Abstract
Purpose
Coffee consumption has been reported to decrease oxidative damage in peripheral white blood cells (WBC). However, effects on the level of spontaneous DNA strand breaks, a well established marker of health risk, have not been specifically reported yet. We analyzed the impact of consuming a dark roast coffee blend on the level of spontaneous DNA strand breaks.
Methods
Healthy men (n = 84) were randomized to consume daily for 4 weeks either 750 ml of fresh coffee brew or 750 ml of water, subsequent to a run in washout phase of 4 weeks. The study coffee was a blend providing high amounts of both caffeoylquinic acids (10.18 ± 0.33 mg/g) and the roast product N-methylpyridinium (1.10 ± 0.05 mg/g). Before and after the coffee/water consumption phase, spontaneous strand breaks were determined by comet assay.
Results
At baseline, both groups exhibited a similar level of spontaneous DNA strand breaks. In the intervention phase, spontaneous DNA strand breaks slightly increased in the control (water only) group whereas they significantly decreased in the coffee group, leading to a 27 % difference within both arms (p = 0.0002). Food frequency questionnaires indicated no differences in the overall diet between groups, and mean body weight during the intervention phases remained stable. The consumption of the study coffee substantially lowered the level of spontaneous DNA strand breaks in WBC.
Conclusion
We conclude that regular coffee consumption contributes to DNA integrity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coffee is one of the most popular beverages worldwide. Several in vivo studies suggest that coffee consumption is associated with prevention or delay of degenerative diseases including diabetes mellitus type two, Parkinson, cardiovascular disease or cancer [1]. The pathogenesis of these diseases is associated with increased production of reactive oxygen species and an imbalance between pro- and antioxidant processes. As a result, oxidative damage to lipids and proteins, and increased DNA damage may occur [2].
Roast coffee is a complex mixture of bioactive compounds, originating from the green bean as well as generated by processing, including minerals, phenolics, roasting-associated components, purine alkaloids and soluble fiber as major components. The antioxidant effectiveness of coffee observed in vitro and in vivo has mainly been ascribed to its content in chlorogenic acids (e.g., caffeoylquinic acid, CQA) and to Maillard reaction products generated during roasting. Such constituents were found to act as radical scavengers, to exhibit metal chelating activity and to induce the expression of antioxidant enzymes such as NAD(P)H:quinone oxidoreductase 1 (NQO1), γ-glutamylcysteine ligase (γ-GCL) and NF-E2 p45 subunit-related factor 2 (Nrf2) [3, 4].
In a previous pilot intervention study, it was reported that coffee rich in both original constituents from green coffee and roast products can markedly reduce total DNA strand breaks in white blood cells (WBC) of human volunteers. Concomitantly, a significant elevation of total glutathione level and of glutathione-S-reductase activity in blood of healthy subjects was observed [5]. In another intervention study after intake of instant coffee rich in chlorogenic acid, a similar decrease of total DNA strand breaks was observed in isolated lymphocytes, together with increased expression of genes involved in the cellular antioxidant defense (e.g., NQO1, Nrf2) [6]. In further human intervention studies, coffee consumption was reported to protect against DNA damage induced by activated forms of chemical carcinogens, including benzo(α)pyrene-7,8-dihydrodiol-9,10-epoxide as well as 3-amino-1-methyl-5H-pyrido[4,3-b]indole acetate (Trp-P-2) and to protect against oxidative DNA damage in isolated lymphocytes [7–9]. These studies followed a sequential and/or cross-over design. In the present investigation, a randomized controlled study was designed to control for potential confounders, including inadvertent changes in body weight or seasonal factors. The control group ingested water, and the study group consumed coffee simultaneously for 8 weeks in parallel. The primary aim was to observe and, if possible, to confirm the protective effect of coffee consumption on DNA integrity in humans, as detected previously in a pilot intervention study [5]. In this randomized controlled study with 84 healthy volunteers, we measured effects on spontaneous DNA strand breaks in WBC. By definition, spontaneous DNA strand breaks are understood as DNA strand breaks resulting from any DNA lesion that may result from exposure to endogenous and/or exogenous DNA damaging agents, encompassing also incomplete repair, detectable as an overall response by comet assay. Compliance to the study design was ascertained by monitoring urinary excretion of trigonelline and N-methylpyridinium (NMP) in all volunteers under study.
Materials and methods
Subjects
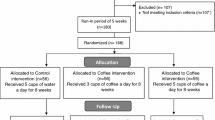
Male healthy volunteers (n = 90, ages 19–50, BMI 19–32) were recruited who fulfilled the inclusion criteria (healthy, non-smokers, non-vegetarians, no intake of pharmaceutical drugs or food supplements during the study period as well as prior to the study, BMI < 32, no alcohol consumption on a regular basis). After informed written consent, the volunteers were subjected to standard medical health check. Thereafter the volunteers were randomly allocated into two groups (control group, n = 45; coffee group, n = 45). A computer-generated randomization list was used with stratification for BMI. In order to obtain mean BMI values matching as closely as possible between the two groups, participants were divided into two cohorts according to their BMI (BMI < 24.9 and BMI ≥ 24.9). Subsequently, participants of each cohort were randomly assigned to control and coffee group, respectively. This strategy led to treatment groups with equal proportions of persons with lower and higher BMI and comparable mean BMI values (24.4 ± 3.5 kg/m2 control group, 24.9 ± 3.0 kg/m2 coffee group). The sample size (target significance level α = 5 %, at a power of 80 %) was calculated based on a previous human intervention study with 33 healthy volunteers [5]. Six individuals dropped out because of private reasons. The remaining 84 volunteers (body weight 80.9 ± 12.4 kg) completed the 8-week study, resulting in n = 42 for the control and the coffee group each.
Study design
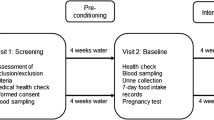
The study was approved by the local ethical committee in Mainz, Germany [No. 837.217.12 (8318-F)]. It consisted of an 8-week prospective, randomized, controlled study with parallel design (time interval: September 19–26 until November 14–21, 2012) and was designed as follows (see Fig. 1): weeks 1–4 washout phase (for both groups, only water 750 mL), weeks 5–8 coffee phase (coffee group) and, in parallel, water phase (control group). In the intervention phase, the coffee group consumed a brew prepared from a coffee blend filled in pads (production see chapter “preparation and analysis of the coffee brews”) and the control group the same volume of water (750 mL). The coffee pads used for the intervention were produced from one batch of coffee beans. Each volunteer was supplied with a coffee pad machine and detailed instructions for the preparation of the coffee brew. During the intervention period, the volunteers of the coffee group consumed 750 mL of coffee (no milk) in three equal portions (morning, noontime, afternoon, six coffee pads of 7.5 g/day). Sugar was used ad libitum (not more than one teaspoon). During the washout phases, coffee was replaced by similar volumes of water (750 mL). Volunteers were instructed to keep their usual dietary habits during the study and to avoid coffee (except study coffee) and caffeine-containing products such as tea or soft drinks, dietary supplements and foods rich in polyphenols. The volunteers received a list of foods and beverages, which were allowed and an exclusion list with additional information of foods to avoid. Records on food intake during a 7-day period were completed by the volunteers in the last week of each study phase, prior to blood sampling. At the days allotted to sampling, the volunteers were asked to come to the study site at the University for urine delivery, body weight measurement and beverage uptake. Two hours after ingestion of coffee or water (baseline point), venous blood samples were drawn from the participants as reported before [5]. Blinding was achieved by coding all blood samples, and comet assays were performed and evaluated by an experienced person unaware of the treatment group allocation.
Preparation and analysis of the coffees
The study coffee brew consisted of a special roasted and blended Arabica coffee, provided by Tchibo GmbH (Hamburg, Germany). The coffee blend consisted to a major part of dark roast 100 % Arabica coffees, characterized, by high concentrations of roast coffee constituents, especially of NMP. To a smaller part, light roast coffees were present, containing high levels of green coffee constituents such as chlorogenic acids. The resulting blend showed characteristics typical for roast-associated as well as green bean-associated constituents (see Table 1). After blending, the coffee was ground and industrially filled into coffee soft pads which were then packed into appropriate sealed plastic bags using inert gas to prevent oxidation. Brews were freshly prepared in a pad machine immediately before consumption. The ground coffee was analytically characterized by HPLC–DAD and HPLC–MS/MS methods as previously described [10]. Caffeine and CQAs (sum of 5-O-, 4-O- and 3-O-CQA) were quantified by HPLC–DAD (272 and 324 nm, respectively) with external calibration [10]. Trigonelline and NMP were quantified by hydrophilic liquid interaction chromatography (HILIC–HPLC MS/MS)/stable isotope dilution analysis with d3-trigonelline and d3-N-methylpyridinium subsequent to solid phase (SPE) clean up, as reported recently [11].
Processing and storage of blood and urine samples
Venous blood samples were collected in serum tubes containing EDTA and stored at 37 °C for up to 30 min, as required by the individual sampling period. Samples were immediately thereafter worked up for the comet assay. Spot urine samples were immediately frozen at −80 °C.
Determination of spontaneous DNA strand breaks (comet assay)
Alkaline single cell gel electrophoresis was performed according to Collins et al. [12, 13], with slight modifications as reported earlier [5]. Whole blood was used without preceding isolation of lymphocytes to ascertain minimal oxidative cell damage during processing. Briefly, aliquots of blood (6 µL) were mixed with low melting agarose (65 µL), distributed onto a frosted glass microscope slide, pre-coated with a layer of normal melting agarose (2 gels per slide), coverslipped and kept at +4 °C for solidification. After removing the cover glass, slides were immersed in standard lysis solution for 1 h at +4 °C [12]. After cell lysis, slides were washed three times in enzyme buffer. DNA was allowed to unwind (pH 13.5, 20 min, +4 °C) and horizontal gel electrophoresis (Bio-Rad Sub Cell GT) was carried out at +4 °C for 20 min (25 V, 300 mA, 0.89 V/cm), using a Bio-Rad 300 power supply. Then slides were washed three times with Tris buffer (0.4 M, pH 7.5), stained with ethidium bromide (40 µL, 10 µg/mL), viewed microscopically with a Zeiss Axioskop 20 (Zeiss, Jena, Germany) and equipped with a filter set 15 (excitation: BP 546/12; emission: LP 590), magnification 63×/1.25 oil. Slides were analyzed by computerized image analysis (Comet IV, Perceptive Instruments), scoring 2 × 50 images per slide (2 gels per slide). DNA migration was directly expressed as mean tail intensity (TI %) from 2 gels. Data are presented as spontaneous DNA strand breaks. The coefficient of variation of spontaneous DNA strand break determination was 7.2 % (intra-assay, n = 5). This compares very well with the literature and is regarded as acceptable variability [14, 15].
Quantification of NMP and trigonelline in human spot urine by HPLC–MS/MS
For compliance within the long-term intervention study, NMP, trigonelline and creatinine were measured in human spot urine samples by HILIC–HPLC–MS/MS as reported recently [16]. Briefly, the spot urine (10 µL) sample was diluted with an aliquot (1 mL) of a solution of the internal standards d3-creatinine (10 µM), d3-trigonelline (1 µM) and d3-N-methylpyridinium (1 µM) in acetonitrile/water (95/5, v/v). After centrifugation, the supernatant (1 µL) was subjected to HPLC–MS/MS analysis [16].
Body weight measurement and dietary intakes
Body weight and height (digital precision scale, Seca delta 707, Hamburg, Germany) of the volunteers (in underwear) were measured, and the BMI (kg/m2) was calculated.
On the basis of 7-day food records, dietary food intake was documented by the volunteers prior to both blood drawings during the 8-week intervention study. Individual food intake was evaluated by the nutrition software package PRODI 5 (Nutri-Science GmbH, Hausach, Germany), to assess intake of kcal and nutrients (carbohydrates, fat and proteins).
Statistical methods
For statistical analysis, the two measurements (resulting from two gels per slide and blood sample, total 84 gels per group) were averaged and arithmetic means with standard deviations given. Additionally, changes in means over time were presented graphically.
All statistical hypotheses were tested against a two-sided alternative at a 5 % level of significance. The hypothesis of normality was tested by the Shapiro–Wilk test. Since normality could not be rejected, and further inductive analysis could be implemented by test procedures assuming normality. In order to verify homogeneity of study groups, a two-sample t test was applied for the baseline measurements (first blood sample). Changes in the control group over time were analyzed by a t test for paired samples. Coffee-intake-related effects were evaluated by analysis of covariance using “coffee intake” as a classification variable and incorporation of baseline measurements as a covariate. This strategy allows modeling intra-individual changes in an appropriate way. Since no multiple comparisons were conducted, an adjustment of p values was not necessary.
Results
Concentration of selected constituents in the coffee samples under study
Data on selected compounds determined in the ground coffee under study are summarized in Table 1. The study coffee consisted of a special roasted and blended Arabica coffee. The caffeine level was in the range of conventional ground coffee [17]. The high concentration of roast products such as NMP (1.10 mg/g) is in the upper range of dark roasts [18], whereas the concentration of CQAs (10.18 mg/g) is in the range expected in medium roasted coffee. These compounds have previously been shown to be extracted practically quantitatively (>90 %) during brewing at the selected ratio of water volume to coffee weight of >16 [19]. Thus, the coffee brew reflected the specific composition of the ground study coffee, containing substantial contents of both original antioxidant constituents (chlorogenic acids) and roast products such as NMP.
Urine analysis to ascertain compliance
Monitoring of NMP in the human urine after the 4-week washout period showed levels below the limit of quantification (LOQ). In the intervention phase, the control group urine samples showed no indication for NMP, while study participants of the coffee intervention group showed values of 2.0–28.7 mg NMP/g creatinine. Likewise, urinary trigonelline was significantly elevated in the coffee-consuming group (Figs. 2, 3). These NMP and trigonelline data were in agreement with the data reported recently [20].
Modulation of spontaneous DNA strand breaks
To ensure processing of the samples with minimal damage, direct processing of full blood for electrophoresis was first compared to isolating WBC by centrifugation prior to electrophoresis. Since in our hands increased spontaneous DNA strand breaks as a consequence of centrifugation became apparent [21], we decided to keep our established procedure of applying blood samples directly onto the agarose beds (see “Materials and methods” section).
Results are shown in Fig. 4a, b. At baseline (first blood collection after 4-week washout), no differences in the level of spontaneous DNA strand breaks between the two intervention groups were observable. After 4-week intervention, the consumption of water resulted in a significant increase of spontaneous DNA strand breaks in the control group (p = 0.0169). A direct comparison of control versus coffee group at the end of the intervention showed a highly significant difference (p = 0.0002) (see Fig. 4a). The observed elevation of DNA strand breaks in the control group as compared to a concomitant decrease in the coffee group is suggestive for DNA protective effects brought about by consumption of the study coffee. Fig. 4b illustrates the differential response to the intervention of the coffee group in comparison with the control group in the form of boxplots, demonstrating the marked overall effectiveness of daily coffee consumption in protecting DNA from spontaneous strand breaks.
a Spontaneous DNA strand breaks in WBC of 84 volunteers (control group n = 42, coffee group n = 42) after the study phases. Data are expressed as tail intensity in % (TI %) showing means and SD; significantly different spontaneous DNA strand breaks: ***p < 0.001, **p < 0.01, *p < 0.05. b Boxplot of control and coffee group responses in comparison with baseline (solid line) showing mean (black circle), median (dashed line) and confidence intervals. BS blood sampling
Nutrient intake and body weight
No changes in body weight of volunteers were found during the whole study period (data not shown). Daily intake of energy and nutrients of the volunteers were calculated on the basis of 7-day food records per study phase. Results showed no significant effect of coffee consumption on energy and nutrient intake (Table 2).
Discussion
The primary aim of the present investigation was to observe and, if possible, confirm the protective effect of coffee consumption on DNA integrity in humans, as reported in a previous study [5]. Monitoring of trigonelline and NMP in urine samples of all volunteers proved adherence to the study protocol and full compliance of all participants (n = 84). For the control group, water was chosen as a “placebo” because there was no coffee-placebo available that would have satisfied the necessary requirements. Nevertheless, it appeared appropriate to control for potential confounders inherent in such an 8-week intervention (e.g., inadvertent environmental, seasonal and other influences). Consumption of the dark coffee brew for 4 weeks was associated with a highly significant reduction (27 %) of spontaneous DNA strand breaks in WBC of coffee-consuming volunteers, as compared to the levels in the water control group. In contrast, water intake in the control group resulted in an increase of spontaneous DNA strand breaks.
Previously published human intervention trials following a sequential design reported a decrease of oxidative and Trp-P-2 induced DNA damage in lymphocytes (processed with formamidopyrimidine DNA glycosylase or endonuclease III) after uptake of coffee in a 5-day intervention study [8]. Similar results have been reported in a cross-over study with 38 healthy participants, showing marked protective effectiveness on oxidative DNA damage after 5-day consumption of freshly prepared filtered coffee brew rich in CQAs [9]. Likewise, a slight reduction of oxidative DNA strand breaks was reported for lymphocytes of volunteers after ingestion of instant coffee with high chlorogenic acids content, but this effect reportedly did not reach significance [6]. In all these studies, an influence on spontaneous DNA strand breaks, as shown in the present study, had not been observed. In a previous pilot single-arm sequential study by our group, spontaneous DNA strand breaks were already found reduced (down to 41 % of the initial tail intensity in 35 healthy volunteers). In the present randomized parallel study, the difference between control and coffee group (altogether 84 volunteers), although somewhat smaller, was highly significant. In single-arm sequential trials, volunteers are presumed to act as their own controls. These studies may potentially be flawed, however, by confounding factors not adequately controlled. Spontaneous DNA strand breaks may be co-influenced by confounders, such as seasonal and lifestyle effects and potentially other factorial variations with time. Therefore, a sequential design appears inferior to a design using a control group in parallel [22]. The importance of the inclusion of “placebo” groups as well as integrating washout periods to improve the quality and predictive value of such trials has been emphasized [23].
The reduction of spontaneous DNA strand breaks observed in the present study may primarily be attributed to the presence of constituents with chemopreventive properties. In addition to the spectrum of antioxidants known to be present in coffee (such as chlorogenic acids and roast-associated constituents), various physiological responses also need to be taken into consideration. For instance, induction of the Nrf2/ARE signaling pathways has been observed together with increased levels of Nrf2 dependent enzymes after consumption of coffees rich in chlorogenic acids and NMP. Likewise, an inverse association between induced Nrf2 transcription and DNA strand breaks has been observed after coffee consumption [24]. It is not known yet whether also other physiological responses, including effects on DNA repair, on intermediate metabolism or on biokinetics also may play a role. Taken together, the observed DNA protective effects associated with coffee consumption may be considered beneficial to human health.
Abbreviations
- BMI:
-
Body mass index
- TI:
-
Tail intensity
- BS:
-
Blood sampling
- NMP:
-
N-Methylpyridinium
- NQO1:
-
NAD(P)H:quinine oxidoreductase 1
- γ-GCL:
-
γ-Glutamylcysteine ligase
- Nrf2:
-
NF-E2 p45 subunit-related factor 2
References
Natella F, Scaccini C (2012) Role of coffee in modulation of diabetes risk. Nutr Rev 70(4):207–217. doi:10.1111/j.1753-4887.2012.00470.x
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Bakuradze T, Lang R, Hofmann T, Stiebitz H, Bytof G, Lantz I, Baum M, Eisenbrand G, Janzowski C (2010) Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol Nutr Food Res 54(12):1734–1743. doi:10.1002/mnfr.201000147
Boettler U, Sommerfeld K, Volz N, Pahlke G, Teller N, Somoza V, Lang R, Hofmann T, Marko D (2011) Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. J Nutr Biochem 22(5):426–440. doi:10.1016/j.jnutbio.2010.03.011
Bakuradze T, Boehm N, Janzowski C, Lang R, Hofmann T, Stockis JP, Albert FW, Stiebitz H, Bytof G, Lantz I, Baum M, Eisenbrand G (2011) Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: results from an intervention study. Mol Nutr Food Res 55(5):793–797. doi:10.1002/mnfr.201100093
Hoelzl C, Knasmuller S, Wagner KH, Elbling L, Huber W, Kager N, Ferk F, Ehrlich V, Nersesyan A, Neubauer O, Desmarchelier A, Marin-Kuan M, Delatour T, Verguet C, Bezencon C, Besson A, Grathwohl D, Simic T, Kundi M, Schilter B, Cavin C (2010) Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol Nutr Food Res 54(12):1722–1733. doi:10.1002/mnfr.201000048
Steinkellner H, Hoelzl C, Uhl M, Cavin C, Haidinger G, Gsur A, Schmid R, Kundi M, Bichler J, Knasmuller S (2005) Coffee consumption induces GSTP in plasma and protects lymphocytes against (±)-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide induced DNA-damage: results of controlled human intervention trials. Mutat Res 591(1–2):264–275
Bichler J, Cavin C, Simic T, Chakraborty A, Ferk F, Hoelzl C, Schulte-Hermann R, Kundi M, Haidinger G, Angelis K, Knasmuller S (2007) Coffee consumption protects human lymphocytes against oxidative and 3-amino-1-methyl-5H-pyrido[4,3-b]indole acetate (Trp-P-2) induced DNA-damage: results of an experimental study with human volunteers. Food Chem Toxicol 45(8):1428–1436
Misik M, Hoelzl C, Wagner KH, Cavin C, Moser B, Kundi M, Simic T, Elbling L, Kager N, Ferk F, Ehrlich V, Nersesyan A, Dusinska M, Schilter B, Knasmuller S (2010) Impact of paper filtered coffee on oxidative DNA-damage: results of a clinical trial. Mutat Res 692(1–2):42–48. doi:10.1016/j.mrfmmm.2010.08.003
Weiss C, Rubach M, Lang R, Seebach E, Blumberg S, Frank O, Hofmann T, Somoza V (2010) Measurement of the intracellular pH in human stomach cells: a novel approach to evaluate the gastric acid secretory potential of coffee beverages. J Agric Food Chem 58(3):1976–1985. doi:10.1021/jf903614d
Lang R, Wahl A, Stark T, Hofmann T (2012) Identification of urinary and salivary biomarkers for coffee consumption. In: Recent advances in the analysis of foods and flavors. ACS symposium series edited by Toth S et al
Collins AR, Dusinska M, Gedik CM, Stetina R (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104(Suppl 3):465–469
Collins AR (2013) Measuring oxidative damage to DNA and its repair with the comet assay. Biochim Biophys Acta. doi:10.1016/j.bbagen.2013.04.022
Azqueta A, Gutzkow KB, Priestley CC, Meier S, Walker JS, Brunborg G, Collins AR (2013) A comparative performance test of standard, medium- and high-throughput comet assays. Toxicol In Vitro 27(2):768–773. doi:10.1016/j.tiv.2012.12.006
Azqueta A, Collins AR (2013) The essential comet assay: a comprehensive guide to measuring DNA damage and repair. Arch Toxicol 87(6):949–968. doi:10.1007/s00204-013-1070-0
Lang R, Wahl A, Stark T, Hofmann T (2011) Urinary N-methylpyridinium and trigonelline as candidate dietary biomarkers of coffee consumption. Mol Nutr Food Res 55(11):1613–1623. doi:10.1002/mnfr.201000656
Fujioka K, Shibamoto T (2008) Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem 106(1):217–221
Stadler RH, Varga N, Milo C, Schilter B, Vera FA, Welti DH (2002) Alkylpyridiniums. 2. Isolation and quantification in roasted and ground coffees. J Agric Food Chem 50(5):1200–1206
Lang R, Yagar EF, Wahl A, Beusch A, Dunkel A, Dieminger N, Eggers R, Bytof G, Stiebitz H, Lantz I, Hofmann T (2013) Quantitative studies on roast kinetics for bioactives in coffee. J Agric Food Chem 61(49):12123–12128. doi:10.1021/jf403846g
Kotyczka C, Boettler U, Lang R, Stiebitz H, Bytof G, Lantz I, Hofmann T, Marko D, Somoza V (2011) Dark roast coffee is more effective than light roast coffee in reducing body weight, and in restoring red blood cell vitamin E and glutathione concentrations in healthy volunteers. Mol Nutr Food Res 55(10):1582–1586. doi:10.1002/mnfr.201100248
Bakuradze T, Baum M, Richling E (2011) Sample preparation modulating the results of the comet assay; abstracts of UKEMS/Dutch EMS-sponsored workshop on biomarkers of exposure and oxidative DNA damage and 7th GUM 32P-Postlabelling workshop. Münster, Germany. March 28–29, 2011. Mutagenesis 26(5):718. doi:10.1093/mutage/ger026
Hoelzl C, Knasmuller S, Misik M, Collins A, Dusinska M, Nersesyan A (2009) Use of single cell gel electrophoresis assays for the detection of DNA-protective effects of dietary factors in humans: recent results and trends. Mutat Res 681(1):68–79. doi:10.1016/j.mrrev.2008.07.004
Moller P, Loft S (2006) Dietary antioxidants and beneficial effect on oxidatively damaged DNA. Free Radic Biol Med 41(3):388–415. doi:10.1016/j.freeradbiomed.2006.04.001
Volz N, Boettler U, Winkler S, Teller N, Schwarz C, Bakuradze T, Eisenbrand G, Haupt L, Griffiths LR, Stiebitz H, Bytof G, Lantz I, Lang R, Hofmann T, Somoza V, Marko D (2012) Effect of coffee combining green coffee bean constituents with typical roasting products on the Nrf2/ARE pathway in vitro and in vivo. J Agric Food Chem 60(38):9631–9641. doi:10.1021/jf302258u
Acknowledgments
We are grateful for the contribution of the participants in the study. We thank Dirk Galan and Axel Stachon for their support during the study. The authors thank Sylvia Schmidt for performing the comet measurements and Anja Beusch for technical assistance.
Conflict of interest
This study has been supported by Tchibo GmbH, Hamburg. G. Eisenbrand is scientific advisor within the BMBF cluster projects, grants no 0313843 and 0315692, with Tchibo GmbH and with the Institute for Scientific Information on Coffee, La Tour de Peilz, Switzerland (ISIC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakuradze, T., Lang, R., Hofmann, T. et al. Consumption of a dark roast coffee decreases the level of spontaneous DNA strand breaks: a randomized controlled trial. Eur J Nutr 54, 149–156 (2015). https://doi.org/10.1007/s00394-014-0696-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0696-x