Abstract
Introduction
Milk is a good source of bioavailable calcium compared with other foods. Recent in vitro and in vivo studies have shown that milk whey protein, especially its basic protein fraction (milk basic protein, MBP), contains several components capable of promoting bone formation and inhibiting bone resorption. The objective of this study was to examine the effects of MBP on bone mineral density (BMD) and bone metabolism of healthy young women.
Methods
Eighty-four healthy young women were randomly assigned to three groups: control group, whole milk group or MBP group treated with milk containing 40 mg MBP for 8 months. The bone mineral density of total body, the lumbar vertebrae L2–L4 and the left forearm of each subject were measured by dual-energy X-ray absorptiometry (DEXA) at 0 and 8 months of treatment. Serum indexes of bone metabolism were measured at 0, 3, 6 and 8 months. Eighty-one subjects who completed the study in accordance with the protocol were included in the analysis.
Results
Total BMD in all groups significantly increased compared with baseline values. However, no significant difference on the mean rate of gain of total BMD was observed among the MBP group (2.19%), the whole milk group (2.63%) and the control group (1.61%). Serum cross-linked N-teleopeptides of type-I collagen (NTx) in MBP group at 8 months and in whole milk group at 6 months were significantly decreased from baseline. There were no significant differences between whole milk group and MBP group; however, after combining the milk groups, NTx had significantly decreased from baseline. No significant increase was observed in serum bone-specific alkaline phosphatase (BAP) in both whole milk group and MBP group.
Conclusion
No significant effect of MBP on bone mineral density and bone metabolism was observed, but milk supplementation was effective in suppressing bone resorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a disease characterized by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased susceptibility to fractures [18]. If for such reasons as estrogen deficiency in menopause, low calcium intake, or lack of exercise, bone resorption may exceed bone formation, and this imbalance would increase the risk of osteoporosis. Optimal management of osteoporosis consists of maximizing peak bone mass in early adulthood and preventing rapid bone loss occurring after menopause for women [8]. However, it is thought to be difficult to completely prevent rapid bone loss by menopause or aging, so building strong bones by maximizing the peak bone mass before the age of 30, can be an effective way against osteoporosis. Bone is in a continuously remodeling process through repeating cycles of destruction and rebuilding [9]. However, the bone turnover rate is relatively slow. Therefore, nutrition becomes very important for maximizing peak bone mass. Some nutrients, such as calcium and magnesium, are components of bone. Several dietary factors such as vitamin D, vitamin K [4], casein phosphopeptides [10], and isoflavones [6], which may affect bone metabolism, have been widely noticed.
Comparing with other food, milk is a good source of bioavailable calcium. Recent studies have demonstrated that milk whey protein plays a functional role in bone remodeling [11, 12]. In these studies, the active components responsible for the promotion of bone formation and the suppression of bone resorption were found to be in its basic protein fraction (milk basic protein, MBP). Studies in rats showed that milk whey protein and fractionated whey protein enhanced femoral bone strength in young ovariectomized rats [13, 14], and MBP prevented bone loss in aged ovariectomized rats, used as a model of osteoporosis [15].
In human studies, MBP was reported to suppress bone resorption and increase BMD [2, 3, 16, 17, 19]. However, the effect of MBP on bone formation remains unclear. Most studies [2, 3, 17, 19] did not find a clear effect of MBP on bone formation whereas Uenishi [19] reported that serum osteocalcin concentration was significantly higher after 40 mg MBP supplementation per day for 6 months. Further studies are needed to determine the effect of MBP on bone formation. Therefore, the present study was designed to examine whether MBP had positive effect on BMD and biochemical markers of bone metabolism in healthy young women.
Subjects and methods
Subjects
Eighty-four healthy young female students [mean age (±SD), 19.6 ± 0.6 years] from Peking University Health Science Center were recruited. Written informed consent was obtained from each subject. The ethical committee of Peking University Health Science Center approved this protocol. All subjects had a moderate level of physical activity. Exclusion criteria included endocrine disease, metabolic disease, osteoporosis and other known medications that affect bone metabolism. Those who had bone fracture history or could not drink the milk consecutively for 8 months were also excluded.
Study design and supplements
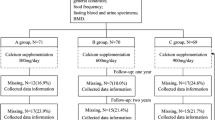
In this 8-month placebo-controlled trial, 84 women were randomly assigned to 1 of 3 groups: control group, whole milk group, or MBP group to respectively consume nothing, a carton of 250 ml whole milk, or a carton of 250 ml whole milk added with 40 mg MBP, with stratification based on their body weight, height, body mass index and BMD.
Both kinds of milk were prepared by MengNiu Milk Products Corporation (Inner Mongolia, China). MBP extracted from skimmed milk was prepared by Aoe’s method [2]. Skimmed milk was loaded onto a column that had been packed with 500 g of cation exchange resin, sulfonated chitopearl (Fuji-bouseke, Tokyo, Japan). The column was washed with deionized water, and the bound proteins were eluted with 1 M sodium chloride. All eluted fractions were collected. MBP was obtained by freeze-drying after dialysis of the eluted fraction in a cellulose membrane tube (Sanko Junyaku Co., Ltd, Tokyo, Japan). Protein concentration of the MBP was 98%. The yield of MBP from skimmed milk showed that approximately 800 ml of milk is equivalent to 40 mg MBP. Women in whole milk group and in MBP group were instructed to drink a carton (250 ml) of milk prepared daily in a fixed clean room. They were advised to maintain their usual diets and to avoid taking supplemental minerals and vitamins throughout the 8-month study. The study was conducted from Autumn (October) to Summer (June). Blood measurements were performed for each subject. At baseline and at the 8-month evaluation, total BMD, lumbar (L2–L4) BMD and left forearm (1/10 portion from the distal end of the radius and ulna, Dist R+U 1/10) BMD were also measured. During the study period, values for the nutrients gained by FFQ were completed at 4 and 8 months. Volunteers were asked to record the amounts or portion sizes of food and meals. The nutrient content of their diet was quantified using a computer program based on the Standard Tables of China Food Composition [7].
Status of subjects and compliance
During the 8-month study period, women in whole milk group and in MBP group were asked to drink the milk on the spot at 7:00–8:00 am or 20:00–21:00 pm. Two women in whole milk group and one woman in MBP group dropped out because they did not drink the milk provided for several days. No bloating, diarrhea or allergies were reported in all groups. We conducted a per protocol analysis of the 81 subjects who completed the study according to the protocol; this was not an intention to treat analysis.
Analytic methods
Bone mineral density (BMD) of total body, lumbar (L2–L4) and dist R+U at 1/10 portion were measured by dual-energy X-ray absorptiometry using a DXA scanner (Norland model XR36, Norland. Corp., Fort Atkinson, WI). The coefficient of variation for the measurements of the phantom was less than 1.0%. A phantom consisting of bone ash embedded in a 12-cm block was scanned everyday as a control; the BMD of the phantom remained unchanged throughout the study.
Overnight fasting venous blood specimens (4.0 ml) were obtained from all the subjects by certified laboratory technicians at 0, 3, 6 and 8 months. Aliquots of samples were frozen at −70°C until analysis. Serum bone-specific alkaline phosphatase (BAP) was measured by an enzyme-linked immunosorbent assay (Metra™ BAP, Quidel Corp., San Diego, USA). Serum cross-linked N-teleopeptides of type-I collagen (NTx) were measured by an enzyme-linked immunosorbent assay (Osteomark NTx, Wampole Laboratories, Princeton, NJ, USA). The intra-assay variability ranged from 5.0 to 8.0%. All members of the study team successfully completed a training programme on the aims of the study and the specific methods used. The biochemical markers of bone metabolism were analyzed by laboratories according to the criteria of Peking University.
Statistical analysis
Dunnett’s t test was used to compare the difference in all of the parameters between the test groups and control, and Bonferroni’s t test was used to compare the difference with the adjacent group with the better MBP supplementation. Comparisons of the values of the bone density at 0 and 8 months in each group were made with the paired samples t test. Repeated measures analysis was used to compare the difference of indexes of bone metabolism at each period in each group. Univariate correlation was used to analyze the relationship among the variables. All the analyses were conducted with SPSS 10.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of subjects
The baseline clinical characteristics of the subjects are shown in Table 1. Overall, there was no significant difference of age, weight, height, body mass index and total BMD among the three groups.
Bone mineral density
The initial mean values for total BMD, lumbar BMD and dist R+U 1/10BMD were similar among the three groups (Table 2). Comparing with initial values, the mean values of total BMD were significantly increased in control group (P = 0.015), whole milk group (P = 0.000) and MBP group (P = 0.005). The effect of MBP on total BMD was estimated as the percentage of gain of BMD after 8 months of treatment. As shown in Fig. 1, the mean rate of gain of total BMD in the MBP group (2.19%), the whole milk group (2.63%) and the control group (1.61%) had no significant difference. There is no significant difference for lumbar BMD and dist R+U 1/10 BMD among the three groups at the same period or comparing with the initial values in each group.
Biochemistry
The biochemical indexes of bone metabolism in the three groups are listed in Fig. 2. The mean values of serum BAP and NTx in each group were similar at baseline. NTx in whole milk group at 6 months (P = 0.048) and in MBP group at 8 months (P = 0.040) were significantly decreased from baseline values, whereas serum NTx remained stable from baseline in control group. Then repeated measure analysis revealed that the mean values of NTx in MBP group at 3 months (P = 0.002) and 8 months (P = 0.017) were significantly lower than that of the control group during the same period. However, there was no difference in change of NTx between whole milk group and MBP group. In addition, BAP have no significant differences in inner-group and inter-group. Therefore, after combining the two milk groups, the results were shown in Table 4. NTx in combined milk group were significantly decreased at 3, 6 and 8 months from baseline, and NTx at 3 months in combined milk group was significantly lower than that of the control group. BAP still had no difference between the two groups.
The biochemical indexes of bone metabolism (serum BAP and serum NTx concentration) in healthy young women for 8 months. Asterisks indicate the significant differences comparing with initial values in the same group (P < 0.05). There is no difference in change of BAP and NTx between whole milk group and MBP group
Dietary minerals and vitamins
The mean dietary intakes of energy, protein, calcium, phosphorus, magnesium, vitamin D, K and C are shown in Table 3. There were wide individual differences in the dietary intakes. No significant difference was observed in energy, protein, phosphorus, magnesium, vitamin D, vitamin K or vitamin C among the three groups by Bonferroni’s test, except that the means of calcium in whole milk group at 4 and 8 months were significantly higher than those in control group. Correlation coefficients between the gain of BMD and intake of minerals and vitamins were also calculated. There was no significant correlation between the gain of BMD and intake of any dietary minerals or vitamins in the all three groups.
Discussion
This study investigated the effect of MBP on bone mineral density in healthy young female university students [mean age (±SD), 19.6 ± 0.6 years]. MBP is extracted from fresh skimmed milk, and 40 mg of MBP was added into whole milk. Our study provided evidence of the effect of MBP added into milk on BMD and bone metabolism in healthy young women.
In this study, comparing with the baseline values, total BMD increased significantly after 8 months supplementation, while lumbar BMD and dist R+U 1/10 BMD had no significant difference. However, there was no significant difference among the three groups in the gain of total BMD, indicating that no significant effect of MBP on BMD in all parts was observed. In the present study, the mean rate of gain of total BMD in control group is 1.61%, while in the MBP group is 2.19%. Thus, it is possible that the natural growth rate of bone mass has stronger effect on the BMD than MBP supplementation in healthy young women. In the present study, we added 40 mg MBP into 250 ml of milk which differ from 50 ml of beverage containing 40 mg MBP in other studies. Since 800 ml of milk is equivalent to 40 mg MBP [2], 250 ml milk contains about 12.5 mg MBP. Therefore, the dose of MBP in MBP group is 52.5 mg while in whole milk group is 12.5 mg. However, the mean rate of gain of total BMD in whole milk group is 2.63%, while in the MBP group it is 2.19%. The reason why the mean rate of the gain of total BMD in whole milk group was faster than that in the MBP group remains unclear. In the present study, no significant correlation between the gain of BMD and dietary intake of any minerals or vitamins was detected, and dietary intake was similar between whole milk group and MBP group. Thus the gain of BMD was comparative and can reflect the effects of MBP.
Biochemical parameters in serum are used clinically to assess the rate of bone formation and resorption. In the present study, there was no significant difference among the three groups in serum BAP concentrations, a bone formation marker. Serum NTx, a bone resorption marker, was lower at 3 and 8 months in MBP group compared to the control group; however, there was no difference in change between whole milk group and MBP group. Thus, after combining the two milk groups, NTx in combined milk group was significantly decreased. These results indicated that milk supplementation was effective in suppressing bone resorption, consistent with Adolphi’s study [1]. In short, although we did not find the significant effect of MBP on NTx, we observed an effect of milk on NTx. It is reported that NTx is more sensitive than deoxypyridinoline to MBP supplementation [5]. Also, we could not find a clear effect of MBP on bone formation judging from the data on bone-specific alkaline phosphatase. These results are consistent with the study of Aoe [2, 3]. It is possible that MBP has no strong effect on either bone resorption or bone formation, since the longitudinal effect on bone markers in women who consumed MBP were not different from those in the control group. Long-term investigations on the effects of MBP on BMD are required to clarify the discrepant findings between our study and those of Aoe et al.
One limitation needed to be considered is the natural growth rate of bone mass. As mentioned above, all the subjects are young women, so their bone mineral density is still increasing until attaining their peak bone mass by their 30s. Thus, the natural growth rate of bone mass will influence the gain of BMD by MBP supplementation, and may have stronger effect on BMD than MBP supplementation in healthy young women.
In conclusion, we report that no significant effect of MBP on bone density and bone metabolism is observed, but milk is effective in suppressing bone resorption. As mentioned above, the effects of MBP on bone mineral density and on bone metabolism are needed to confirm. Further long-term and large-scale studies in different countries are needed to investigate the effects of MBP supplementation on the bone health and peak bone mass of young adult women.
References
Adolphi B, Scholz-Ahrens KE, de Vrese M, Acil Y, Laue C, Schrezenmeir J (2008) Short-term effect of bedtime consumption of fermented milk supplemented with calcium, inulin-type fructans and caseinphosphopeptides on bone metabolism in healthy, postmenopausal women. Eur J Nut 48(1):45–53. doi:10.1007/s00394-008-0759-y
Aoe S, Toba Y, Yamamura J, Kawakami H, Yahiro M, Kumegawa M, Itabashi A, Takada Y (2001) Controlled trial of the effects of milk basic protein supplementation on bone metabolism in health adult women. Biosci Biotechnol Biochem 65(4):913–918
Aoe S, Koyama T, Toba Y, Itabashi A, Takada Y (2005) A controlled trial of the effect of milk basic protein (MBP) supplementation on bone metabolism in healthy menopausal women. Osteoporos Int 16:2123–2128
Bugel S (2003) Vitamin K and bone health. Proc Nutr Soc 62:839–843
Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR (1992) A specific immunoassay for monitoring human bone resorption: quantization of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res 7:1251–1258
Ishimi Y, Miyaura C, Ohmura M, Onoe Y, Sato T, Uchiyama Y, Ito M, Wang XX, Suda T, Ikegami S (1999) Selective effects of genistein, a soybean isoflavone, on B-lymphopoiesis and bone loss caused by estrogen deficiency. Endocrinology 140:1893–1900
Institute of Nutrition and Food Safety, Center of Disease Control, China (2004) China food composition. Peking University Medical Press, Beijing
Keen R (2007) Osteoporosis: strategies for prevention and management. Best Pract Res CL RH 21(1):109–122
Ott SM (1996) Theoretical and methodological approach. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology. Academic Press, San Diego, pp 231–241
Scholz-Ahrens KE, Schrezenmeir J (2000) Effects of bioactive substances in milk on mineral and trace element metabolism with special reference to casein phosphopeptides. Br J Nutr 84(Suppl 1):S147–S153
Takada Y, Aoe S, Kumegawa M (1996) Whey protein stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun 223:445–449
Takada Y, Kobayashi N, Mastsuyama H, Kato K, Yamamura J, Yahiro M, Kumegawa M, Aoe S (1997) Whey protein suppresses the osteoclast-mediated bone resorption and osteoclast cell formation. Int Dairy J 6(7):821–825
Takada Y, Kobayashi N, Kato K, Mastsuyama H, Yahiro M, Aoe S (1997) Effect of whey protein on calcium and bone metabolism in ovariectomized rats. J Nutr Sci Vitaminol 43:199–210
Takada Y, Mastsuyama H, Kato K, Kobayashi N, Yamamura J, Yahiro M, Ace S (1997) Milk whey protein enhances the bone breaking force in ovariectomized rats. Nutr Res 17(12):1709–1720
Toba Y, Takada Y, Yamamura J, Tanaka M, Matsuoka Y, Kawakami H, Itabashi A, Aoe S, Kumegawa (2000) Milk basic protein: a novel protective function of milk against osteoporosis. Bone Miner 9(27):403–408
Toba Y, Takada Y, Matsuoka Y, Morita Y, Motouri M, Hirai T, Suguri T, Aoe S, Kawakami H, Kumegawa M, Takeuchi A, Itabashi A (2001) Milk basic protein (MBP) promotes bone formation and suppresses bone resorption in healthy adult men. Biosci Biotechnol Biochem 65(12):1353–1357
Uenishi K, Ishida H, Toba Y, Aoe S, Itabashi A, Takada Y (2007) Milk basic protein increase bone mineral density and improves bone metabolism in healthy young women. Osteoporos Int 18:385–390
World Health Organization (2003) Prevention and management of osteoporosis. WHO Technical Report Series no. 921. WHO, Geneva
Yamamura J, Aoe S, Toba Y, Motouri M, Kawakami H, Kumegawa M, Itabashi A, Takada Y (2002) Milk basic protein (MBP) increases radial bone mineral density in healthy adult women. Biosci Biotechnol Biochem 66(3):702–704
Acknowledgments
This study was conducted at Department of Nutrition and Food Hygiene, School of Public Health, Peking University, with funding from MengNiu Research Center. The authors gratefully acknowledge the technical help of Mr. Chang-Yong Xue (People’s Liberation Army General Hospital, Beijing, China) with food analysis records, and also acknowledge the instruction of statistical analysis by Mr. Wei-Ning Yi, Mr. Xiao-Dong Sun (Peking University).
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, ZY., Lin, XM., Xu, XR. et al. Evaluation of milk basic protein supplementation on bone density and bone metabolism in Chinese young women. Eur J Nutr 48, 301–306 (2009). https://doi.org/10.1007/s00394-009-0014-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-009-0014-1