH 500 mg (500 mg GSH, 15 mg cellulose); and placebo (470 mg cellulose). Both subjects and investigators were blinded to the group assignment. At baseline, trained nurse interviewers administered a structured questionnaire to each subject to collect information on demographics, occupation, lifestyle habits, medical history, usage of medication and dietary supplements, alcohol consumption and past cigarette smoking history.
University pharmacists dispensed either active supplements or placebo at the baseline visit. Subjects were provided instructions for supplement usage and instructed to continue taking the capsules for 6 months, maintain a daily pill diary and return all unused capsules. There was a 1-month washout period between 6 and 7 months where subjects discontinued their supplementation to assess the reversibility of any GSH-related changes. After 1, 3 and 6 months, subjects returned to the clinic to return their unused capsules and, at the 1- and 3-month time points, to receive new capsules. At baseline and after 1, 3, 6 and 7 months, biological samples including blood, urine and exfoliated buccal mucosal cells were collected and processed as described below. Outcome measures included GSH levels in whole blood, erythrocytes, plasma and lymphocytes at baseline and 1, 3, 6 and 7 months and in exfoliated buccal mucosal cells at baseline and 3, 6 and 7 months. Secondary outcomes included immune function activities including NK cell cytotoxicity and lymphocyte proliferation at baseline and 3 months and respiratory burst and neutrophil phagocytosis at baseline and 3 and 6 months.
Subjects
The clinical phase of the study was conducted from February, 2010, to September, 2011, over which time, 61 subjects were enrolled (Fig. 1). A total of 60 received intervention, of which 6 dropped out for reasons including relocation (n = 1), enrollment in competing trials (n = 2) and reports of adverse effects (n = 2, both in the placebo group) and one was dropped for lack of compliance. A total of 54 completed at least 6 months of the study protocol and were included in the analyses. The characteristics of these 54 participants are summarized in Table 1 including, age, sex, race and BMI. All subjects were non-smokers (having not smoked for >1 year) and had no history of chronic disease or antioxidant or GSH supplement usage within the past month. Most subjects were female (76 %), white (92 %) and ranged in age from 28 to 72 years (mean = 46.6 years). Fifteen of subjects had a BMI > 30 kg/m2. There were no significant differences in subject characteristics by treatment group. Compliance was assessed by pill count and daily pill diary entries.
Collection and processing of biological samples
Exfoliated buccal mucosal cells were obtained after a mouth rinse with distilled water and brushing of the cheeks and gums with a soft tooth brush. Subjects then rinsed with 20 ml of saline, which was collected and stored at 4 °C until centrifugation (6,000×g for 10 min) on-site within 1 h after collection. Cells were washed three times with saline, and packed cells were stored at −80° C until analysis.
Blood samples were collected between 9:00 am and 1:00 pm from an antecubital vein into three tubes containing sodium heparin as an anticoagulant and immediately placed on ice. Tubes were mixed by gentle shaking, and a 2.5-ml aliquot of whole blood was removed for analysis of neutrophil phagocytosis and respiratory burst (see below). Two 0.5-ml aliquots of whole blood were removed and stored at −80 °C for future analyses. The remaining blood was centrifuged for 10 min at 1,300×g to obtain plasma, buffy coat and red cell fractions. Multiple 0.5-ml aliquots of plasma were placed into 1.5-ml cryovials and immediately frozen at −80 °C. Packed red cells were washed three times in saline, aliquoted (0.5 ml each) into multiple cryovials and frozen at −80 °C. Buffy coat fractions were combined, and lymphocytes were isolated by Ficoll–Hypaque density gradient centrifugation. In brief, after addition of 3 ml of Ficoll, buffy coats were centrifuged at 400g for 30 min at 19 °C. Lymphocyte layers were removed and washed two times in PBS, followed by centrifugation at 250g for 10 min. After the final wash, lymphocytes were re-suspended in 5 ml PBS. Cell number was assessed after addition of 40 μl trypan blue to 10 μl of cell suspension using a hemocytometer. Cells were re-suspended in 95 % FBS, 5 % DMSO at concentrations of 2.5 × 106 cells/ml, frozen at −80 °C and stored in liquid nitrogen until analysis of GSH, lymphocyte proliferation or NK cell cytotoxicity (see below). Buccal cells were mixed with an equal volume of PBS and centrifuged at 5,000g for 2 min. Cells were kept on ice until acid extraction as described below.
Analytical procedures
Glutathione
The synthesis and regulation of GSH can vary in different cells and tissues. Consequently, we measured GSH in a variety of compartments including whole blood, erythrocytes, plasma, lymphocytes and exfoliated buccal mucosal cells. For whole blood or red cells, 0.8 ml of 5 % (w/v) metaphosphoric acid (MPA) was added to 0.2 ml of blood or packed cells. Samples were centrifuged for 2 min at 14,000g, and supernatants were stored at −80° until analysis of free GSH. Levels of GSH and GSSG were determined in MPA extracts as described previously [38, 39]. GSSP was measured in acid-insoluble pellets after reduction with KBH4 and re-acidification with MPA as described previously [32]. While GSH in whole blood is concentrated in erythrocytes, lower but measurable levels can also be detected in plasma. GSH in plasma is present mostly in its oxidized forms, GSSG and GSSP [40]. Therefore, plasma samples were first reduced with sodium borohydride prior to analysis to allow for the measurement of total GSH (free + bound) [40].
To prevent the oxidation of lymphocyte and buccal cell GSH that can occur during sample processing, these samples were also first reduced with sodium borohydride. In brief, 400 μl of 5 % MPA was added to aliquots of packed cells containing ~5 × 106 cells. After vigorous mixing and incubation at room temperature for 15 min, samples were centrifuged at 14,000g for 2 min. Supernatants were stored at −80 °C until analysis for GSH.
Glutathione levels were expressed on a per milliliter basis for plasma and whole blood, per gram hemoglobin for erythrocytes, per 106 cells for lymphocytes and per gram protein for buccal cells. Protein concentrations were measured by the bicinchoninic acid procedure (Pierce, Rockford, IL). Hemoglobin was determined spectrophotometrically using Drabkin’s reagent [41].
Glutamate-cysteine ligase activity was determined in red cells by measuring the product, γ-glutamylcysteine, formed after incubating cell lysates with cysteine and glutamic acid, as described previously [42].
Cyst(e)ine (cystine and cysteine) was analyzed in plasma according to the spectrophotometric method of Gaitonde [43].
Immune function
Neutrophil phagocytosis and respiratory burst assays were performed on fresh whole blood samples, which were available from 16 subjects from the placebo group, 18 subjects from the 250 mg/day GSH group and 20 subjects from the 1,000 mg/day GSH group at baseline and 3 and 6 months. Lymphocyte and NK cell assays were performed on frozen purified lymphocyte cell fractions. Fewer samples were available for these analyses; samples with a sufficient number of viable cells were available from 8 to 9 subjects per group at baseline and 3 months for the lymphocyte proliferation assay and 5 to 6 subjects per group at baseline and 3 months for the NK cell cytotoxicity assay.
Neutrophil phagocytosis was determined in fresh whole blood samples (0.1 ml aliquots) incubated with E. coli labeled with a pH-sensitive dye using the pHrodo™E. coli BioParticles® Phagocytosis kit (Invitrogen). Dye-containing cells were analyzed with a FACScan flow cytometer (Becton–Dickinson) using the 488-nm laser for excitation and emission within the FL2 channel. Results were expressed as a phagocytosis index calculated as the FL2 geometric mean of granulocytes incubated at 37 °C/FL2 geometric mean of granulocytes incubated at 4 °C. Thus, the higher the index value, the greater the extent of phagocytosis.
Neutrophil respiratory burst in fresh whole blood samples was determined by measuring the oxidation of dihydrorhodamine 123 by hydrogen peroxide produced by activated neutrophils as described previously [44]. In brief, 0.1-ml aliquots of whole blood in triplicate were exposed to 1 μM phorbol 12-myristate 13-acetate (PMA) for 15 min to activate granulocytes and then exposed to the fluorescence probe dihydrorhodamine 123. Fluorescent cells were detected using a FACSCanto flow cytometer, and the portion of granulocytes that showed increased fluorescence was determined along with the geometric mean of FL1 fluorescence intensity per cell. Results are expressed as the respiratory burst index calculated as the FL1 geometric mean of PMA-stimulated cells/non-PMA-stimulated cells. Thus, the higher the index, the greater the respiratory burst activation.
Lymphocyte proliferation was measured at 2 time points. Lymphocytes were thawed, washed three times and counted to determine cell viability. Cells were plated in triplicate at both 1 × 105 and 5 × 104 cells per well of a 96-well flat-bottom plate in RPMI-1640 plus GlutaMAX supplemented with 10 % fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, 25 ng/ml sodium pyruvate, 10 mM HEPES and 50 μM 2-mercaptoethanol. Cells were rested for 48 h at 37 °C and 5 % CO2 followed by addition of media alone or 2 μg/ml of the T-cell mitogen phytohemagglutinin (PHA). Cultures were incubated for further 72 h, and 1 μCi of 3H-thymidine was added for the last 6 h of the assay. Cells were harvested using a PHD™ cell harvester (Brandel, model 290) per the manufacturer’s instructions. Intracellular radioactivity was measured by liquid scintillation counting, and results were expressed as CPM.
Natural killer cell cytotoxicity was assessed using a standard 51Chromium-release assay. Briefly, lymphocyte samples from each of the five time points were thawed, washed three times, counted to determine cell viability, added in triplicate to 96-well V-bottom plates in complete RPMI-1640 supplemented with 10 % FBS and rested for 48 h at 37 °C and 5 % CO2. Human K562 cells [45] grown in complete RPMI-1640 medium were labeled overnight with 200 μCi sodium 51Chromate in saline, washed three times and then added to lymphocytes (1 × 104 cells per well) at an effector/target cell ratio of 10:1. After incubation for 4 h at 37 °C and 5 % CO2, cells were pelleted by centrifugation and supernatants were analyzed for radioactivity by gamma counting. Results are expressed as percent of target cells lysed (% lysis) calculated as (cpm experimental − cpm spontaneous release)/(cpm maximum − spontaneous) × 100 as described previously [46].
Statistics
Sample size and power estimates were based on a two-tailed type I error of 0.05, 15 % standard deviation, n = 16 per group and >80 % power. Minimal detectable percent differences in GSH between treatment groups and placebo were ~11 %. Descriptive statistics were provided as means and standard deviations. The normality of data distribution was assessed using the Kolmogorov–Smirnov goodness-of-fit test. Group differences at baseline were assessed by ANOVA followed by Tukey’s post hoc test or χ 2 where appropriate. General linear mixed models with repeated measurement (Proc mixed) were used to test for the effects of intervention, period and their interactions with changes in all outcome variables. Potential confounding by age and gender was assessed, and none were observed. Subgroup analyses of age groups (<40 years of age, >40–50 years of age and >50 years of age) and gender revealed no interactions. Correlations of changes in outcomes with levels at baseline or between measures were evaluated using Pearson (r) correlations. All statistical tests were two-sided, and significance was set at P < 0.05. SAS 9.3 software (SAS Institute, Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Compliance and adverse effects
Overall, compliance was ≥94 % based on both pill counts and pill diary entries. By pill count, compliance was 95.5 ± 6.25 % (mean ± SD) in all subjects and did not differ between treatment arms (placebo, 97.6 ± 3.01; 250 mg GSH, 93.7 ± 8.38; and 1,000 mg GSH, 95.5 ± 5.54). Four subjects had an overall compliance rate of <90 % including three in the low-dose GSH group and one in the high-dose GSH group.
No serious adverse effects were reported by the study participants regardless of arm. All potential adverse events reported were minor including colds, stomach virus, lightheadedness, back pain, hot flashes, soft stools, eye twitching, headaches, ear infection, urinary tract infection and constipation. None were attributed to any specific treatment arm. The number of these events reported was similar in each arm of the study (placebo, 20; 250 mg GSH, 18; and 1,000 mg GSH, 19). Two individuals dropped out because of allergy-like symptoms, both of which were in the placebo group.
Effects of oral GSH supplementation on body GSH stores
In whole blood, mean GSH levels significantly increased in both low- and high-dose GSH groups after 1, 3 and 6 months of administration versus baseline (Fig. 2). No increase was observed in the placebo group. At 6 months, the increased levels were higher in the 1,000 mg dose group (31 %) than in the 250 mg group (20 %) (P < 0.05). In the 1,000 mg group, GSH levels increased in a time-dependent manner from 1 to 6 months of supplement administration. In the 250 mg group, equivalent increases were observed after 1, 5 and 6 months. In both dose groups, GSH levels decreased toward baseline levels following the 1-month washout period. However, the levels in the 1,000 mg group remained significantly greater than baseline after washout (P < 0.05).
Effect of oral GSH supplementation on GSH levels in whole blood and erythrocytes. Subjects were randomized to placebo (n = 16), 250 mg/day GSH (n = 18) and 1,000 mg/day GSH (n = 20). GSH or placebo supplementation continued for 6 months followed by a 1-month washout. Blood was collected at baseline and after 1, 3, 6 and 7 months. Free and protein-bound GSH was determined in whole blood (upper panel) and in packed erythrocytes (lower panel) as described in text. Erythrocyte GSH levels, expressed on a gram hemoglobin basis, are presented as changes from baseline. Bars are mean ± SE. General linear models with repeated measurement were used to test for effects of intervention, period and their interaction with study outcomes. *Change from baseline within group is statistically significant, P < 0.05. †Change from baseline is significantly different from placebo group, P < 0.05
In erythrocytes, mean GSH levels significantly increased after 1, 3 and 6 months in the 1,000 mg group and after 6 months in the 250 mg group (Fig. 2). Similar to whole blood, maximum increases in GSH levels in erythrocytes of about 35 % were observed after 6 months. In both dose groups, GSH levels decreased toward baseline levels after the 1-month washout period.
The effects of oral GSH on plasma total GSH levels are summarized in Fig. 3. Concentrations ranged from ~0.2 to 8 nmol/ml, which are <1 % of levels in whole blood. Increases in plasma GSH levels were observed in the 1,000 mg group after 3 and 6 months of administration. While the mean level at 6 months was higher than baseline in the low-dose group, this increase was not statistically significant. In the high-dose group, the increase appeared to be time dependent with levels progressively increasing from 3 to 6 months of administration, after which time GSH levels decreased toward baseline levels after the 1-month washout period. Nonsignificant time-dependent increases were found in the 250 mg group. There were no changes in the placebo group.
Effect of oral GSH supplementation on total GSH in plasma, lymphocytes and buccal mucosa. Subjects were randomized to placebo (n = 16), 250 mg/day GSH (n = 18) and 1,000 mg/day GSH (n = 20). GSH or placebo supplementation continued for 6 months followed by a 1-month washout. Blood and buccal cells were collected at baseline and after 1, 3, 6 and 7 months. Total GSH was determined in plasma, lymphocytes and buccal cells as described in text. Lymphocyte and buccal cell GSH levels were expressed on a 106 cells and milligram protein basis, respectively, and are presented as changes from baseline. Bars are mean ± SE. General linear models with repeated measurement were used to test for effects of intervention, period and their interaction with study outcomes. *Change from baseline within group is statistically significant, P < 0.05. †Change from baseline is significantly different from change from baseline in the placebo group, P < 0.05
The effects of oral GSH administration on lymphocyte total GSH levels are summarized in Fig. 3. Values are expressed on a per million cell basis and ranged from ~0.7 to 6.7 μmol/106 cells. Increases in lymphocyte GSH levels were observed in the high-dose GSH group after 1, 3 and 6 months of administration. While the mean level at 3 and 6 months was higher than baseline in the low-dose group, these increases were not statistically significant. No changes were observed in the placebo group. A maximum increase of about 30 % was observed after 6 months in the high-dose GSH group. In the high-dose group, the increase appeared to be time dependent with levels progressively increasing from 1–3 to 6 months of administration after which time GSH levels decreased toward baseline levels during the 1-month washout period.
The effects of oral GSH administration of exfoliated buccal cell levels are summarized in Fig. 3. Values are expressed on a per milligram protein basis and ranged from ~0.6 to 11 μmol/mg protein. While variation in GSH levels was high, a significant increase was observed in the high-dose GSH group after 6 months of administration. No changes were observed at other time points or in either the low-dose GSH or placebo groups.
GSH levels in whole blood, erythrocytes, plasma, lymphocytes and buccal cells at baseline and after 6 months are summarized for each treatment group in Supplemental Table 1. There were no effects of either sex or age observed for GSH levels in any blood compartment or buccal cells. Likewise, sex and age had no impact on changes in GSH levels due to treatment.
The effects of GSH supplementation on the levels of the GSH precursor cysteine in plasma and the activity of the rate-limiting GSH biosynthetic enzyme GCL in erythrocytes were also examined after 6 months (Supplemental Table 2). No changes were observed in cyst(e)ine concentrations or GCL activity in any of the groups.
Effects of oral GSH supplementation on GSH redox status in blood
The major oxidized forms of GSH, GSSG and GSSP were analyzed in whole blood, and the impact of oral GSH supplementation on the ratio of oxidized to reduced forms is summarized at baseline and after 6 months in Fig. 4. No differences were observed in the placebo group for any of the following ratios: GSSG/GSH, GSSP/GSH or (GSSG + GSSP)/GSH. However, GSSG/GSH and (GSSG + GSH)/GSSG ratios were both decreased significantly in the high-dose GSH supplementation group and GSSG/GSH was decreased significantly in the low-dose GSH supplementation group after 6 months.
Effect of oral GSH supplementation on oxidized to reduced glutathione ratios in whole blood. Subjects were randomized to placebo (n = 16), 250 mg/d GSH (n = 18) and 1,000 mg/day GSH (n = 20). GSH or placebo supplementation continued for 6 months followed by a 1-month washout. Blood was collected at baseline and after 1, 3, 6 and 7 months. GSH and its major oxidized forms, GSSG and GSSP were determined in whole blood as described in text. Symbols and bars are mean ± SE
Effect of oral GSH supplementation by baseline GSH levels
In order to determine whether effects of oral GSH differed by baseline GSH levels, changes by treatment arm in each of the measured outcomes were compared between individuals with levels below the median (<0.89 μmol/ml) and above the median (>0.89 μmol/ml). No significant differences were observed between the two groups at 6 months for GSH in blood, erythrocytes, plasma, lymphocytes and buccal cells (data not shown). Likewise, changes in GSH levels after 6 months in blood, erythrocytes, lymphocytes, plasma and buccal cells were not significantly correlated with GSH levels at baseline for subjects in the GSH-treated groups (n = 38) (blood, r = 0.35; erythrocytes, r = 0.043; plasma, r = −0.11; lymphocytes, r = 0.01; and buccal cells, r = 0.018).
Correlation of GSH changes in different blood compartments and buccal cells
To examine whether changes in GSH levels within individuals were consistent among the different blood compartments and buccal cells, correlational analyses were conducted for individuals in the GSH-treated groups (Table 2). Changes in blood GSH were highly correlated with those in erythrocytes at 6 months (r = 0.83). In addition, changes in lymphocyte GSH were significantly correlated with those in both blood (r = 0.55) and erythrocytes (r = 0.45). Changes in plasma GSH were weakly correlated with those in erythrocytes (r = 0.33). No associations were observed for GSH changes in buccal cells.
Effects of oral GSH on immune function markers in blood
The effects of GSH administration on lymphocyte proliferation are summarized in Fig. 5a. Increases in mean proliferative capacity were observed after 3 months in both GSH groups, but were not significant. No changes were evident in the placebo group. The effects of GSH administration on NK cell cytotoxicity are summarized in Fig. 5a. Increases in mean % lysis values were observed after 3 months in both GSH groups, but were only significant in the high GSH dose arm (P paired = 0.01). No changes were evident in the placebo group. The effects of GSH administration on neutrophil phagocytosis and respiratory burst are summarized in Fig. 5b. No consistent or significant change in either index was observed by study arm after either 3 or 6 months.
Effect of oral GSH supplementation on immune function. Subjects were randomized to placebo, 250 mg/day GSH and 1,000 mg/day GSH. GSH or placebo supplementation continued for 6 months followed by a 1-month washout period. Blood and buccal cells were collected at baseline and after 1, 3, 6 and 7 months. Lymphocytes collected at baseline and at 3 months were isolated by density gradient centrifugation of whole blood on Ficoll–Hypaque and utilized for analysis of NK cell cytotoxicity and lymphocyte proliferation (a). NK cytotoxicity was assessed using 51Cr-labeled human K562 cells as the target and measuring the percent of target cells lysed after incubation with lymphocytes for 4 h at 37 °C. Lymphocyte proliferation was assessed by measuring 3H-thymidine incorporation after incubation with phytohemagglutinin (PHA) as described in the text. Results are expressed as CPM. Bars are mean ± SE. For NK cytotoxicity n = 6 for placebo and 1,000 mg/day GSH groups and n = 5 for 250 mg/day GSH group. For lymphocyte proliferation, n = 9 for placebo and 1,000 mg/day GSH groups and n = 8 for 250 mg/day GSH group. Fresh whole blood collected at baseline and at 3 and 6 months was used for assessment of neutrophil phagocytosis and respiratory burst (b). Phagocytosis was measured by incubation of whole blood with E. coli labeled with a pH-sensitive dye using the pHrodo™ BioParticles kit (Invitrogen). Dye-containing cells were analyzed by flow cytometry, and results were expressed as a phagocytosis index calculated as the geometric mean of gated granulocytes incubated at 37 °C/geometric mean of granulocytes incubated at 4 °C. Respiratory burst was measured after incubation of whole blood with PMA followed by addition of the fluorescence probe dihydrorhodamine 123 and detection of fluorescent neutrophils by flow cytometry. Results are expressed as a respiratory burst index calculated as the geometric mean of PMA-stimulated cells/non-PMA-stimulated cells. Symbols and bars are mean ± SE. For phagocytosis and respiratory burst assays, n = 16, 18 and 20 for the placebo, 250 mg/day GSH and 1,000 mg/day GSH groups, respectively. *Significantly different from baseline by Student’s t test, P < 0.05
Discussion
The results of this randomized, double-blinded, placebo-controlled study of long-term (6 months) supplementation with GSH at two doses (250 mg/day and 1,000 mg/day) demonstrate for the first time that orally administered GSH in supplement form increased body GSH stores in humans. These findings support the use of oral GSH supplementation as a strategy for increasing tissue GSH levels. Overall, these results are consistent with previous in vivo studies in laboratory animals and findings of specific intestinal transport systems for GSH in laboratory animals in humans [18–23, 30]. In two previous clinical investigations, oral GSH supplementation was not associated with increased tissue GSH levels. In one study, a single oral dose of GSH (0.15 mmol/kg) had no impact on plasma GSH levels after 4.5 h [31]. However, due to the very short half-life (1–2 min) of GSH in human plasma resulting from its rapid removal by tissues such as kidney that are rich in γ-glutamyl transpeptidase [47, 48], the accurate measurement of plasma GSH status in vivo is difficult and the ability to detect an increase in plasma GSH levels after a single oral dose is unlikely [40]. Our results were also not consistent with those from a recent study of oral GSH supplementation (1,000 mg/day) that showed no effects on erythrocyte GSH concentration and biomarkers of oxidative stress after 1 month [49]. However, in this study, GSH and GSSG measurements did not account for possible differences in erythrocyte volume and number which can significantly impact GSH levels. In addition, erythrocytes were not directly acidified immediately after collection, but rather after an initial hemolysis step which can greatly decrease the stability of both GSSG and GSH and lead to inaccurate measurement [50].
The increases in free GSH levels in whole blood and erythrocytes in the current study were dose dependent with the greatest increases occurring in the high-dose (1,000 mg/day) group. Increases in GSH were also time dependent, particularly in the high-dose group, where levels increased from 1–3 to 6 months and decreased toward baseline after the 1-month washout period. Using this experimental design, the mechanism of GSH induction could not be ascertained. In animal models, direct intestinal absorption of GSH has been observed with transport being facilitated by specific proteins including the cystic fibrosis transmembrane conductance regulator (CFTR) [18, 19, 23, 30]. Facilitated transport of GSH has also been observed and characterized in human intestinal epithelial cells in vitro [30] and in buccal mucosal cells in vivo [23]. The extent to which direct absorption may be responsible for the present findings is not known. The progressive nature of the increase in GSH levels may suggest that changes in GSH metabolism may be occurring as a result of long-term GSH supplementation leading to greater steady-state levels. While it is possible that changes in dietary intake of precursor amino acids resulting in increased GSH biosynthesis could account for some of the observed changes, it is unlikely that group-specific changes in intake would occur with the randomized clinical trial design used. This is supported by our finding that plasma cyst(e)ine levels of erythrocyte GCL activity were unchanged.
In order to gain a more comprehensive assessment of supplementation on body GSH stores, we measured GSH levels in plasma, lymphocytes and exfoliated buccal mucosal cells in addition to whole blood and erythrocytes. In lymphocytes and buccal cells, only total GSH levels (free + bound) were measured since the in vivo levels of protein-bound GSH are very low in these cells and the extent of artifactual GSH oxidation during processing can be quite high. Total GSH was also measured in plasma since overall GSH levels in plasma are low and the majority of GSH is present in the oxidized state [40]. For most measures, GSH increases were both time- and dose responsive and were not impacted by baseline levels of blood GSH. Increases in erythrocyte GSH were highly correlated with those in lymphocytes and plasma supporting a generalized treatment effect of oral GSH on different blood compartments. While increases in buccal cells were not correlated with those in blood, it should be noted that buccal cell GSH levels are highly variable due to heterogeneity of cells obtained in the sampling procedure and the small number cells that are used for measurement which severely limits the power to detect changes and correlations. Overall, GSH was highly tolerated and its administration was not associated with any signs of adverse effects. The trial had high levels of compliance (95.5 % based on pill count), a low dropout rate and no reported significant adverse events in all treatment arms.
The impact of oral GSH on buccal cells is of interest given the previous epidemiologic findings where increased dietary intake of GSH from fruits and vegetables, and higher blood GSH levels were associated with decreased risk of oral cancer [17, 51]. Also, we recently identified a trinucleotide repeat polymorphism in the gene encoding the rate-limiting enzyme in GSH biosynthesis (GCL) which is linked with decreased GCL activity and GSH levels in vivo and, in case control studies, observed an association between this polymorphism and risk of oral and lung cancers [52, 53]. Our present findings suggest that the mechanism of GSH protection against oral cancer development may involve increased GSH levels in oral tissues. It is of interest to note that, in the previous study by Flagg et al. [17], the differences in dietary GSH intake between low and high oral cancer risk groups were 32–358 mg/day in men and 34–126 mg/day in women, well within the dose ranges used in the present intervention trial.
While the majority of GSH in cells is in the reduced form, GSH oxidation can occur resulting in the formation of GSSG or GSSP. The levels of both GSSG and GSSP are increased during periods of oxidative stress. The formation of GSSP, a process known as protein glutathionylation, is thought to play an important redox-sensitive regulatory role in the cell [4, 54]. The ratio of GSSG or GSSP to GSH has often been used as indicators of redox status or biomarkers of oxidative stress [32]. In the present study, we observed a significant decrease in the GSSG/GSH ratio as a result of both low- and high-dose GSH supplementation. This decrease may be indicative of a reduction in oxidative stress resulting from long-term GSH supplementation. Slight reductions in the ratio of GSSP/GSH were also observed, but did not reach the level of significance. Since the subjects were healthy non-smokers, the basal levels of oxidative stress would be expected to be low, thus limiting the ability to detect reductions by GSH. Larger differences in these ratios would likely be expected in individuals exposed to higher levels of free radicals and reactive oxygen species such as tobacco smokers.
Regarding immune parameters, in the high-dose GSH group after 3 months, a significant increase in NK cell cytotoxicity was observed. An effect of GSH supplementation on lymphocyte proliferation was suggested, but the sample sizes for these assays were small and results were not statistically significant. While the mechanisms for these effects are not known, they are consistent with previous in vitro studies demonstrating the importance of intracellular GSH levels in NK cytotoxic activity [55–58]. These studies show that even partial depletion of intracellular GSH can inhibit the effector phase of cytotoxic cell response as well as IL-2-dependent functions. Our results are also consistent with a previous clinical study in which low intracellular GSH levels were correlated with NK cell activity [59]. Overall, the present findings provide a basis for conducting larger studies focusing on immune function.
A limitation of the trial was that there were few male participants. We did not find significant differences in GSH levels by sex at any time point in the study, but caution should be used when generalizing these findings to men. Gender-related differences in blood GSH levels have been noted in some studies [60, 61], but not in most others including those conducted by our group [32, 38, 62, 63]. Also, there were limited samples available for analysis of NK cell cytotoxicity and lymphocyte proliferation. While significant increases in NK cell cytotoxicity were observed, future studies with a larger sample size and additional time points are required to provide more detailed information on the impact of GSH on this important NK cell activity.
These findings may have implications regarding the treatment of diseases associated with reduced GSH levels [11]. HIV infection represents one such disease where GSH depletion is thought to be an important factor leading to impairments in immune function and disease progression [33, 37, 64]. GSH precursors including cysteine and N-acetylcysteine have been tested for their potential use in the control of symptoms of HIV infection with improvements being noted for some endpoints [65–67]. The use of GSH itself in HIV infection may have advantages over its precursors since it would not require GSH re-synthesis within cells via GCL, the activity of which is reduced in HIV+ macrophages [68]. Indeed, in vitro studies indicate that GSH is more effective in restoring immune function in macrophages from HIV-infected individuals that is N-acetylcysteine [68].
Overall, results from this study demonstrate for the first time the effectiveness of long-term GSH supplementation at increasing body stores of GSH in humans. GSH was well tolerated and was not associated with any negative side effects. Beneficial effects on immune function were observed, but additional studies are required to further elucidate the nature of these effects. Importantly, no negative impacts on immune function were observed. These new findings are consistent with studies in laboratory animals [18–23, 30] and suggest that oral GSH supplementation is an effective strategy for increasing body stores of GSH.
References
Lu SC (1999) Regulation of hepatic glutathione synthesis: current concepts and controversies. Faseb J 13:1169–1183
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Vina J (1990) Glutathione: metabolism and physiological functions. CRC Press, Boca Raton
Giustarini D, Rossi R, Milzani A, Colombo R, Dalle-Donne I (2004) S-glutathionylation: from redox regulation of protein functions to human diseases. J Cell Mol Med 8:201–212
Forman HJ, Zhang H, Rinna A (2009) Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 30:1–12. doi:10.1016/j.mam.2008.08.006
Griffith OW (1999) Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27:922–935
Zhang H, Forman HJ (2012) Glutathione synthesis and its role in redox signaling. Semin Cell Dev Biol 23:722–728. doi:10.1016/j.semcdb.2012.03.017
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X (1999) Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15:269–290
Jollow DJ (1980) Glutathione thresholds in reactive metabolite toxicity. Arch Toxicol Suppl 3:95–110
Ellouk-Achard S, Levresse V, Martin C, Pham-Huy C, Dutertre-Catella H, Thevenin M, Warnet JM, Claude JR (1995) Ex vivo and in vitro models in acetaminophen hepatotoxicity studies. Relationship between glutathione depletion, oxidative stress and disturbances in calcium homeostasis and energy metabolism. Arch Toxicol Suppl 17:209–214
Townsend DM, Tew KD, Tapiero H (2003) The importance of glutathione in human disease. Biomed Pharmacother 57:145–155
Nuttall SL, Martin U, Sinclair AJ, Kendall MJ (1998) Glutathione: in sickness and in health. Lancet 351:645–646
Julius M, Lang CA, Gleiberman L, Harburg E, DiFranceisco W, Schork A (1994) Glutathione and morbidity in a community-based sample of elderly. J Clin Epidemiol 47:1021–1026
Vogt BL, Richie JP Jr (1993) Fasting-induced depletion of glutathione in the aging mouse. Biochem Pharmacol 46:257–263
Jaeschke H, Wendel A (1985) Diurnal fluctuation and pharmacological alteration of mouse organ glutathione content. Biochem Pharmacol 34:1029–1033
Flagg EW, Coates RJ, Eley JW, Jones DP, Gunter EW, Byers TE, Block GS, Greenberg RS (1994) Dietary glutathione intake in humans and the relationship between intake and plasma total glutathione level. Nutr Cancer 21:33–46
Flagg EW, Coates RJ, Jones DP, Byers TE, Greenberg RS, Gridley G, McLaughlin JK, Blot WJ, Haber M, Preston-Martin S et al (1994) Dietary glutathione intake and the risk of oral and pharyngeal cancer. Am J Epidemiol 139:453–465
Favilli F, Marraccini P, Iantomasi T, Vincenzini MT (1997) Effect of orally administered glutathione on glutathione levels in some organs of rats: role of specific transporters. Br J Nutr 78:293–300
Kariya C, Leitner H, Min E, van Heeckeren C, van Heeckeren A, Day BJ (2007) A role for CFTR in the elevation of glutathione levels in the lung by oral glutathione administration. Am J Physiol Lung Cell Mol Physiol 292:L1590–L1597. doi:10.1152/ajplung.00365.2006
Aw TY, Wierzbicka G, Jones DP (1991) Oral glutathione increases tissue glutathione in vivo. Chem Biol Interact 80:89–97
Hagen TM, Wierzbicka GT, Sillau AH, Bowman BB, Jones DP (1990) Bioavailability of dietary glutathione: effect on plasma concentration. Am J Physiol 259:G524–G529
Vina J, Perez C, Furukawa T, Palacin M, Vina JR (1989) Effect of oral glutathione on hepatic glutathione levels in rats and mice. Br J Nutr 62:683–691
Hunjan MK, Evered DF (1985) Absorption of glutathione from the gastro-intestinal tract. Biochim Biophys Acta 815:184–188
Furukawa T, Meydani SN, Blumberg JB (1987) Reversal of age-associated decline in immune responsiveness by dietary glutathione supplementation in mice. Mech Ageing Dev 38:107–117
Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP (2003) Inhibition of influenza infection by glutathione. Free Radic Biol Med 34:928–936
Novi AM (1981) Regression of aflatoxin B1-induced hepatocellular carcinomas by reduced glutathione. Science 212:541–542
Trickler D, Shklar G, Schwartz J (1993) Inhibition of oral carcinogenesis by glutathione. Nutr Cancer 20:139–144
Schwartz JL, Shklar G (1996) Glutathione inhibits experimental oral carcinogenesis, p53 expression, and angiogenesis. Nutr Cancer 26:229–236
Shklar G, Schwartz J, Trickler D, Cheverie SR (1993) The effectiveness of a mixture of beta-carotene, alpha-tocopherol, glutathione, and ascorbic acid for cancer prevention. Nutr Cancer 20:145–151
Iantomasi T, Favilli F, Marraccini P, Magaldi T, Bruni P, Vincenzini MT (1997) Glutathione transport system in human small intestine epithelial cells. Biochim Biophys Acta 1330:274–283
Witschi A, Reddy S, Stofer B, Lauterburg BH (1992) The systemic availability of oral glutathione. Eur J Clin Pharmacol 43:667–669
Muscat JE, Kleinman W, Colosimo S, Muir A, Lazarus P, Park J, Richie JP Jr (2004) Enhanced protein glutathiolation and oxidative stress in cigarette smokers. Free Radic Biol Med 36:464–470
Droge W, Breitkreutz R (2000) Glutathione and immune function. Proc Nutr Soc 59:595–600
Hamilos DL, Zelarney P, Mascali JJ (1989) Lymphocyte proliferation in glutathione-depleted lymphocytes: direct relationship between glutathione availability and the proliferative response. Immunopharmacology 18:223–235
Hamilos DL, Wedner HJ (1985) The role of glutathione in lymphocyte activation. I. Comparison of inhibitory effects of buthionine sulfoximine and 2-cyclohexene-1-one by nuclear size transformation. J Immunol 135:2740–2747
Gmunder H, Droge W (1991) Differential effects of glutathione depletion on T cell subsets. Cell Immunol 138:229–237
Morris D, Khurasany M, Nguyen T, Kim J, Guilford F, Mehta R, Gray D, Saviola B, Venketaraman V (2013) Glutathione and infection. Biochim Biophys Acta 1830:3329–3349. doi:10.1016/j.bbagen.2012.10.012
Richie JP Jr, Skowronski L, Abraham P, Leutzinger Y (1996) Blood glutathione concentrations in a large-scale human study. Clin Chem 42:64–70
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Kleinman WA, Richie JP Jr (2000) Status of glutathione and other thiols and disulfides in human plasma. Biochem Pharmacol 60:19–29
Drabkin DL (1949) The standardization of hemoglobin measurements. Am J Med Sci 217:711–714
Nichenametla SN, Ellison I, Calcagnotto A, Lazarus P, Muscat JE, Richie JP Jr (2008) Functional significance of the GAG trinucleotide-repeat polymorphism in the gene for the catalytic subunit of gamma-glutamylcysteine ligase. Free Radic Biol Med 45:645–650. doi:10.1016/j.freeradbiomed.2008.05.012
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Richardson MP, Ayliffe MJ, Helbert M, Davies EG (1998) A simple flow cytometry assay using dihydrorhodamine for the measurement of the neutrophil respiratory burst in whole blood: comparison with the quantitative nitrobluetetrazolium test. J Immunol Methods 219:187–193
Lozzio CB, Lozzio BB (1975) Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45:321–334
Schell TD, Mylin LM, Georgoff I, Teresky AK, Levine AJ, Tevethia SS (1999) Cytotoxic T-lymphocyte epitope immunodominance in the control of choroid plexus tumors in simian virus 40 large T antigen transgenic mice. J Virol 73:5981–5993
Anderson ME, Bridges RJ, Meister A (1980) Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun 96:848–853
Wendel A, Cikryt P (1980) The level and half-life of glutathione in human plasma. FEBS Lett 120:209–211
Allen J, Bradley RD (2011) Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J Altern Complement Med 17:827–833. doi:10.1089/acm.2010.0716
Mills BJ, Richie JP Jr, Lang CA (1994) Glutathione disulfide variability in normal human blood. Anal Biochem 222:95–101
Richie JP Jr, Kleinman W, Marina P, Abraham P, Wynder EL, Muscat JE (2008) Blood iron, glutathione, and micronutrient levels and the risk of oral cancer. Nutr Cancer 60:474–482. doi:10.1080/01635580801956477
Nichenametla SN, Lazarus P, Richie JP Jr (2011) A GAG trinucleotide-repeat polymorphism in the gene for glutathione biosynthetic enzyme, GCLC, affects gene expression through translation. Faseb J 25:2180–2187. doi:10.1096/fj.10-174011
Nichenametla SN, Muscat JE, Liao JG, Lazarus P, Richie JP Jr (2012) A functional trinucleotide repeat polymorphism in the 5′-untranslated region of the glutathione biosynthetic gene GCLC is associated with increased risk for lung and aerodigestive tract cancers. Mol Carcinog. doi:10.1002/mc.21923
Pastore A, Piemonte F (2012) S-Glutathionylation signaling in cell biology: progress and prospects. Eur J Pharm Sci Off J Eur Fed Pharm Sci 46:279–292. doi:10.1016/j.ejps.2012.03.010
Galter D, Mihm S, Droge W (1994) Distinct effects of glutathione disulphide on the nuclear transcription factor kappa B and the activator protein-1. Eur J Biochem 221:639–648
Suthanthiran M, Anderson ME, Sharma VK, Meister A (1990) Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci USA 87:3343–3347
Hargrove ME, Wang J, Ting CC (1993) Regulation by glutathione of the activation and differentiation of IL-4-dependent activated killer cells. Cell Immunol 149:433–443. doi:10.1006/cimm.1993.1168
Liang CM, Lee N, Cattell D, Liang SM (1989) Glutathione regulates interleukin-2 activity on cytotoxic T-cells. J Biol Chem 264:13519–13523
Vojdani A, Mumper E, Granpeesheh D, Mielke L, Traver D, Bock K, Hirani K, Neubrander J, Woeller KN, O’Hara N, Usman A, Schneider C, Hebroni F, Berookhim J, McCandless J (2008) Low natural killer cell cytotoxic activity in autism: the role of glutathione, IL-2 and IL-15. J Neuroimmunol 205:148–154. doi:10.1016/j.jneuroim.2008.09.005
Ilhan N, Kamanli A, Ozmerdivenli R (2004) Variable effects of exercise intensity on reduced glutathione, thiobarbituric acid reactive substance levels, and glucose concentration. Arch Med Res 35:294–300. doi:10.1016/j.arcmed.2004.03.006
Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL (2005) Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci 46:877–883. doi:10.1167/iovs.04-0777
Erden-Inal M, Sunal E, Kanbak G (2002) Age-related changes in the glutathione redox system. Cell Biochem Funct 20:61–66. doi:10.1002/cbf.937
Michelet F, Gueguen R, Leroy P, Wellman M, Nicolas A, Siest G (1995) Blood and plasma glutathione measured in healthy subjects by HPLC: relation to sex, aging, biological variables, and life habits. Clin Chem 41:1509–1517
Morris D, Guerra C, Donohue C, Oh H, Khurasany M, Venketaraman V (2012) Unveiling the mechanisms for decreased glutathione in individuals with HIV infection. Clin Dev Immunol 2012:734125. doi:10.1155/2012/734125
Nguyen D, Hsu JW, Jahoor F, Sekhar RV (2014) Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J Clin Endocrinol Metab 99:169–177. doi:10.1210/jc.2013-2376
Borges-Santos MD, Moreto F, Pereira PC, Ming-Yu Y, Burini RC (2012) Plasma glutathione of HIV(+) patients responded positively and differently to dietary supplementation with cysteine or glutamine. Nutrition 28:753–756. doi:10.1016/j.nut.2011.10.014
Hummelen R, Hemsworth J, Reid G (2010) Micronutrients, N-acetyl cysteine, probiotics and prebiotics, a review of effectiveness in reducing HIV progression. Nutrients 2:626–651. doi:10.3390/nu2060626
Morris D, Guerra C, Khurasany M, Guilford F, Saviola B, Huang Y, Venketaraman V (2013) Glutathione supplementation improves macrophage functions in HIV. J Interf Cytokine Res Off J Int Soc Interf Cytokine Res 33:270–279. doi:10.1089/jir.2012.0103
Acknowledgments
We thank Ashley Knipe for her technical assistance with sample processing and Nate Sheaffer in the Penn State Hershey Flow Cytometry Core Facility. We thank Mr. Yoichiro Sugimura (deceased) and Ms. Erika Hashizume for their support in the design and implementation of this trial. This work was supported by Kyowa Hakko Bio Co., Ltd (JR). Glutathione (Setria®) supplements and placebo were provided by Kyowa Hakko Bio Co., Ltd. Kyowa Hakko Bio Co., Ltd had no role in the conduct, collection, management, analysis and interpretation of the data, or writing of the manuscript. Immunological analyses were supported in part by the Penn State Hershey Cancer Institute through the Clinical Correlative Immunology Laboratory (TS and JH).
Conflict of interest
JPR received research support for this study and travel funds to present previous research findings from Kyowa Hakko Bio Co., Ltd. Kyowa Hakko Bio Co., Ltd is a biotechnology and fermentation company that provides glutathione (Setria®). None of the other authors have any conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Richie, J.P., Nichenametla, S., Neidig, W. et al. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur J Nutr 54, 251–263 (2015). https://doi.org/10.1007/s00394-014-0706-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0706-z