Abstract
Background
In human beings, women are at lower risk of cardiovascular diseases, and respond differently from men to dietary fatty acids.
Aim
The aim of the present study was to investigate (i) the influence of gender on the response of lipid metabolism to dietary n-3 PUFA, and (ii) the contribution of PPARα to this response.
Methods
Male and female mice, wild-type (WT) and PPARα-null (KO), were fed on diets rich in either saturated FA (SFA) or 18:3 n-3 (ALA). Lipid composition, mRNA levels and certain activities of key enzymes and major transcription factors were determined in the liver. WT mice were slightly affected by dietary FA. However, in WT female mice, but not in males, mRNA levels of PPARα-dependent genes (L-FABP, ACO) were higher in the mice fed on the ALA-rich diet. When compared to WT mice, KO female mice exhibited a decreased lipogenesis capacity (40% lower FAS, ACC, and SREBP-1c mRNA level), whereas KO males showed a decrease in peroxisomal β-oxidation (activity and expression of ACO reduced by 20 and 40%, respectively). When compared to SFA-fed KO mice, steatosis was twice lower in KO mice fed on ALA, despite the absence of dietary effect on plasma TG, CPT1 and ACO activities, or ACC and FAS expression. Besides, in mice on the SFA diet, steatosis was alleviated in females, and CPT1 expression was up-regulated to a higher extent in females than in males (2.7- and 3.6-fold, respectively, as compared to the corresponding WT groups).
Conclusions
Our data suggests estrogen to modulate the regulation of hepatic lipid metabolic pathway by dietary fatty acids. Besides, PPARα invalidation resulted in unexpected regulations by ALA of its known targets and was compensated partly in females, which was therefore less sensitive to the detrimental effects of a SFA-rich diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary n-3 polyunsaturated fatty acids (PUFA), on the one hand, and estrogens, on the other hand, exert protective effects against pathologies, such as metabolic syndrome or cardiovascular diseases (CVD) [3, 22]. Part of these beneficial effects is related to common regulations of several lipid metabolism pathway impacting lipid risk factors, such as de novo lipogenesis, fatty acid oxidation, intravascular lipid transport and catabolism, or adipose storage. As a consequence, interactions of dietary fatty acids with sex hormones in lipid metabolism are likely, but remain poorly known. A meta-analysis of 5 intervention studies in normolipidemic subjects concluded that, following dietary change from high to low saturated fatty acids (SFA) to PUFA ratio, women showed a greater reduction in HDL-cholesterol than men [5]. In response to restriction on both dietary SFA and cholesterol, mildly hypercholesterolemic men showed a more pronounced reduction in cholesterol and plasma apolipoprotein B than post-menopausal women [12]. Animal studies also showed that lipid metabolism in females is less sensitive than in males to the nature of dietary fatty acids, and especially to the adverse effects of SFA [15, 18, 19, 25].
Thus, in vivo human and animal studies strongly suggest that gene regulation of lipid metabolism by n-3 PUFA may be influenced by estrogens. This is in accordance with molecular studies showing a cross-talk between PPARα and ER, the main transcription factors involved in lipid metabolism regulation by n-3 PUFA and estrogen, respectively [20, 24]. The interaction of PPARα with the gender specific differences in lipid metabolism has been shown in the mouse. Indeed, PPARα-null (KO) mice exhibit important sexual dimorphism: hypertriglyceridemia is greater in females, whereas hypercholesterolemia and hepatic steatosis are more pronounced in males [6, 13]. Besides, after mitochondrial β-oxidation of fatty acids was inhibited, 100% of KO males, but only 25% of KO females died. Mortality of males decreased to that in females when they received a pre-treatment with estradiol, which confirms in vivo the importance of a regulatory cross-talk between PPARα and ER [7].
The aim of the present study was to investigate (i) the influence of gender on the response of lipid metabolism to dietary n-3 PUFA, and (ii) the contribution of PPARα to this response, by using wild-type (WT) and KO mice. Among sources of n-3 PUFA, we favored a vegetable oil rich in the precursor ALA. Indeed, even if its metabolic effects remain controversial in the human, ALA has been proved, at least in rodent models, to share with its long-chain derivatives typical regulatory effects, such as decreased lipogenesis and increased β-oxidation in the liver, resulting in lower triglyceridemia and hepatic lipid content [16, 17, 19]. Because the liver plays a major role in lipid metabolism and is a key target of PPARα invalidation [6], the study focussed on the key enzymes involved in fatty acid synthesis, transport, and oxidation, and on the major transcription factors involved in the regulation of hepatic lipid metabolism.
Materials and methods
Experimental procedure
Animals
Males and females WT (12 males, 12 females) (Charles River, L’Arbresle, France) and KO mice (12 males, 12 females), kind gift of F. Gonzales (Laboratory of Metabolism, National Institute of Health, Bethesda, MD, USA) in the C57BL/6J background were used. Animals were housed individually in a controlled environment (25 °C, 40–60% humidity, and darkness from 6 p.m. to 6 a.m.).
Diets
Until 8 weeks of age, all mice were fed ad libitum a standard pelleted chow (Formula A04, SAFE, Villemoisson, France), of which ingredients were (as indicated by the manufacturer): 83.9% cereals, 8% fish meal, 4% soya meal + yeast, and 4.1% vitamin + mineral mix. Nutritional composition of the standard chow was (in grams for 100 g chow, as indicated by the manufacturer): protein 16; carbohydrate 64 (including fibers: 4); lipid 3.5 (including cholesterol: 0.032); water 11.5; and minerals 5. Fatty acid composition was (as % of total fatty acids): saturated, 21.2; 18:1 n-9, 20.0; 18:2 n-6, 48.7; 18:3 n-3, 3.7; and others, 6.5. At 8 weeks of age, mice were fed one of the experimental diets for 5 weeks. The two experimental diets consisted (in weight) of 83% of the above commercial ground pellets, and of either 13% butter and 4% sunflower oil in the ‘butter’ (B) diet, or 15.4% linseed oil (kind gift of P. Weill and G. Chesneau, Valorex, Combourtillé, France), 1.6% water and 0.027% cholesterol (5-cholesten-3ß-ol, Sigma, St Louis, USA) in the ‘linseed oil’ (LO) diet. Water and cholesterol were added to the ‘linseed oil’ diet in order to take into account the water and cholesterol content of the butter. The calculated composition (in weight) of the 2 experimental diets was 13.3% protein, 53.1% carbohydrate, 18.4% lipid, 11.1% water, and 4.2% mineral + vitamin mix. The lipid and cholesterol content of the diets and their FA profile was determined as described previously [19] (Table 1).
Experimental design
After 5 weeks on the experimental diets, mice were fasted overnight, then weighed and anesthetized by isoflurane gas. Blood was taken from the axillary artery for determination of plasma lipids. The abdominal cavity was then opened surgically and liver was carefully removed and weighed. A sample of 0.3 g was immediately frozen in liquid nitrogen and stored at −80 °C for RNA extraction. Another 0.3 g sample was stored at −20 °C for further analysis of lipid composition. A 1 g sample was kept on ice for immediate isolation of the mitochondria + peroxisomes fraction [23]. Mice were then killed by decapitation. Epididymal adipose tissue (EAT) of males and periuterine adipose tissue (PUAT) of females were removed and weighed. Blood samples were centrifuged (1,700g, 15 min, 4 °C) and then plasma was stored at −80 °C until analyses. The present work was carried out in agreement with the French legislation on animal experimentation and with the authorization of the French Ministry of Agriculture (Animal Health and Protection Directorate).
Analyses
Plasma and liver lipid composition
Hepatic lipids were extracted in isopropanol [14]. Triglycerides (TG) and total cholesterol (TC) were quantified in plasma and in alcoholic liver extract by colorimetric enzymatic methods using the kits provided by Bio-Merieux (Marcy-l’Etoile, France) [8, 21].
Enzyme activities
The activity of key enzymes of fatty acid oxidation, carnitine palmitoyltransferase (CPT1, EC 2.3.1.21) and acylCoA oxidase (ACO, EC 1.3.3.6) was determined in the hepatic mitochondrial/peroxisomal fraction. Total mitochondrial CPT activity was measured according to Bieber et al. [2], and ACO activity according to Lazarow and De Duve [11].
RNA analysis
Total RNAs were isolated from liver using TRIzol kit (Invitrogen, Carlsbad, USA) adapted from Chomczynski and Sacchi method [4].
Northern blotting
Total RNAs were electrophoresed on a 1% agarose gel and transferred to GeneScreen membranes (NEN Life Science Products) as described previously [1]. Rat liver-fatty acid binding protein (L-FABP) and rat ACO cDNA probes were labeled with [α-32P]dCTP (3,000 Ci/mmol, ICN) using the Prime-It RmT Random Primer Labelling Kit (Stratagene). A 24-residue oligonucleotide specific for rat, 18S rRNA, was used as probe to ensure that equivalent amounts of RNAs were loaded and transferred. This oligonucleotide was 5′ end-labeled using T4 polynucleotide kinase and [γ-32P]-ATP (3,000 Ci/mmol, ICN).
Real time quantitative RT-PCR
Total mRNAs were extracted with Trizol Reagent (Invitrogen) and reverse cDNA transcribed using Superscript II (Invitrogen), according to manufacturer protocol. Real-time quantitative PCR were performed with an Icycler IQ machine (BioRad) using qPCR MasterMix Plus for SYBR® Green I (Eurogentec, Leuven, Belgium). The sequences of the primer sets were: ACC: F/5′-GATCCCCAAATCAGAAAGTG, R/5′-GCCAAAACTCTGGAGCTAA; FAS: F/5′-AGTGGGTGGACTCTCTGAAG, R/5′GAGATGTGTTGCTGAGGTTG; CPT1: F/5′-GACGAAGAACATCGTGAGTG, R/5′-GACCATAGCCATCCAGATTC; PPARα: F/5′-ACGATGCTGTCCTCCTTGATG, R/5′-GTGTGATAAAGCCATTGCCGT; PPARγ: F/5′-CAGCTCTTGTGAATGGAATG, R/5′-ATCAGCTCTGTGGACCTCTC; SREBP1c: F/5′-GCTTCCAGAGAGGAGGCCAG, R/5′-GGAGCCATGGATTGCACATT; SREBP2: F/5′-GCGTGAGTGTGGGCGAATC, R/5′-CCCTTGACTTCCTTGCTGCA; ERα: F/5′-ATGTGGTCCTTCTCTTCCAG, R/5′-GCAGGGAGAAGAGTTTGTGT.
The comparative ΔΔCT-method was used for the relative mRNA quantification, using 18S rRNA as reference gene.
Statistical analyses
Data were analyzed using the Statview 4.5 program (Abacus Concept, Bekerley, CA, USA). Differences between treatments and interactions were assayed with a three-way ANOVA with diet, gender, and genotype as factors. Differences between means of the 8 groups were determined by post-hoc Fisher test. Statistical significance was set at the 5% level.
Results
Body weight and composition
The dietary treatment did not influence the body weight significantly (Table 2). In contrast, the absolute (data not shown) and relative weights (as % of body weight) of the epididymal adipose tissue (EAT) were considerably reduced in males that fed on LO-diet, irrespective of their genotype, whereas those of the periuterine adipose tissue (PUAT) were not influenced by dietary fatty acids in females (Table 2). Conversely, feeding LO increased the liver proportion in females of both genotypes, but not in males (Table 2).
Liver and plasma lipids
As expected, and whatever gender or diet, KO mice exhibited a dramatic hepatic steatosis resulting from TG accumulation (Fig. 1a). There was no overall gender effect, whereas mice that fed on the LO-diet accumulated less TG than those that fed on the B one. Due to a significant interaction between genotype, gender, and diet, the dietary effect in KO mice was more pronounced in males than in females. WT, female, or LO-fed mice accumulated less cholesterol than their respective counterparts. As a consequence, hepatic cholesterol content was the highest in KO males that fed on the B-diet.
Triglyceride and cholesterol concentration in liver (a) and plasma (b) of male and female WT and KO mice fed on either ‘butter’ (B) or ‘linseed oil’ (LO) diet for 5 weeks. Mean values ± standard errors for 6 mice per group. CH, total cholesterol, TG, triglycerides. For a given parameter (triglycerides or cholesterol) mean values within the 8 groups with unlike superscript letters were significantly different at P ≤ 0.05. Gd, Gt, and D correspond to gender, genotype, and diet, respectively. Gt × Gd, Gt × D, Gn × D, and Gt × Gn × D are the interactions between the corresponding parameters after three-way ANOVA
As in the liver, fasting plasma TG concentration was sensitive to the genotype (Fig. 1b). KO mice exhibited a slight hypertriglyceridemia, whereas there was no significant effect of either gender or diet. Fasting plasma concentration of TC (data not shown) paralleled hepatic cholesterol content: it was lower in WT, female or LO-fed mice than in their respective counterparts.
Hepatic enzymes involved in fatty acid transport, synthesis, and oxidation
Intracellular transport (Table 3)
Expression of L-FABP did not differ with gender. Due to a significant interaction between genotype and diet, mRNA levels in KO mice was 2-fold lower than in their WT counterparts when fed on the LO-diet, but did not differ from WT mice when fed on the B-diet.
Synthesis (Table 3)
The ACC and FAS mRNA levels were not sensitive to the diet. Gender and genotype both influenced ACC expression. However, due to an interaction between these two effects, PPARα invalidation decreased ACC expression in females only. FAS expression was lower in KO and male mice than in WT and female mice, respectively. Due to a significant interaction between genotype and gender, the effect of genotype was considerably more pronounced in females than in males.
Oxidation
CPT1 expression was influenced by both genotype and diet, with a strong interaction between these two factors, but not by gender (Table 3). Indeed, CPT1 expression did not differ with the diet in WT mice. It was about 3-fold higher in KO mice on the B-diet than in their WT counterparts, whereas it was not affected in mice on the LO-diet. In consequence, CPT1 expression in KO mice was considerably higher on the B-diet than in those on the LO-diet. In contrast, specific activity of CPT was not influenced by genotype, gender, or diet (Fig. 2).
Specific activity of CPT (expressed as nmol of reduced DTNB/min/mg protein) and ACO (expressed as nmol of NADH/min/mg protein) in the liver of male and female WT and KO mice fed on either ‘butter’(B) or ‘linseed oil’ (LO) diet for 5 weeks. Mean values ± standard errors for 6 mice per group. CPT, carnitine palmitoyl transferase (nmol reduced DTNB/min/mg proteins), ACO, acylCoA oxidase (nmol NADH/min/mg proteins). For a given parameter CPT or ACO) mean values within the 8 groups with unlike superscript letters were significantly different at P ≤ 0.05. Gn, Gt and, D correspond to gender, genotype, and diet, respectively. Gt × Gn, Gt × D, Gt × D, Gn × Gt × D are the interactions between the corresponding parameters after three-way ANOVA
Overall ACO expression was lower in KO than in WT mice. However, the effect of PPARα deficiency was considerably more pronounced in males (2-fold decrease) than in females. Indeed, there was no effect of the genotype in females on the B-diet (Table 3). There was no dietary effect in either genotype or gender. ACO specific activity paralleled its expression (Fig. 2). It was not sensitive to the diet, and was sensitive to the genotype in males only. Indeed, values in KO males were lower than in the WT males, and did not differ from those in either KO or WT females.
Hepatic transcription factors (Table 4)
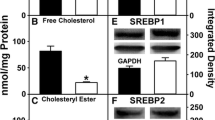
In WT mice, the expression of PPARα did not differ between males and females, but was twice higher in those fed the LO-diet than in those fed the B one. The expression of PPARγ was higher in male than in female mice. It was also increased by PPARα invalidation, but this effect was more marked in the B-diet-fed mice. Expression of SREBP1c was influenced by genotype mainly, but also by diet and gender, that both interacted significantly with the genotype. Indeed, in WT mice, mRNA level was lower in males than in females and was not diet-responsive. In KO mice, however, mRNA level was higher in LO-fed mice and was not gender-responsive. Expression of SREBP2 was influenced by both genotype and gender, but not by diet. Indeed, SREBP2 mRNA level was lower in KO and male mice than in their respective WT and females counterparts. The level of ER mRNA was 4-fold higher in females than in males, and was affected neither by genotype nor by diet.
Discussion
Despite that the mouse is largely used to investigate the regulation of energy metabolism, very few data are available in this species related to the effects of dietary fatty acids on lipid metabolism. Besides, the systemic assessment of sex-biased gene expression has shown extensive differences not only in reproductive tissues, but also in somatic ones, and especially in the liver [9]. However, data on lipid metabolism-related genes are scarce, and not obtained in a controlled nutritional context. The present study describes the effects of dietary ALA on hepatic lipid metabolism and related gene expression in the mouse, and shows that these effects may depend on gender, and may also rely on PPARα functionality.
Effect of dietary ALA in WT mice, and interaction with gender
A few target genes of fatty acid metabolism appeared to be regulated by dietary ALA, and that in gender-dependent manner for some of them. Indeed, in WT males, L-FABP and ACO expression were identical in response to either the B- or the LO-diet (Table 3), in accordance with a recent study performed only in male mice [16]. In contrast, in females, L-FABP and ACO expression were higher in mice that fed on the LO-diet than in those that fed on the B-diet. Besides, PPARα expression was twice higher in mice on the LO-diet, whatever their gender was (Table 4). This indicates that, in the WT mouse, only hepatic genes known to be regulated essentially via PPARα (L-FAPB, ACO, and PPARα, but not CPT) may be up-regulated by the precursor ALA as they are up-regulated by long-chain derivatives [10]. Moreover, the present data suggest that up-regulations of L-FABP and ACO expression by n-3 fatty acids is effective only in females, and thus may depend on the functionality of ER. Nevertheless, the physiological relevance of the up-regulation of ACO expression in females that fed on the LO-diet may be questioned, since ACO specific activity and hepatic lipid content did not parallel these variations (Figs. 1a and 2).
Effect of PPARα deficiency
Interaction with gender
The effects of PPARα deficiency on the regulation of genes involved in fatty acid synthesis and oxidation differed with gender. As concerns lipogenesis, mRNA levels of ACC, FAS, and SREBP-1c in KO males did not differ from those in WT males, whereas they decreased in KO females to the levels found in males (Tables 3 and 4). This suggests that interactions between the transcription factors involved in the regulation of TG metabolism, such as PPARα, SREPB-1c, and ER, make females more sensitive to the effects of PPARα deficiency on hepatic lipogenesis. This down-regulation of hepatic lipogenesis is paralleled by a more efficient TG secretion by female PPARα-null mice than by their male counterparts [13], and both mechanisms may contribute to limit hepatic TG storage in females. As concerns fatty acid β-oxidation, mRNA level and specific activity of ACO were decreased in KO males compared to the WT ones (Table 3 and Fig. 2). However, it was not the case in KO females, which were therefore less sensitive to the effects of PPARα deficiency on peroxisomal fatty acid β-oxidation. Taken together, the present data contribute to explain why hepatic steatosis is less pronounced in female KO mice than in their male counterparts (Fig. 1a), as previously shown in the seminal study of Costet et al. [6]. This better resilience of females could result from a higher metabolic flexibility. Indeed, the lesser impact of PPARα deficiency in females may result from (i) a female-specific decrease in de novo lipogenic capacity, and (ii) a lower decrease, as compared to KO males, in fatty acid peroxisomal β-oxidation.
Despite the fact that the present study was an end-point one, a limitation might be a possible heterogeneity of enzyme activities and mRNA levels in the females, due to variations in sex steroid hormone concentrations during the estrus cycle (4 days in the mouse). However, as concerns somatic cells, and especially hepatocytes, the nature of growth hormone secretion and the level of ER, which is gender-related and thus dramatically differ between males and females, are the major determinants of metabolic regulations, rather than the plasma concentration of sex steroid hormones [9]. Besides, female mice most likely had synchronized estrus cycles, due to both grouping in collective cages and exposure to male pheromones. This is consistent with the absence of a greater heterogeneity of measured parameters (as estimated by SD) in females than in males. Taken together, these physiological features allowed considering that, in the present experimental conditions, variations with estrus cycle in the concentration of sex hormone concentrations, and especially of estradiol, marginally affected enzyme regulations when compared to the global effect of gender-related hormonal status.
Interaction with diet
In KO mice, whatever the gender, hepatic steatosis was lesser in mice that fed on the LO-diet than in those that fed on the B one (Fig. 1a). This beneficial effects of an ALA-rich diet on hepatic steatosis was unexpected, since the known targets of lipid metabolism regulation by n-3 FA were not affected by the diet (gene expression of ACC, FAS, and ACO, specific activity of CPT and ACO) (Table 3 and Fig. 2), which is consistent with PPARα invalidation. Moreover, in these KO mice that fed on the LO-diet, mRNA level of SREBP1c was twice higher and that of CPT1 was twice lower than in those that fed on the B-diet, which should favor hepatic steatosis instead of decreasing it. Under our experimental conditions, hepatic steatosis associated with PPARα deficiency is, at least partly, counteracted by a diet rich in ALA, of which regulatory mechanisms seem to be PPARα-independent, and remain to be explored.
Moreover, considerable interactions between diet and gender occurred in KO mice. Indeed, in the B-diet group, hepatic steatosis was less pronounced in KO females than in males, in accordance with the suggested mechanisms pointed above. In contrast, in the LO-diet group, hepatic steatosis was indeed lower than in the B-diet one, but remained the same in males and females (Fig. 1a). Thus, the beneficial effect of the hormonal status, which limits hepatic steatosis in KO females, is not additive to the beneficial effect of the diet, and is effective only to limit the extent of hepatic steatosis in response to the B-diet. This may be puzzling, since the expression and activity of enzymes involved in hepatic fatty acid metabolism did not show interaction between diet and gender in KO mice. The only exception is that of CPT1 mRNA level. Indeed, CPT1 expression was unexpectedly up-regulated by PPARα deficiency in mice on the B-diet, and that in a significantly higher proportion in females than in males (Table 3). Interestingly, hepatic steatosis and mortality of KO mice given an inhibitor of CPT was higher in males than in females or in estradiol-treated males [7]. The present study confirms that the beneficial effects of estrogen on the degree of hepatic steatosis involve CPT regulation. However, this protective effect appears only when hepatic steatosis is not otherwise limited by other factors, such as dietary LO.
Conclusion
In conclusion, in the WT mouse, the nature of dietary fatty acids (SFA or ALA) has a limited influence on hepatic lipid metabolism. In contrast, the influence of dietary fatty acids was more pronounced in PPARα-null mice, i.e. when lipid metabolism is altered. Indeed, TG accumulation, which was massive in the liver and the adipose tissue of mice on the SFA-rich diet, was partially prevented by the ALA-rich diet.
Moreover, our data show complex gender- and genotype-dependent interactions on the response to dietary fatty acids. In the WT mice, the up-regulation by the ALA-rich diet of target genes of PPARα occurs only in females. This supports our hypothesis that some gender-specific responses to dietary fatty acids could be due to an interaction between estrogens and PPARα on lipid regulatory pathways. In the PPARα null mice, in the absence of the classical regulations of lipid metabolism by n-3 fatty acids, both genders develop only a limited hepatic steatosis when fed on an ALA-rich diet, of which effects appear to be PPARα-independent. When fed on a SFA-rich diet, it is likely that the tandem estrogen/ER may compensate, at least partly, the PPARα deficiency, and therefore limit the extension of hepatic steatosis and adiposity only in females, and not in males.
The present study was an end-point one. The precise regulation of TG metabolism by dietary PUFA has to be now investigated in longitudinal studies to determine the initial events that limit hepatic steatosis in females on a SFA-rich diet, before the setting of counter-regulations. Besides, comprehensive investigations of cross-talks between estrogen and n-3 FA call now for studies in ER- and/or aromatase- (estrogen deficient) null mice.
References
Besnard P, Mallordy A, Carlier H (1993) Transcriptional induction of the fatty acid binding protein gene in mouse liver by bezafibrate. FEBS Lett 327:219–223
Bieber LL, Abraham T, Helmrath T (1972) A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem 50:509–518
Calder PC (2004) n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 107:1–11
Chomczynski P, Qasba P, Topper YJ (1986) Transcriptional and post-transcriptional roles of glucocorticoid in the expression of the rat 25,000 molecular weight casein gene. Biochem Biophys Res Commun 134:812–818
Cobb M, Greenspan J, Timmons M, Teitelbaum H (1993) Gender differences in lipoprotein responses to diet. Ann Nutr Metab 37:225–236
Costet P, Legendre C, More J, Edgar A, Galtier P, Pineau T (1998) Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem 273:29577–29585
Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP (1998) A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha-deficient mice. J Clin Invest 102:1083–1091
Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28:2077–2080
Isensee J, Ruiz Noppinger P (2007) Sexually dimorphic gene expression in mammalian somatic tissue. Gend Med 4(Suppl B):S75–S95
Kim HJ, Takahashi M, Ezaki O (1999) Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAs. J Biol Chem 274:25892–25898
Lazarow PB, De Duve C (1976) A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci USA 73:2043–2046
Li Z, Otvos JD, Lamon-Fava S, Carrasco WV, Lichtenstein AH, McNamara JR, Ordovas JM, Schaefer EJ (2003) Men and women differ in lipoprotein response to dietary saturated fat and cholesterol restriction. J Nutr 133:3428–3433
Linden D, Alsterholm M, Wennbo H, Oscarsson J (2001) PPAR{alpha} deficiency increases secretion and serum levels of apolipoprotein B-containing lipoproteins. J Lipid Res 42:1831–1840
Loison C, Mendy F, Serougne C, Lutton C (2002) Dietary myristic acid modifies the HDL-cholesterol concentration and liver scavenger receptor BI expression in the hamster. Br J Nutr 87:199–210
Luz Fernandez M, West KL, Roy S, Ramjiganesh T (2001) Dietary fat saturation and gender/hormonal status modulate plasma lipids and lipoprotein composition. J Nutr Biochem 12:703–710
Martin PGP, Guillou H, Lasserre F, Déjean S, Lan A, Pascussi JM, SanCristobal M, Legrand P, Besse P, Pineau T (2007) Novel aspects of PPARα mediated regulation of lipid and xenobiotic metabolism revealed through a nutrigenomic study. Hepatology 45:767–777
Morise A, Combe N, Boue C, Legrand P, Catheline D, Delplanque B, Fenart E, Weill P, Hermier D (2004) Dose effect of alpha-linolenic acid on PUFA conversion, bioavailability, and storage in the hamster. Lipids 39:325–334
Morise A, Serougne C, Gripois D, Blouquit M-F, Lutton C, Hermier D (2004) Effects of dietary alpha linolenic acid on cholesterol metabolism in male and female hamsters of the LPN strain. J Nutr Biochem 15:51–61
Morise A, Mourot J, Boue C, Combe N, Amsler G, Gripois D, Quignard-Boulange A, Yvan-Charvet L, Fenart E, Weill P, Hermier D (2006) Gender-related response of lipid metabolism to dietary fatty acids in the hamster. Br J Nutr 95:709–720
Nunez SB, Medin JA, Braissant O, Kemp L, Wahli W, Ozato K, Segars JH (1997) Retinoid X receptor and peroxisome proliferator-activated receptor activate an estrogen responsive gene independent of the estrogen receptor. Mol Cell Endocrinol 127:27–40
Richmond W (1973) Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem 19:1350–1356
Roeters van Lennep JE, Westerveld HT, Erkelens DW, van der Wall EE (2002) Risk factors for coronary heart disease: implications of gender. Cardiovasc Res 53:538–549
Souidi M, Parquet M, Ferezou J, Lutton C (1999) Modulation of cholesterol 7alpha-hydroxylase and sterol 27-hydroxylase activities by steroids and physiological conditions in hamster. Life Sci 64:1585–1593
Wang X, Kilgore MW (2002) Signal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol Cell Endocrinol 194:123–133
Wilson TA, Nicolosi RJ, Lawton CW, Babiak J (1999) Gender differences in response to a hypercholesterolemic diet in hamsters: effects on plasma lipoprotein cholesterol concentrations and early aortic atherosclerosis. Atherosclerosis 146:83–91
Acknowledgments
The authors gratefully acknowledge the financial support of ONIDOL (Organisation Nationale Interprofessionnelle des Graines et Fruits Oléagineux) and ONIOL (Office National Interprofessionnel des Oléagineux, protéagineux et cultures textiles). They also thank sincerely Nicole Combe and Carole Boué (ITERG, Talence, France) for dietary fatty acid analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morise, A., Thomas, C., Landrier, JF. et al. Hepatic lipid metabolism response to dietary fatty acids is differently modulated by PPARα in male and female mice. Eur J Nutr 48, 465–473 (2009). https://doi.org/10.1007/s00394-009-0037-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-009-0037-7