Abstract
Background
Mild iodine deficiency (MID) is endemic in Belgium. Previous surveys, which assessed iodine nutrition in Belgium, focused on children. The iodine status of adults and the influence of ethnicity or seasonality on urinary iodine concentrations (UIC) have not been investigated. Since the nutritional profile of children differs from that of adults, we may anticipate similar differences in iodine status. Seasonal fluctuations in UIC have also been reported from other MID regions.
Aim of the study
We aimed at assessing iodine status and its association with ethnicity and seasonality in adults.
Methods
A stratified random sample of 401 healthy subjects aged between 40 and 60 years, of Belgian, Moroccan, Turkish and Congolese descent residing in Brussels was obtained. Iodine status and thyroid function were determined.
Results
Median UIC was 68 μg/L. The frequency of UIC below 100 μg/L was 73.3%, of which 41.9% fell between 50 and 99 μg/L, and 29.8% between 49 and 20 μg/L. There was no difference in UIC and thyroid function between subjects of different ethnic origins. The frequency of UIC below 50 μg/L was higher in the fall-winter compared to spring-summer periods (P = 0.004). Serum FT3 concentrations, but not FT4 and TSH, were significantly greater in winter than in summer.
Conclusion
Seasonal fluctuations in UIC suggest that the risk of iodine deficiency among adults living in Brussels is higher in fall-winter than in spring-summer. The prevalence of MID in Brussels is high among adults but ethnicity does not appear to influence iodine status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iodine deficiency (ID) has profound effect on human health, with endemic goiter and mental retardation being the most serious consequences of severe ID. The effect of mild iodine deficiency (MID) is more insidious because of an extended latency period before visible thyroid abnormalities occur. To some extent, this may explain the common perception that MID is not a public health problem [1]. This in untrue since, for example, the prevalence of autonomous thyroid nodules (ATN) and multinodular goiter (MNG) was shown to be higher in MID areas compared to areas of iodine sufficiency, such as Switzerland and the United States of America [2–5]. Incidentally, both ATN and MNG are the main causes of hyperthyroidism in the adult population. In addition, MID may affect thyroid function in both pregnant women and their offspring [6, 7]. A recent, prospective study, designed to investigate the benefits of MID correction on the neurobehavioral outcome of offspring, demonstrated the importance of early dietary iodine fortification during pregnancy [8]. Several studies from MID areas have reported an association between intelligence quotient, auditory threshold and the adequacy of iodine intake in children [9–13]. Hypothyroxinemia, common in pregnant women, particularly in iodine-deficient areas is associated with impaired neurodevelopment among their offspring [14–17].
The majority of the past surveys into the iodine status in Belgium concerns children [18]. However, the nutritional profile of children may differ from that of adults, and so the outcome of these previous studies may not present an accurate picture of iodine deficiency in the population as a whole. The influence of ethnicity and seasonality on iodine status has never been investigated in Belgium, although these factors have been shown to be an important determinant of iodine status in other European countries [19, 20]. The aim of this study was to asses the iodine status and its association with ethnicity and seasonality in adults aged over 40 years residing in Belgium.
Methods
A stratified random sample of 1,000 subjects, aged between 40 and 60 years, of Belgian, Moroccan, Turkish and Congolese descent was obtained from 7 of the 19 municipalities of Brussels as described in details elsewhere [21]. The sample was stratified according to the four different ethnic groups living in Brussels: autochthonous Belgians, and first-generation immigrants from Morocco, Turkey and the Democratic Republic of Congo. Ethnicity classification was observer-assigned and based on the subject’s name and own or parents’ country of birth. Second-and third-generation immigrants were excluded from the sample. In a second stage, a random subsample of each ethnic group was invited to Erasme Hospital for further assessment of the iodine status and thyroid function. Only healthy adults without known history of thyroid disease or medications affecting thyroid function were included in the study.
MID was defined as a UIC between 50 and 99 μg/L, moderate ID as a UIC between 20 and 49 μg/L and severe ID below 20 μg/L, according to WHO recommendations.
The protocol for this study was approved by the Ethics Committee of Erasme University Hospital.
Laboratory measurements
Serum free thyroxine (FT4), free triiodothyronine (FT3), thyrotropin (TSH) were measured by chemoluminescence using the Modular Analytic System (Elecsys, Roche, Mannheim, Germany). The normal ranges for adults in Belgium were as follows: serum FT4, 0.8–1.7 ng/dL; serum FT3, 1.8–4.6 pg/mL; serum TSH 0.4–4.0 mIU/L. Urinary iodine was measured by inductively coupled plasma mass spectrometry (ICPMS), as previously described [22].
Statistical analysis
Continuous variables were summarized by their mean (±SD), and means were compared using one-way analysis of variance. The Scheffé multiple-comparison test was used to compare pairs of means. All P values were two-sided. For discrete variables, we compared proportions using Chi-square tests and computed odds ratios with a 95% confidence interval. To control for possible confounding factors, adjusted odds ratios were computed by logistic regression. Statistical analyses were performed using SPSS (Chicago, Illinois).
Results
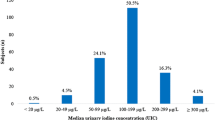
Among the 386 subjects from Brussels in whom spot UIC were measured, the median was 68 μg/L (Fig. 1). Based on the UIC values, the overall prevalence of iodine deficiency was 73.3% (95% CI 69–78%) of adults residing in Brussels, with 41.9% scoring a UIC of between 50 and 99 μg/L, 29.8% scoring between 49 and 20 μg/L and 1.6% below 20 μg/L.
There was no significant difference in UIC subjects of different ethnicity (Table 1). Within the four ethnic groups studied, Belgian subjects were slightly older when compared with the other subjects. Overall, the prevalence of thyroid dysfunction (TSH <0.4 or >4 mIU/L) was greater than 6% but there were no differences in thyroid function between the groups.
The frequency of moderate iodine deficiency fluctuated significantly according to seasons (Fig. 2). The frequency of low UIC was nearly twice as high between January and March than between April and June. After controlling for age, sex, ethnicity and BMI, this seasonal difference was confirmed by multivariate analysis (Table 2), which also showed the absence of association between iodine status and ethnicity. Similarly to UIC, serum FT3 concentrations also fluctuated with the seasons. Serum FT3 concentrations were significantly greater in winter than in summer, while serum FT4 and serum TSH concentrations showed no seasonal fluctuation (Fig. 3).
Distribution of serum FT4, FT3 and TSH concentrations according to season. Box plots show median values (the horizontal line in center of each box), as well as 25th and 75th percentile (bottom and top of each box), and lowest and highest value (bottom and top of error bar). P values were derived from one-way analysis of variance
Discussion
The prevalence of mild (41.9%) and moderate (29.8%) iodine deficiency among adults residing in Brussels was high. Iodine deficiency in the adult population we studied was associated with seasonal fluctuations in UIC, but not with ethnicity.
The frequency of iodine deficiency was higher in the population studied than that reported in the most recent survey of Belgian schoolchildren survey [18]. Our finding suggests that iodine deficiency in Belgium may be more pronounced in adults than that detected among children as reported previously in other iodine-deficient regions [19, 23].
There was no difference in UIC between the different ethnic groups residing in Brussels. Low UIC have been reported among immigrant pregnant women in Italy [20]. In this study, a positive correlation was found between the length of residence in Italy and UIC. The authors attributed the difference to pronounced iodine depletion, prior to their arrival in Italy. Unlike the subjects studied by Mian et al., our Brussels subjects were long-term Belgian residents, which could explain the absence of association between ethnicity and iodine status.
The frequency of low UIC (<50 μg/L) in adults was significantly greater in the fall-winter period than during spring-summer. Seasonal variations in the iodine content of nontoxic goiter, revealed by X-ray fluorescence, have previously been reported in Belgium [24]. In line with the findings of this study, the authors reported that the iodine content of the thyroid was found to peak in April–May and decline during September–October. By contrast, other European studies have reported lower UIC during the summer months and higher values in winter for both children and adults [19, 25, 26]. Seasonal variation has been attributed to variations in the content of iodine in milk. Indeed, during the winter months, cattle are housed indoors and fed with iodine supplements. Because the consumption of dairy product in Belgium among the adult population is low [27], seasonal variations in the iodine content of milk are unlikely to impact upon the iodine status of the adult population. Nevertheless, there are few foods other than milk that have demonstrated such seasonal fluctuations. One recent study suggests that, in Belgium, milk is an important dietary source of iodine [28]. Consequently, variations in the iodine content of milk cannot be completely ruled out as the explanation for UIC seasonal fluctuations which we observed.
The proportion of household iodized salt represents approximately 10% of all salt consumption in Belgium. However, iodized salt may become a more important source of iodine in Belgium because it was decided recently to use iodized salt in bread baking. Although this measure is not compulsory by law, the use of iodized salt in bread is expected to provide an extra iodine daily intake of approximately 30 μg.
Seasonal fluctuations in UIC were associated with increased serum FT3 concentrations in winter when compared to summer. In Belgium, seasonal variations in the concentrations of circulating T3 concentrations have been reported previously and attributed to exposure to cold [29]. Because in conditions of low iodine availability the thyroid favors T3 production, an alternative explanation might be that the aggravation of iodine deficiency during the winter months enhances the production of T3.
In the adult population studied, 50% were women and, of these women, 31% were women of childbearing age. The iodine status of these women may be a matter of public health concern. Our findings suggest that those women who fall pregnant in winter may be exposed to a greater risk of iodine deficiency. In MID areas, the impact of the seasonal fluctuations in UIC on the thyroid function of pregnant women has not been investigated. Further research is needed to confirm the fluctuation in UIC across the seasons over larger sample of women in order to assess the impact of these fluctuations on the thyroid function and the neurodevelopment of their offspring.
The measurement iodine status among adults residing in Brussels was deliberately conducted in municipalities of the city containing large immigrant communities of low socio-economic status. Therefore, extrapolating of this data to the entire Belgian adult population should be undertaken cautiously, as the autochthonous Belgians in our sample did not necessarily represent a cross-section of the total Belgian population.
In conclusion, seasonal fluctuations in urinary iodine concentrations suggest that the risk of iodine deficiency in adults is higher in fall-winter than in spring-summer. The prevalence of mild iodine deficiency is high among the adult population in Brussels but ethnicity has no impact on iodine status.
References
de Benoist B, McLean E, Andersson M, Rogers L (2008) Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull 29:195–202
Baltisberger BL, Minder CE, Burgi H (1995) Decrease of incidence of toxic nodular goitre in a region of Switzerland after full correction of mild iodine deficiency. Eur J Endocrinol 132:546–549
Knudsen N, Jorgensen T, Rasmussen S, Christiansen E, Perrild H (1999) The prevalence of thyroid dysfunction in a population with borderline iodine deficiency. Clin Endocrinol (Oxf) 51:361–367
Laurberg P, Nohr SB, Pedersen KM, Hreidarsson AB, Andersen S, Bulow PI, Knudsen N, Perrild H, Jorgensen T, Ovesen L (2000) Thyroid disorders in mild iodine deficiency. Thyroid 10:951–963
Brush BE, Altland JK (1952) Goiter prevention with iodized salt: results of a thirty-year study. J Clin Endocrinol Metab 12:1380–1388
Glinoer D, Delange F, Laboureur I, De NP, Lejeune B, Kinthaert J, Bourdoux P (1992) Maternal and neonatal thyroid function at birth in an area of marginally low iodine intake. J Clin Endocrinol Metab 75:800–805
Glinoer D, De NP, Delange F, Lemone M, Toppet V, Spehl M, Grun JP, Kinthaert J, Lejeune B (1995) A randomized trial for the treatment of mild iodine deficiency during pregnancy: maternal and neonatal effects. J Clin Endocrinol Metab 80:258–269
Berbel P, Mestre JL, Santamaria A, Palazon I, Franco A, Graells M, Gonzalez-Torga A, de Escobar GM (2009) Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid 19:511–519
Santiago-Fernandez P, Torres-Barahona R, Muela-Martinez JA, Rojo-Martinez G, Garcia-Fuentes E, Garriga MJ, Leon AG, Soriguer F (2004) Intelligence quotient and iodine intake: a cross-sectional study in children. J Clin Endocrinol Metab 89:3851–3857
Valeix P, Preziosi P, Rossignol C, Farnier MA, Hercberg S (1994) Relationship between urinary iodine concentration and hearing capacity in children. Eur J Clin Nutr 48:54–59
Soriguer F, Millon MC, Munoz R, Mancha I, Lopez Siguero JP, Martinez Aedo MJ, Gomez-Huelga R, Garriga MJ, Rojo-Martinez G, Esteva I, Tinahones FJ (2000) The auditory threshold in a school-age population is related to iodine intake and thyroid function. Thyroid 10:991–999
Vermiglio F, Lo PV, Moleti M, Sidoti M, Tortorella G, Scaffidi G, Castagna MG, Mattina F, Violi MA, Crisa A, Artemisia A, Trimarchi F (2004) Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab 89:6054–6060
Vermiglio F, Sidoti M, Finocchiaro MD, Battiato S, Lo PV, Benvenga S, Trimarchi F (1990) Defective neuromotor and cognitive ability in iodine-deficient schoolchildren of an endemic goiter region in Sicily. J Clin Endocrinol Metab 70:379–384
Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL (1999) Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 50:149–155
Moleti M, Lo PV, Campolo MC, Mattina F, Galletti M, Mandolfino M, Violi MA, Giorgianni G, De Domenico D, Trimarchi F, Vermiglio F (2008) Iodine prophylaxis using iodized salt and risk of maternal thyroid failure in conditions of mild iodine deficiency. J Clin Endocrinol Metab 93:2616–2621
Velasco I, Carreira M, Santiago P, Muela JA, Garcia-Fuentes E, Sanchez-Munoz B, Garriga MJ, Gonzalez-Fernandez MC, Rodriguez A, Caballero FF, Machado A, Gonzalez-Romero S, Anarte MT, Soriguer F (2009) Effect of iodine prophylaxis during pregnancy on neurocognitive development of children during the first two years of life. J Clin Endocrinol Metab 94:3234–3241
Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, Hooijkaas H, de Muinck Keizer-Schrama SM, Hofman A, Jaddoe VV, Visser W, Steegers EA, Verhulst FC, de Rijke YB, Tiemeier H (2010) Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 95:4227–4234
Delange F, Van Onderbergen A, Shabana W, Vandemeulebroucke E, Vertongen F, Gnat D, Dramaix M (2000) Silent iodine prophylaxis in Western Europe only partly corrects iodine deficiency; the case of Belgium. Eur J Endocrinol 143:189–196
Als C, Haldimann M, Burgi E, Donati F, Gerber H, Zimmerli B (2003) Swiss pilot study of individual seasonal fluctuations of urinary iodine concentration over two years: is age-dependency linked to the major source of dietary iodine? Eur J Clin Nutr 57:636–646
Mian C, Vitaliano P, Pozza D, Barollo S, Pitton M, Callegari G, Di GE, Casaro A, Acamulli DN, Busnardo B, Mantero F, Girelli ME (2009) Iodine status in pregnancy: role of dietary habits and geographical origin. Clin Endocrinol (Oxf) 70:776–780
Moreno-Reyes R, Carpentier YA, Boelaert M, El MK, Dufourny G, Bazelmans C, Leveque A, Gervy C, Goldman S (2009) Vitamin D deficiency and hyperparathyroidism in relation to ethnicity: a cross-sectional survey in healthy adults. Eur J Nutr 48:31–37
Macours P, Aubry JC, Hauquier B, Boeynaems JM, Goldman S, Moreno-Reyes R (2008) Determination of urinary iodine by inductively coupled plasma mass spectrometry. J Trace Elem Med Biol 22:162–165
Gowachirapant S, Winichagoon P, Wyss L, Tong B, Baumgartner J, Melse-Boonstra A, Zimmermann MB (2009) Urinary iodine concentrations indicate iodine deficiency in pregnant thai women but iodine sufficiency in their school-aged children. J Nutr 139:1169–1172
Jonckheer M, Coomans D, Broeckaert I, Van PR, Deconinck F (1982) Seasonal variation of stable intrathyroidal iodine in nontoxic goiter disclosed by x-ray fluorescence. J Endocrinol Invest 5:27–31
Rasmussen LB, Ovesen L, Bulow I, Jorgensen T, Knudsen N, Laurberg P, Pertild H (2002) Dietary iodine intake and urinary iodine excretion in a Danish population: effect of geography, supplements and food choice. Br J Nutr 87:61–69
Nelson M, Phillips DI, Morris JA, Wood TJ (1988) Urinary iodine excretion correlates with milk iodine content in seven British towns. J Epidemiol Community Health 42:72–75
Devriese S, Huybrechts I, Moreau M, Van Oyen H (2006) Enquête de consommation alimentaire Belge 1—2004. Service d’Epidémiologie. D/2006/2505/16, IPH/EPI REPORTS No. 2006—014. 2006. Institut Scientifique de Santé Publique
Guyot H, Saegerman C, Lebreton P, Sandersen C, Rollin F (2009) Epidemiology of trace elements deficiencies in Belgian beef and dairy cattle herds. J Trace Elem Med Biol 23:116–123
Maes M, Mommen K, Hendrickx D, Peeters D, D’Hondt P, Ranjan R, De MF, Scharpe S (1997) Components of biological variation, including seasonality, in blood concentrations of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers. Clin Endocrinol (Oxf) 46:587–598
Acknowledgments
The advice of M. Boelaert for statistical analysis and P. Vine for editing are gratefully acknowledged. This work was supported by grants from the Belgian Fonds de la Recherche Scientifique Médicale (no. 3.4578.09). Daniel Glinoer acknowledges the support of the «Ministère de la Communauté Française, Administration Générale de l’Enseignement & Recherche Scientifique», within the framework of the «Actions de Recherche Concertées» (Convention no. ARC 04/09–314).
Conflict of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreno-Reyes, R., Carpentier, Y.A., Macours, P. et al. Seasons but not ethnicity influence urinary iodine concentrations in Belgian adults. Eur J Nutr 50, 285–290 (2011). https://doi.org/10.1007/s00394-010-0137-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-010-0137-4