Abstract
Purpose
Obesity increases the risk of diabetes. The dysregulation of estrogen metabolism has been associated with the susceptibility to obesity and diabetes. Here, we explore the role estrogen plays in sex differences in obesity and glucose metabolism, specifically adipocyte biology.
Methods
We randomized C57BL/6 J male, non-ovariectomized female, ovariectomized female, and ovariectomized female mice supplemented with 17β estradiol to receive a calorie-restricted, low- or a high-fat diet (15 mice per group). We measured weight gained, calories consumed, percent body fat, abdominal adipose tissue, adipocyte size, lipogenic and adipogenic gene expression, and glucose tolerance.
Results
Male mice had a higher susceptibility to obesity than intact female mice. However, removal of the ovaries in female mice eliminated the protection to obesity and estrogen supplementation restored this protection. Male and ovariectomized female mice gained weight predominately in the form of abdominal adipose tissue possibly due to an increase in adipocyte size. Moreover, for mice consuming the high-fat diet, male and ovariectomized female mice had significantly higher levels of leptin mRNA and lower hormone-sensitive lipase mRNA relative to intact female mice and ovariectomized female mice supplemented with estrogen. Additionally, estrogen had a strong inhibitory effect on key adipogenic genes in non-ovariectomized female and ovx-female mice supplemented with estrogen. Finally, we show that male and ovariectomized female mice consuming the high-fat diet had a higher incidence of glucose intolerance.
Conclusion
Estrogen protects female mice from obesity and impaired glucose tolerance possibly by modulating the expression of genes regulating adipogenesis, lipogenesis, and lipolysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of obesity has grown at an epidemic rate at both a national and global level [1]. One-third of Americans are considered to be obese, which is defined as a body mass index (BMI) of >30 kg/m². Obesity increases the risk of numerous diseases, such as diabetes mellitus (type 2 diabetes). The role sex plays in the susceptibility to obesity is not fully understood, specifically the role of ovarian hormones in response to different amounts of caloric consumption. Previously, our lab demonstrated that male mice were more likely to become obese compared to novx-female mice [2]. Furthermore, it has been established that the protection against obesity in female mice is eliminated by ovariectomy (surgical removal of the ovaries) and can be reversed through the administration of estrogen [3–5]. Various mouse models have been used to assess the significance of estrogen in the regulation of abdominal adiposity. In both ERαKO and ARKO mice, results show that a decrease in systemic estrogen levels and signaling can cause a significant increase in abdominal adiposity [6–8]. Moreover, estrogen has been shown to decrease food consumption and promote energy expenditure, thus creating a negative energy balance and preventing obesity [9–11]. The role of estrogen in the regulation of lipogenesis is unclear. Previous research has shown that estrogen suppresses lipoprotein lipase (LPL) transcription possibly due to an estrogen response element that is located in the promoter region of this gene [12]. Moreover, others have established that estrogen stimulates lipolysis in adipocytes possibly through the regulation of perilipin [13]. The effect of estrogen in adipogenesis is still convoluted and needs further exploration. Specifically, it has yet to be determined whether estrogen affects the early or late events of adipogenesis and if it is modulating key adipogenic genes, such as peroxisome proliferator-activated receptor-gamma (PPARγ), ccaat-enhancer-binding proteins (CEBPβ), and lipin1. PPARγ and CEBPβ are key transcription factors that are highly regulated during adipogenesis, and lipin1 has recently been shown to be essential for adipocyte development [14–16]. Adipocyte biology has the ability to affect many physiological processes, particularly glucose metabolism. Our laboratory and others have demonstrated that the higher susceptibility to obesity in male mice is associated with a higher propensity of becoming insulin resistant [17, 18]. Moreover, studies have identified a significant correlation between small adipocyte size and improved insulin sensitivity in female mice [19]. However, it is not clear how estrogen modulates the adipocyte biology process and how these differences may affect other morbidities. Thus, the objective of the present study is to determine if estrogen can protect female mice from obesity and its morbidities by modulating key genes involved in adipocyte biology.
To determine the role of estrogen in the sex differences in the susceptibility to obesity and diabetes, we used the following groups of C57BL/6 J mice in our studies: (1) males, (2) non-ovariectomized females (novx-females), (3) ovariectomized females (ovx-females), and (4) ovariectomized females supplemented with estrogen (ovx-females + E2), which were randomized to receive one of three different diets: 15–30% calorie-restricted, low-fat or high-fat diet (CR, LF, and HF). Our results show that when exposed to a high-fat diet, male mice became obese sooner than female mice and that removal of the ovaries eliminated the protection against obesity in female mice. In fact, ovx-female mice weight gain patterned that of male mice. However, supplementation of 17β-estradiol to ovx-female mice restored the protection against obesity observed in novx-female mice. This difference in bodyweight was also reflected in body adiposity, specifically abdominal adiposity and adipocyte size. Estrogen also had a significant effect on lipogenic and adipogenic genes. Moreover, male and ovx-female mice consuming a high-fat diet had significantly impaired glucose tolerance suggesting that males and ovx-females may have a higher susceptibility to become insulin resistance. This finding was further supported by the higher insulin and resistin serum levels observed in the male and ovx-female mice consuming the high-fat diet.
Materials and methods
Mouse husbandry and diets
A total of 195 pathogen-free C57BL/6 J male, non-ovariectomized female, ovariectomized female, and sham-ovariectomized female mice were purchased from Jackson Labs (Bar Harbor, Maine, USA) at 6 weeks of age and housed according to NIH guidelines (National Research Council, 1996) in the Animal Resources Center at the University of Texas at Austin. The animal protocol was approved by the Institutional Animal Care and Use Committee at UT-Austin. The mice were singly housed and maintained on a 12-h light–12-h dark cycle and at a temperature of 22–24 °C. After 2 weeks of acclimation, the mice were randomized, 15 mice per group, to receive one of three semi-purified diet regimens: (1) a 15–30% calorie-restricted diet (CR; D03020702), (2) a low-fat diet (LF; 10% fat from kcals, D12450B), and (3) a high-fat diet (HF; 60% fat from kcals, D12492). To control for the effects of surgery on bodyweight and glucose metabolism, we included sham-ovariectomized female mice who consumed the low-fat diet (n = 15). All diets were obtained from Research Diets, Inc. and are semi-purified diets (New Brunswick, NJ, USA). A table with detailed information on these diets was previously described [20]. Briefly, the fiber content is identical in all three diets (50 g), and the caloric content is identical in both the CR and LF diet (3.8 kcal/g), but the HF diet is hypercaloric (5.2 kcal/g). The CR diet was modified so that the mice received 70–85% (2.7 g/day) of the mean daily caloric consumption of their respected control (LF) group, but 100% of the vitamins and minerals. Mice were fed ad libitum or calorie restricted (one 2.7 g pellet/day); body weight, food, and liquid consumption were recorded weekly. All mice, including the sham-ovariectomized mice, were killed after 10 weeks.
Estrogen supplementation
To further characterize the role estrogen plays in the susceptibility to obesity and glucose metabolism, we implanted a 0.72 mg 17β estradiol pellet into ovariectomized female mice, which delivered 5 μg/d (Innovative Research of America, Sarasota, USA). This dosage protocol is similar to the estradiol supplementation used by others and has been shown to re-establish physiological estradiol levels in ovariectomized female mice [21]. Control mice were implanted with placebo pellets. At 9 weeks of age, ovariectomized mice were randomized to receive either a placebo or an estradiol pellet. Mice were anesthetized with isoflurane, the dorsal area between the ear and shoulder was shaved and sterilized with 70% isopropyl alcohol, and a trochar was used to implant the 4.5-mm pellet subcutaneously.
Body composition
Body composition was determined using magnetic resonance imaging (MRI), specifically, the EchoMRI QNMR from Jackson Labs (Bar Harbor, Maine, USA). This device allowed us to measure lean mass, percent body fat, and water content without sedating the mice.
Assessment of abdominal adiposity
After necropsy, all mice were thawed at room temperature and visceral adipose tissue was removed from the entire abdominal cavity of the mouse and weighed. The abdominal fat depots collected included gonadal, perirenal, and omental fat pads. The percent abdominal fat represents the total weight of the abdominal adipose tissue divided by the total weight of the mouse.
Assessment of other adipose tissue depots
After necropsy and the removal of all abdominal adipose tissue, the remaining adipose tissue content was determined by dual-energy X-ray absorptiometry (DEXA) using a GE Lunar Piximus II densitometer (Madison, WI, USA). The percent of other adipose tissue depots represents the weight of the remaining adipose tissue after the removal of the abdominal fat determine by DEXA divided by the final body weight.
Measurement of adipocyte size
At necropsy, perigonadal adipose tissue was fixed in 10% neutral buffered formalin for 48 h and then transferred to 70% ethanol indefinitely. Adipose tissue was paraffin-embedded and cut 5 μm thick. For histological analysis, tissues were hematoxylin and eosin-stained. The size of the adipocytes was determined using Nikon’s NIS Elements AR software (Melville, NY, USA). Eight samples from each group were randomly selected for analysis.
QRTPCR
Total RNA was extracted from frozen white adipose tissue using an RNAeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. RNA content was determined by measuring the absorbance at 260 and 280 nm. Reverse transcription was conducted with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), using 2 μg of RNA for each reaction. Real-time PCR was performed with a SYBR GreenER qPCR kit (Invitrogen, Carlsbad, CA) and a Mastercycle Realplex Thermocycler (Eppendorf, Hamburg, Germany). The relative expression level of each target gene was normalized to the endogenous reference control gene 18 s rRNA. Moreover, the male mice were used as the calibrator to which all other groups were compared against using the CT method. The primers are available upon request.
Glucose tolerance test
To establish the role of estrogen in glucose metabolism, ten randomly selected mice were fasted for 14 h and then intraperitoneally injected with 20% glucose, 2 g/kg of bodyweight. Blood glucose was measured using a Glucometer Elite (Bayer, Elkhart, IN). Approximately half a drop of blood was drawn from each mouse tail. Blood glucose levels were measured at 0, 15, 30, 60, and 120 min from injection time and area under the curve was calculated.
Measurement of serum hormones
In order to assess the sex differences in serum hormone levels, we measured serum levels of leptin, insulin, and resistin in high-fat fed mice. Mice were fasted 3 h prior to necropsy and serum was collected. Serum leptin, insulin, and resistin were detected using Millipore’s Milliplex Map Mouse Serum Adipokine Panel (Billerica, MA).
Statistics
To determine the effects of both diet and sex, results were analyzed by ANOVA with pairwise comparisons and a post hoc comparison of means using Tukey’s Honestly Significant Difference. All results are presented as mean ± standard error mean (SEM). SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical comparisons. p values ≤ 0.05 were considered statistically significant.
Results
Sex differences in bodyweight
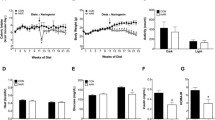
The three different diet regimens (CR, LF, and HF) induce three different body phenotypes (lean, overweight, and obese). Figure 1 shows the body weights induced by the diets. There were no significant differences in body weights in the CR groups. In the LF groups, male and ovx-female mice weighed significantly more compared to novx-female and ovx-female + E2 mice (p < 0.05). After 10 weeks of consuming a high-fat diet, male mice weighed significantly more than novx-female mice (39.7 ± 0.95 vs. 26.8 ± 1.2 g, p < 0.001). Moreover, when the ovaries were removed, the ovx-female mice mimicked male mice in their weight gain (37.1 ± 0.87 g, p < 0.001). However, estrogen supplementation restored the protection against obesity, and ovx-female + E2 mice resembled the body weight of novx-female mice (24.6 ± 1.0 g, p < 0.001).
Diet-induced obesity. a mice were maintained on one of three different diet regimens (calorie restricted (CR), low fat (LF), high fat (HF)) for 10 weeks with n = 15 per diet group. *Significantly different compared to novx-female and ovx-female + E2 mice in the low-fat diet group, p < 0.05 (n = 15). **Significantly different compared to novx-female and ovx-female +E2 mice in the high-fat diet group, p < 0.001 (n = 15). b images were taken at the time of necropsy (week 10) and were captured from mice exposed to a high-fat diet
Food consumption
To determine if the differences in bodyweight were due to differences in food consumption, the average daily caloric intake (kcal/d) was assessed for each group. This data are presented in Table 1. As expected, caloric consumption between CR, LF, and HF groups was significantly different; CR consumed the least amount of calories and HF consumed the most. All of the mice received the same amount of calories per day in the CR groups. In the groups consuming the low-fat diet, there were no significant differences in calorie consumption between novx-female, ovx-female, and ovx-female + E2 mice. In the mice consuming the high-fat diet, calorie consumption was not significantly different between male, novx-female, and ovx-female mice.
Estrogen alters body composition by modulating abdominal adiposity
Figure 2 shows the sex differences in adipose tissue deposition. Panel A represents abdominal adiposity. Overall, there was a diet interaction within each gender, with CR mice displaying the least amount of abdominal adiposity and HF mice displaying the most abdominal adiposity. However, after 10 weeks on the high-fat diet, male and ovx-female mice had significantly higher abdominal fat levels compared to novx-female and ovx-females + E2 mice (9.67 ± 0.54% and 11.41 ± 0.27% vs. 6.99 ± 0.8% and 2.86 ± 1.28%, respectively, p < 0.001). Panel B represents the percentage of other adipose tissue depots, which primarily consists of subcutaneous adipose tissue. There were no significant differences in these adiposity levels between male, novx-female, and ovx-female mice; however, ovx-female + E2 mice had lower other adiposity levels compared to the other groups. Panel C represents total adiposity. Within the high-fat diet group, male and ovx-female mice had significantly higher levels of total adiposity compared to novx-female and ovx-female + E2 mice (36.6 ± 1.9% and 50.1 ± 1.2% vs. 24.3 ± 3.6% and 17.2 ± 4.2%), respectively, p < 0.001. Our results suggest that the difference in adiposity identified between the sexes is due to alterations in abdominal adiposity.
Body composition. a shows sex differences in abdominal adiposity. b represents other adipose tissue depots, which was assessed by DEXA after the complete removal of abdominal adipose tissue. c shows total body adiposity which was determined in 8 mice per group with a magnetic resonance imaging (MRI) machine. *Significantly different compared to novx-female and ovx-female + E2 mice within the low-fat diet groups (n = 8). **Significantly different compared to novx-female and ovx-female + E2 mice in high-fat diet group, p < 0.001 (n = 8). +Significantly different compared to ovx-female mice consuming the low-fat diet group, p < 0.05. ++Significantly different compared to all other groups consuming the high-fat diet, p < 0.05
Estrogen decreases adipocyte size
To determine if this increase in abdominal adiposity was due to adipocyte hypertrophy, we assessed adipocyte size. Results in Fig. 3 show CR mice had the smallest, LF had the intermediate, and HF mice had the largest adipocytes. We also found a strong relationship between sex and adipocyte size. When exposed to a high-fat diet, male mice had significantly larger fat cells compared to novx-female mice (p < 0.001). However, removal of the ovaries in female mice made the size of the fat cells between male and ovx-female mice similar. Treatment of ovx-female mice with estrogen significantly decreased the size of their fat cells (p < 0.001), which tended to be of similar size or smaller than those found in novx-female mice. Moreover, in groups consuming the low-fat diet, ovx-female mice had significantly larger adipocytes compared to novx-female and ovx-female + E2 mice (p < 0.001).
Adipocyte area. a Adipose tissue was collected from the perigonadal region of each mouse and stained with hematoxylin and eosin. Two images were taken from each slide at 10× magnification. b shows the average size of the fat cells for each group. Adipocyte size was determined by Nikon’s NIS Elements AR software, scale bar equals 100 μm. *Significantly different compared to novx-female and ovx-female + E2 mice consuming the low-fat diet (n = 7). **Significantly different compared to novx-female and ovx-female + E2 mice in high-fat diet group, p < 0.001 (n = 7)
Sex differences in lipogenic, lipolytic, and adipogenic gene expression
To gain a better understanding of the role of estrogen in the expression of adipogenic genes, we measured their mRNA levels. As a positive control, we measured leptin mRNA levels, which have been shown to correlate with total adiposity [10]. We selected to detect the expression of adipogenic genes only in the mice consuming the high-fat diet, because in this group of mice we observed the most drastic increases in adiposity. Briefly, we found that similar to the total adiposity levels, male and ovx-female mice had significantly higher leptin mRNA levels relative to novx-female and ovx-female + E2 mice, p < 0.05. In addition, we measured mRNA levels of lipoprotein lipase (LPL) and hormone-sensitive lipase (HSL). There was no significant difference in LPL mRNA levels, but novx-female (2.5×) and ovx-female + E2 mice (2.76×) had significantly higher mRNA levels of HSL compared to male and ovx-female mice, Fig. 4. Results also show that ovx-female mice had significantly higher PPARγ mRNA levels compared to novx-female and ovx-female + E2 mice (1.3× vs. −2.79× and −4.63×, respectively, p < 0.05). Moreover, male mice had significantly higher mRNA levels of cebpβ compared to novx-female and ovx-female + E2 mice. Interestingly, estrogen had a strong effect of lipin1 mRNA levels. Novx-female and ovx-female + E2 mice had significantly lower mRNA levels compared to male mice (−15.76× and −10.89× vs. 1×, respectively, p < 0.05), Fig. 5.
Lipogenic and lipolytic genes. As a positive control, we measured leptin mRNA levels in the high-fat fed mice, which were consistent with adiposity levels. *Significantly different compared to novx-females and ovx-females + E2 with the same gene, p < 0.05. The relative expression level of each target gene was normalized to the endogenous reference control gene 18 s rRNA and the male mice were used as the calibrator to which all other groups were compared against using the CT method. MRNA levels less than 1 are presented by their negative reciprocal
Adipogenic genes. To determine how estrogen affects key adipogenic genes, we measured mRNA levels of PPARγ, cebpβ, and lipin1 from adipose tissue collected from the perigonadal depot of high-fat fed mice. *Significantly different compared to novx-female and ovx-female + E2 mice, p < 0.05. +Significantly different compared to ovx-female + E2 mice, p < 0.05. The relative expression level of each target gene was normalized to the endogenous reference control gene 18 s rRNA and the male mice were used as the calibrator to which all other groups were compared against using the CT method. MRNA levels less than 1 are presented by their negative reciprocal
Estrogen protects female mice from glucose intolerance
To determine if there were sex differences in the susceptibility to diabetes, we conducted a glucose tolerance test. Briefly, Fig. 6a shows that male mice were glucose intolerant (glucose disappearance rate was lower) compared to novx-female mice, and removal of the ovaries caused the female mice to mimic the glucose intolerance observed in the male mice. However, supplementation with estrogen to the ovx-female mice improved their glucose tolerance to levels similar of novx-female mice. Figure 6b is a graphical representation of the calculated area under the curve for the glucose tolerance test.
Glucose tolerance test. On the 10th week, GTT was performed after a 14-h overnight fast. Mice were injected with 20% glucose intraperitoneally, and a small drop of blood was collected from the tail to measure blood glucose levels at 0, 15, 30, 60, 120 min after injection. a after 10 weeks of consuming a high-fat diet, male and ovx-female mice had significantly impaired glucose tolerance compared to novx-female and ovx-female + E2 mice. Area under the curve was calculated and is graphically shown in b. *Significantly different compared to novx-female and ovx-female + E2 mice consuming the low-fat diet, p < 0.05 (n = 10). **Significantly different compared to novx-female and ovx-female + E2 mice consuming the high-fat diet, p < 0.001 (n = 10)
Sex differences in serum hormone levels
To assess the systemic effects of estrogen on serum hormone levels, we measured serum leptin, insulin, and resistin in mice consuming the high-fat diet. Briefly, in Fig. 7a, we detected significantly higher serum leptin levels in male and ovx-female mice. These results are comparable to the leptin mRNA levels found in the adipose tissue of male and ovx-female mice. Moreover, in Fig. 7b and c, we detected higher serum levels of insulin and resistin in the male and ovx-female mice, which is suggestive of insulin resistance and supports our findings of impaired glucose tolerance in the male and ovx-female mice.
Serum hormone levels. After 3 h of fasting, serum was collected from the mice. Using mice consuming the high-fat diet, we detected serum leptin, insulin, and resistin levels using Millipore’s Milliplex Map Mouse Serum Adipokine Panel (a, b, c, respectively). *Significantly different compared to novx-female and ovx-female + E2 mice consuming the high-fat diet, p < 0.05 (n = 8)
Discussion
The specific role estrogen plays in obesity and diabetes sex disparities is not completely understood. Our studies suggest male mice are more susceptible to obesity and impaired glucose tolerance due to an increase in abdominal adiposity secondary to adipocyte hypertrophy. Moreover, we show estrogen has a significant role in altering genes regulating adipogenesis, lipogenesis, and lipolysis and could therefore be a major player in the prevention of obesity and its co-morbidities.
We propose that estrogen modulates central adipose tissue metabolism to protect female mice from the obesogenic effects of a high-fat diet. Our data confirm what others have shown: as body weight increases, percent body fat becomes elevated and adipocyte size expands [22]. Moreover, we observed that body composition was modulated by estrogen. Briefly, our results show estrogen had a dramatic effect on abdominal adiposity. Others have shown that the effects of estrogen on adiposity occur predominantly in abdominal fat depots as opposed to subcutaneous fat depots [13]. Additionally, even though body weights between male and ovx-female mice were similar, ovx-female mice tended to have more abdominal and subcutaneous fat depots. Furthermore, the adipocytes of ovx-female mice were larger than those of male mice and their adipocyte gene expression differed. Specifically, ovx-female mice exhibited much lower cebpβ and lipin1 mRNA expression levels relative to male mice. Although not significant, mRNA expression levels were considerably decreased compared to male mice which might suggest sex differences in adipogenesis independent of estrogen. Male mice have more testosterone than ovx-female mice; on the other hand, as the male mice become obese, testosterone levels decrease which can contribute to the loss of lean body mass [23]. Furthermore, research has shown that low levels of testosterone in men and high testosterone levels in post-menopausal women increase their susceptibility to diabetes [24].
It is well established that adipocyte size strongly correlates with the incidence of certain chronic diseases, such as diabetes [25, 26]. Small fat cells are strongly associated with insulin sensitivity independent of BMI [27]. Moreover, the expression of lipogenic genes is inversely correlated with adipocyte size [27]. The presence of large adipocytes has been linked to the increased production of hormones and other bioactive substances, such as leptin, insulin, IGF-1, pro-inflammatory cytokines, and reactive oxygen species [28–30]. Our results show that when fed a high-fat diet, the adipocytes of male and ovx-female mice increased in size which was accompanied by higher serum levels of leptin, insulin, and resistin. However, the adipocytes of novx-female and ovx-female + E2 mice were resistant to enlargement, suggesting that estrogen could prevent the increase in adipocyte size by possibly interfering with the expression of certain lipogenic genes [31]. In fact, our results show that estrogen increased HSL mRNA levels and others have demonstrated that estrogen inhibits lipogenesis through interactions with lipoprotein lipase [32, 33]. Our findings also imply a strong role for estrogen in adipogenesis as evidenced by significantly lower PPARγ, cebpβ, and lipin1 mRNA levels. PPARγ and cebpβ are key transcription factors shown to be essential in adipocyte development [34]. The Lipin1 gene is also essential for adipocyte development as mice deficient in lipin1 suffer from lipodystrophy and display hepatic steatosis [35]. Therefore, our data suggest that estrogen could be important in adipocyte development and as a result could play a significant role in protecting female mice from obesity and its co-morbidities.
Although estrogen has a prominent effect on genes regulating adipocyte morphology, it is important to note that estrogen could prevent obesity through other means. For instance, estrogen has been documented to increase physical activity by stimulating ERα in the hypothalamus [11]. Moreover, the removal of the ovaries can cause a drastic decrease in the metabolic rate of mice [11]. Additionally, studies have shown a potential role for ERβ in the prevention of obesity; therefore, this could be another alternative mechanism employed by estrogen to protect female mice from obesity [36]. Furthermore, estrogen also has a stimulatory role in lipid oxidation in both the liver and the muscle by up-regulating genes involved in lipid oxidation [37–39]. Thus, it is conceivable that the positive effect of estrogen on energy expenditure could also play an important role in preventing obesity.
Conclusion
In summary, we show that estrogen protects female mice from obesity and impaired glucose tolerance by modulating genes regulating lipogenesis, lipolysis, and adipogenesis.
References
Flegal K, Carroll M, Ogden C, Johnson C (2002) Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727
Hong J, Stubbins R, Smith R, Harvey A, Núñez N (2009) Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J 8:11–16
Cooke PS, Naaz A (2004) Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood) 229:1127–1135
Wade GN, Gray JM, Bartness TJ (1985) Gonadal influences on adiposity. Int J Obes 9 Suppl 1:83–92
Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, Di Carlo C, Nappi C, Di Carlo R (2004) Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology 145:3115–3121
Jones M, Thorburn A, Britt K, Hewitt K, Wreford N, Proietto J, Oz O, Leury B, Robertson K, Yao S, Simpson E (2000) Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97:12735–12740
Hao L, Wang Y, Duan Y, Bu S (2010) Effects of treadmill exercise training on liver fat accumulation and estrogen receptor alpha expression in intact and ovariectomized rats with or without estrogen replacement treatment. Eur J Appl Physiol 109:879–886
Heine P, Taylor J, Iwamoto G, Lubahn D, Cooke P (2000) Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97:12729–12734
Brown LM, Clegg DJ (2010) Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol 122:65–73
Eckel LA (2011) The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav 104:517–524
Musatov S, Chen W, Pfaff D, Mobbs C, Yang X, Clegg D, Kaplitt M, Ogawa S (2007) Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 104:2501–2506
Homma H, Kurachi H, Nishio Y, Takeda T, Yamamoto T, Adachi K, Morishige K, Ohmichi M, Matsuzawa Y, Murata Y (2000) Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J Biol Chem 275:11404–11411
D’Eon T, Souza S, Aronovitz M, Obin M, Fried S, Greenberg A (2005) Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280:35983–35991
Wu Z, Xie Y, Bucher NL, Farmer SR (1995) Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes Dev 9:2350–2363
Phan J, Peterfy M, Reue K (2005) Biphasic expression of lipin suggests dual roles in adipocyte development. Drug News Perspect 18:5–11
Tontonoz P, Hu E, Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79:1147–1156
Yakar S, Nunez N, Pennisi P, Brodt P, Sun H, Fallavollita L, Zhao H, Scavo L, Novosyadlyy R, Kurshan N, Stannard B, East-Palmer J, Smith N, Perkins S, Fuchs-Young R, Barrett J, Hursting S, LeRoith D (2006) Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology 147:5826–5834
Giovannucci E, Ascherio A, Rimm E, Colditz G, Stampfer M, Willett W (1995) Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 122:327–334
Macotela Y, Boucher J, Tran TT, Kahn CR (2009) Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 58:803–812
Núñez N, Carpenter C, Perkins S, Berrigan D, Jaque S, Ingles S, Bernstein L, Forman M, Barrett J, Hursting S (2007) Extreme obesity reduces bone mineral density: complementary evidence from mice and women. Obesity (Silver Spring) 15:1980–1987
Karas RH, Schulten H, Pare G, Aronovitz MJ, Ohlsson C, Gustafsson JA, Mendelsohn ME (2001) Effects of estrogen on the vascular injury response in estrogen receptor alpha, beta (double) knockout mice. Circ Res 89:534–539
Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner A, Cushman S, Periwal V (2009) Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol 5:e1000324
Hinz S, Rais-Bahrami S, Kempkensteffen C, Weiske W, Miller K, Magheli A (2010) Effect of obesity on sex hormone levels, antisperm antibodies, and fertility after vasectomy reversal. Urology 76:851–856
Oh J, Barrett-Connor E, Wedick N, Wingard D, Study RB (2002) Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care 25:55–60
de Ferranti S, Mozaffarian D (2008) The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem 54:945–955
Imbeault P, Lemieux S, Prud’homme D, Tremblay A, Nadeau A, Després J, Mauriège P (1999) Relationship of visceral adipose tissue to metabolic risk factors for coronary heart disease: is there a contribution of subcutaneous fat cell hypertrophy? Metabolism 48:355–362
Roberts R, Hodson L, Dennis A, Neville M, Humphreys S, Harnden K, Micklem K, Frayn K (2009) Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia 52:882–890
Fenton J, Nuñez N, Yakar S, Perkins S, Hord N, Hursting SD (2009) Diet-induced adiposity alters the serum profile of inflammation in C57BL/6 N mice as measured by antibody array. Diabetes Obes Metab 11:343–354
Frederich R, Hamann A, Anderson S, Löllmann B, Lowell B, Flier J (1995) Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1:1311–1314
Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, Prabhala A, Afzal A, Garg R (2001) The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 86:355–362
Lundholm L, Zang H, Hirschberg A, Gustafsson J, Arner P, Dahlman-Wright K (2008) Key lipogenic gene expression can be decreased by estrogen in human adipose tissue. Fertil Steril 90:44–48
Hewitt K, Pratis K, Jones M, Simpson E (2004) Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology 145:1842–1848
Abraham S, Hillyard L, Hansen F, Lin C (1980) Tissue specificity for the effect of estrogen on lipogenic activity in male and female rats. Biochim Biophys Acta 620:167–171
Hu E, Tontonoz P, Spiegelman BM (1995) Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA 92:9856–9860
Koh YK, Lee MY, Kim JW, Kim M, Moon JS, Lee YJ, Ahn YH, Kim KS (2008) Lipin1 is a key factor for the maturation and maintenance of adipocytes in the regulatory network with CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma 2. J Biol Chem 283:34896–34906
Yepuru M, Eswaraka J, Kearbey J, Barrett C, Raghow S, Veverka K, Miller D, Dalton J, Narayanan R (2010) Estrogen receptor-{beta} selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem 285:31292–31303
Campbell SE, Mehan KA, Tunstall RJ, Febbraio MA, Cameron-Smith D (2003) 17beta-estradiol upregulates the expression of peroxisome proliferator-activated receptor alpha and lipid oxidative genes in skeletal muscle. J Mol Endocrinol 31:37–45
Campbell S, Febbraio M (2001) Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am J Physiol Endocrinol Metab 281:E803–E808
Morise A, Thomas C, Landrier J, Besnard P, Hermier D (2009) Hepatic lipid metabolism response to dietary fatty acids is differently modulated by PPARalpha in male and female mice. Eur J Nutr 48:465–473
Acknowledgments
This work was supported by American Cancer Society grant ACS RSG CNE-113703 and by grants from the National Institutes of Health: National Cancer Society grant NCI 1K22CA127519-01A1 and National Institute of Environmental Health Sciences Center grants ES09145 and ES007784.
Conflict of interest
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stubbins, R.E., Holcomb, V.B., Hong, J. et al. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr 51, 861–870 (2012). https://doi.org/10.1007/s00394-011-0266-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0266-4