Abstract
Extracorporeal cardiopulmonary resuscitation (eCPR) may be considered as a rescue attempt for highly selected patients with refractory cardiac arrest and potentially reversible aetiology. Currently, there are no randomised, controlled studies on eCPR. Thus, prospective validated predictors of benefit and outcome are lacking. Currently, selection criteria and procedure techniques differ across hospitals and standardised algorithms are lacking. Based on expert opinion, the present consensus statement provides a first standardised treatment algorithm for eCPR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At least 275,000 patients in Europe suffer an out-of-hospital cardiac arrest (OHCA) every year [1]. In the US, approximately 568,500 cardiac arrests occur annually. Of these, 359,400 (63%) occur outside hospital (OHCA) and 209,000 (37%) inside hospital (in-hospital cardiac arrest, IHCA) [2]. With 60%, cardiac aetiology is presumed to be the most common reason [3, 4]. In approximately 20–30% of cases, the origin is not cardiac [5, 6]. Even with immediate initiation of conventional cardiopulmonary resuscitation (CPR), survival rate with a favourable neurological outcome (mild to moderate neurological impairment, equivalent to a cerebral performance category (CPC) of 1–2) is low, both for IHCA and for OHCA [5–10% (OHCA) versus 15% (IHCA)] [7,8,9]. Extracorporeal cardiopulmonary resuscitation (eCPR) can be considered a rescue attempt for selected patients with refractory cardiac arrest of potentially reversible cause (e.g. myocardial infarction or pulmonary embolism) [10,11,12]. Observational studies suggest that eCPR can increase survival rate up to 30% in these patients [4, 13,14,15,16,17,18,19,20,21,22]. A meta-analysis showed an absolute increase of 30 day survival of 13% compared with conventional CPR (95% CI 6–20%; p < 0.001; number needed to treat 7.7) [23].
Currently, highly selected patients (see Table 1) receive venoarterial extracorporeal cardiovascular life support (VA-ECLS) under conventional or mechanical CPR [mCPR with, for instance, the LUCAS® (Physio-Control, Inc., Redmond, WA, USA) or AutoPulse® (ZOLL (Resuscitation Products), Chelmsford, MA, USA) system]. Selection criteria and procedures vary across institutions. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is commonly defined in the literature and in this consensus paper as being synonymous with extracorporeal life support system (ECLS) [24]. The present consensus paper offers a proposal for a standardised algorithm for eCPR with the use of an ECLS. The consensus paper has been written with representatives of the German Society of Medical Intensive Care and Emergency Medicine (DGIIN), the German Cardiac Society (DGK), the German Society of Thoracic, Cardiac and Vascular Surgery (DGTHG), the German Society of Cardiotechnics (DGfK), the German Society of Neuro-Intensive Care and Emergency Medicine (DGNI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Interdisciplinary Association for Intensive Care and Emergency Medicine [DIVI, section group Neurological Medicine (Studies and Standards in Neurological Medicine), the section group Emergency Medicine (Resuscitation and Post-Resuscitation Therapy), section group Ethics], and the German Resuscitation Council (GRC).
Previous guidelines and recommendations
Neither the guidelines of the European Resuscitation Council [10] nor the guidelines of the American Heart Association [8] on CPR recommend the routine use of eCPR for patients with cardiac arrest [Class IIb (benefit ≥ risk), evidence level C-LD (limited data)]. A separate guideline on eCPR from the ‘Extracorporeal Life Support Organisation (ELSO)’ [26] is limited to general aspects of eCPR only.
Current studies
Randomised controlled trials (RCT) on eCPR are lacking so far, and there are no prospectively validated criteria for selecting patients and determining indications for eCPR [27]. Defining predictors of risk and benefit, which could help to determine indications for eCPR, remain to be a major clinical challenge. A meta-analysis by Debaty et al. [19] investigated the prognostic value of various risk factors which might help guiding the specialist in acute settings when the question to initiate eCPR or not raises. Primary outcome was significantly better in the presence of an initially defibrillatable heart rhythm (OR 2.2, 95% CI 1.30–3.72, p = 0.003), a shorter low-flow time (time from CPR initiation to eCPR, geometric mean ratio 0.90, 95% CI 0.81–0.99, p = 0.04), a higher pH (Δ pH (arterial) 0.12, 95% CI 0.03–0.22, p = 0.01) and a low serum lactate concentration (Δ − 3.52 mmol/L, 95% CI − 5.05 to − 1.99, p < 0.001).
Together with data from other major observational studies, a defibrillatable heart rhythm appears to be an important prognostic factor for patients with OHCA [19]. At present, however, it does not seem justified to deny eCPR categorically for patients without defibrillatable heart rhythms. The prognostic value of the low-flow time has already been documented, both for in-hospital and out-of-hospital cardiac arrest [19, 28]. The time factor seems to play a particularly important part. A Japanese registry study showed that strict adherence to the ‘collapse-to-start eCPR’ of < 40 min and the ‘collapse-to-coronary reperfusion’ of < 60 min was accompanied by the best prognostic outcome [29]. However, even in cities with established eCPR programmes, low-flow time to ECLS support exceeded 70 min. Analogous to the treatment of ST-segment elevation myocardial infarction it will be very important to reduce low-flow time in future [17, 28]. Current and future studies [e.g. the CAREECMO study (NCT0335299)] are investigating whether a considerably accelerated CPR algorithm for selected patients, with the focus on rapid transportation following the ‘load-and-go’ principle or preclinical ECLS initiation [30] have more impact on mortality. Moreover, early arrival of the first-aider, a low serum lactate, a higher etCO2 before arrival at the cardiac catheterisation laboratory and the presence of coronary heart disease as a reversible cause for collapse are associated with a significantly higher probability of survival [31].
As biochemical markers, pH and serum lactate have emerged as ‘the’ prognostic factors at many sites [19]. However, any prolonged CPR causes deviations of pH and serum lactate levels, owing to a metabolic imbalance at cellular level. One-off elevations should therefore be interpreted with caution, whereas clearance might be more important over time [32, 33]. Comparing both markers, pH seems to predict neurological outcome better than lactate levels [34]. By the way, venous pH shows a good correlation with the arterial pH [35]. There are no well-validated cut-off values, neither for pH nor for serum lactate. These values of these parameters must be interpreted in clinical context only. In the past pH values below 6.8 were supposed to be incompatible with life, according to textbook opinion [36]. However, some current case reports show a good neurological outcome with even lower pH values (CPC 1–2) [37, 38]. Nevertheless, a retrospective analysis of prospective registry data of OHCA patients showed that a pH < 6.8 was not associated with a good neurological outcome [39].
Therefore, decision to initiate eCPR should always be made by a multi-professional ‘eCPR team’ (see Recommendation 2, below), taking into account all available indicators individually (Table 1). Besides these event-related variables, general patient-related, variables are also of prognostic significance. Obesity is often discussed in this context, as it might hinder the placement of ECLS cannula. However, a retrospective observational study showed that the body mass index (BMI < 18.5 to ≥ 30 kg/m2) was not associated with either an increased mortality or a poorer neurological outcome in eCPR patients [40]. A retrospective study showed that the 1-year survival rate was significantly lower for patients with malignant disease than for those with non-malignant disease (1.7% versus 11.4%) [41]. Moreover, elderly and frail patients with OHCA show a low probability of survival [42]. Age itself does not seem to have a negative effect on the survival rate and therefore should not be listed as an absolute contraindication [22, 43, 44]. In summary, observational studies have yielded a series of variables of prognostic significance—none of these markers can be regarded as a ‘no-go’ decision aid.
Although observational studies showed a survival advantage for eCPR over conventional CPR after IHCA and OHCA, RCT data are still missing. Some RCTs on this topic have recently been initiated [e.g. the INCEPTION study (NCT03101787, scheduled completion 2019), the EROCA study (NCT03065647, scheduled completion 2019), the Prague OHCA study (NCT01511666, scheduled completion 2018), and the ACPAR2 study (NCT02527031, scheduled completion 2018)], and until these studies are completed it will not be possible to make any statement about clinical endpoints.
Besides the medical aspects of eCPR, it is also necessary to consider the ethical aspects (such as diagnosis of brain death under ECLS) and the potential psychological burdens on the treatment team and the family [45,46,47,48,49].
Treatment pathways—current evidence
Out-of-hospital cardiac arrest (OHCA)
No standardised treatment pathway for patients under CPR has been clarified yet. Even in the case of a favourable ventricular rhythm event over 60% of the patients display refractory ventricular fibrillation and rarely achieve a return of spontaneous circulation (ROSC), or die before hospital admission [50]. In a prospective study by Yannopoulos et al. [31], an algorithm was established for selected patients with refractory ventricular fibrillation (age 18–75 years, transfer time < 30 min to the cardiac catheterisation lab, patients received at least three defibrillations and 300 mg of amiodarone without achieving ROSC). Patients in this specific group were transported rapidly to a 24-h/7-day/365-day hospital with readiness for cardiac catheterisation under mCPR (LUCAS® chest compression system). Placement of an ECLS was done immediately on arrival in the cardiac catheterisation laboratory if there were no specific exclusion criteria (etCO2 < 10 mmHg, paO2 < 50 mmHg or SO2 < 85%, serum lactate > 18 mmol/L). Left-heart catheterisation, if necessary with coronary intervention was performed in the same setting. Certain patients were excluded from this rapid emergency transport: the presence of a cardiac arrest of non-cardiac aetiology (e.g. trauma, haemorrhage), contraindications to the placement of a LUCAS® device, known pregnancy, nursing home patients, the existence of a ‘Do-Not Resuscitate/Do-Not Intubate’ situation (e.g. an advance directive), and the presence of a terminal disease (e.g. cancer or terminal heart or kidney failure). 9% of the patients experienced ROSC on the way to the cardiac catheterisation laboratory, 91% of the patients (50/55) were cannulated for eCPR and 84% received a successful coronary intervention. Complications associated with ECLS placement were retroperitoneal bleeding (8%) and other vascular complications (6%). 42.0% of the patients survived with a good neurological status (CPC 1–2) versus 15.3% in a historical comparison group. Fluoroscopy-guided cannulation of the femoral vessels under CPR conditions is safe [51] and leads to a reduction of complication rates without loss of time [52]. Up-to-date, there is no randomised controlled or prospective study comparing fluoroscopically guided with ultrasonically guided ECLS placement. A small retrospective single-centre observational study compared the cannulation time for anatomical ‘landmark’ based vessel puncture plus the use of conventional wires with that of ultrasound guided vessel puncture using stiff wires [53]. The median cannulation time was 17 (12–26) min (landmark technique) versus 8 (6–12) min (ultrasonic technique, p < 0.001), which favours the use of ultrasound and stiff wires. From an interventional point of view, the ‘stiff-wire’ method generally is the preferred procedure—irrespective of whether the landmark or the ultrasonic technique is used. In this study, cannulation was performed by interventional cardiologists only. Some answers to the question of the optimum cannulation method are still missing [53]. Given that early coronary angiography in resuscitated patients is accompanied by lower mortality [54, 55]—and as no other algorithm has yet been studied—the ‘CPR-cardiac catheterisation pathway’ should be preferred [31]. Direct transportation of selected patients with OHCA to a cardiac arrest centre with cardiac catheterisation readiness should be the aim [56, 57]. Hospitals with an ECMO/ECLS programme should be able to implant an ECLS at various sites in the hospital (e.g. the trauma room or cardiac catheterisation laboratory) [57]. This will ensure that an eCPR can be done even with non-cardiac aetiology (e.g. accidental hypothermia) [58].
In-hospital cardiac arrest (IHCA)
The treatment pathways within hospital departments depend on the prevailing conditions and resources and therefore vary widely. In the case of IHCA, it might be appropriate to cannulate the patient an ECLS on the spot (e.g. in the intensive-care ward), to save transport time.
For hospitals without the structural and staffing requirements for ECLS placement, it is recommended to have predefined contact persons at the nearest hospital with an ECMO–ECLS programme. A brief discussion will reveal wether it is more advisable to rapidly transport the patient under CPR to ECLS centre or to send an ECLS team to the patient.
Organisational requirements and recommendations for eCPR
-
1.
Seamless eCPR readiness requires a 24-h/7-day/365-day availability of the eCPR team with correspondingly short assembly time.
-
2.
The multi-professional eCPR team ideally consists of a doctor who is additionally qualified in emergency medicine or a medical specialist who is additionally qualified in intensive care medicine and the ECLS implantation team. The ECLS implantation team should meet medical specialist standards from at least two of the three specialities of cardiology, cardiac surgery and anaesthesiology and should also include a cardiovascular perfusionist, or—especially in institutions without a cardiovascular perfusion unit—a professional care staff specifically trained in ECLS with the qualifications listed in the following sentence. The assistants and/or nurses who are involved with implanting and operating the ECLS are trained nursing professionals—ideally with further specialist training in intensive or emergency nursing—and are experienced in the therapy of patients with ECLS. For further information, please consult the appropriate European recommendations [30].
-
3.
The eCPR programme should ideally be attached to a hospital with an intensive-care unit and many years of experience in the care of ECLS patients and also the option of further treatments (for instance, the implantation of ventricular support systems or heart transplants) [30, 59].
-
4.
Availability of portable ECLS/ECMO systems is not given all over Germany. For that, the patient should be admitted to a collaborating hospital with 24-h/7-day/365-day cardiac catheterisation and ECLS readiness. If mobile extracorporeal support systems are used, e.g. in patients with massive pulmonary embolism under CPR, please consult the recommendations for inter-hospital transfer under ECLS [60,61,62].
-
5.
A telephone notification call and a shared checklist-based indication review should be made with the doctor responsible in the ECLS team. Ideally, the review should be done within the first 15 min of low-flow time (refractory CPR [20, 30]) und includes age, possible comorbidities, initial rhythm, no-flow time and ROSC status.
-
6.
Valid procedural instructions must be implemented which reliably define the structured handover and the sites of intervention to improve and maintain communication between the parties [57].
-
7.
After the structured handover (including team time-out), a general clinical examination and immediate focused ultrasound/echocardiography under mCPR should be done to rule out or detect any reversible causes (pneumothorax, signs of right ventricular strain indicating pulmonary embolism, pericardial tamponade, left ventricular dysfunction and hypovolaemia) [63,64,65].
-
8.
The final decision about performing an ECLS placement should be made by the ECLS implantation team after weighing up the pro and contra criteria. Until then the CPR should be continued uninterruptedly and in accordance with the guidelines [66,67,68].
-
9.
An arterial access should be installed for haemodynamic monitoring under CPR and to determine the chemical laboratory prognostic factors (serum lactate, pH). Ideally, an arterial catheter should be placed in the common femoral artery for this purpose immediately on arrival of the patient. Besides the arterial blood gas analysis and the invasive monitoring of blood pressure arterial cannulation for the ECLS can be performed at this site.
-
10.
A separate intensive care or trauma team, consisting of a doctor with experience in the critical care of resuscitated patients (if possible > 1 year) and nursing staff should be present continuously during ECLS placement and take care of the hemodynamic and respiratory support as well monitoring.
-
11.
A ‘collapse-to-start eCPR interval’ of 60 min [69] and a ‘door-to-ECLS implantation time’ of less than 30 min should be adhered to depending on local conditions [70].
-
12.
The ECLS placement via the femoral artery (15–19 Fr) and femoral vein (19–23 Fr) should ideally be done either in the cardiac catheterisation laboratory under fluoroscopic guidance (if necessary by vascular ultrasound) or in the emergency department/trauma room under ultrasonic guidance [71, 72].
-
13.
After ECLS placement a distally oriented catheter should be inserted for anterograde leg perfusion under ultrasonic guidance. If placement is unsuccessful and there are clinical or technical signs of critically low perfusion (e.g. via near-infrared spectroscopy on the lower leg) open surgical implantation should be persued. The correct site and functioning of the distally oriented catheter must be evaluated early by appropriate diagnostic methods (e.g. vascular ultrasound or (CT) angiography).
-
14.
There should be a low threshold for considering a whole-body CT scan, depending on the clinical situation after ECLS placement, to identify undetected causes of cardiac arrest (especially central processes), secondary injuries after CPR and complications due to the ECLS placement [73].
-
15.
Guideline-compliant temperature management (32–36 °C constantly for 24 h) should be carried out, taking into account the current blood coagulation status and bleeding complications [66, 74].
-
16.
The additional implantation of a left ventricular microaxial pump [Impella® (Abiomed U.S., Danvers, MA, USA)] can be considered over time if there is no pulsatility or only minimal left ventricular contractility consistent with left ventricular unloading in the form of ‘venting’ [75, 76].
-
17.
Prognostication in eCPR patients remains difficult. In particular, the question if and when an eCPR should be terminated should be decided—given the lack of scientific evidence to date—within the interdisciplinary intensive care and ECLS team, taking account of the medical and ethical aspects, as a decision specific to the patient as an individual. The current guidelines on resuscitation generally recommend a neurological prognosis assessment and treatment decision not earlier than 72 h after ROSC [10].
-
18.
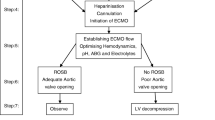
An eCPR process flowchart in the form of a standardised operating procedure (SOP) should be established in the eCPR team and evaluated at regular intervals (Fig. 1).
eCPR algorithm. BGA blood gas analysis, CPR cardiopulmonary resuscitation, CT computed tomography, ECMO extracorporeal membrane oxygenation, ECLS extracorporeal life support system, eCPR extracorporeal cardiopulmonary resuscitation, FEEL focused echocardiographic evaluation in life support, PCI percutaneous coronary intervention, TRO-CT triple-rule-out CT angiography, to rule out or detect simultaneously coronary heart disease, an acute pulmonary embolism and acute aortic disease, VA venoarterial, X-blood blood for cross-match
Quality criteria
Besides technical skills, implementing eCPR requires social, economic and medical–ethical skills [69].
-
1.
For the care of patients with pre-hospital cardiac arrest, please consult the quality indicators and structural requirements for ‘cardiac arrest centres’ [57].
-
2.
Implementing eCPR demands considerable resources and requires very good communication and co-operation between all members and associates of the eCPR team—similar to regional infarct networks [77]. Especially, because of the great importance of early notification and shared eCPR assessment in the form of a ‘rapid decision-making’ process management and the focus on rapid transportation a close dialogue and binding structural collaboration with the local emergency medical services is essential.
-
3.
Under the direction of a qualified mentor, a multi-professional training for team-focused eCPR contributes to quality assurance [78].
-
4.
At regular meetings, quality criteria/features (e.g. optimising the eCPR-SOP), the current study results and case reports should be reported and discussed. Participation in national and international multicentre studies is desirable.
-
5.
Since many undesirable events and complications arise from the complexity of treatment, uncertainty, lack of team management or misunderstandings between members of the eCPR team in the ad hoc emergency situation, all participants in the eCPR team should receive the appropriate quality of training and instruction. For that reason, there should be clearcut rules governing the allocation of responsibilities with regard to both medicine and logistics, and training sessions should be held regularly.
-
6.
To achieve the appropriate quality, the requirement is a caseload of at least 30 ECLS/ECMO placements (elective plus under CPR) per year and per hospital with an ECMO/ECLS programme [30, 79].
-
7.
To maintain quality in line with current recommendations and study data, the aim is to update this present consensus paper every 5 years.
References
Atwood C, Eisenberg MS, Herlitz J, Rea TD (2005) Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation 67(1):75–80
Go AS, Mozaffarian D, Roger VL et al (2014) Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129(3):399–410
Hawkes C, Booth S, Ji C et al (2017) Epidemiology and outcomes from out-of-hospital cardiac arrests in England. Resuscitation 110:133–140
Spangenberg T, Schewel J, Dreher A et al (2018) Health related quality of life after extracorporeal cardiopulmonary resuscitation in refractory cardiac arrest. Resuscitation 127:73–78
Kuisma M, Alaspää A (1997) Out-of-hospital cardiac arrests of non-cardiac origin. Epidemiology and outcome. Eur Heart J 18(7):1122–1128
Hess EP, Campbell RL, White RD (2007) Epidemiology, trends, and outcome of out-of-hospital cardiac arrest of non-cardiac origin. Resuscitation 72(2):200–206
Sandroni C, Nolan J, Cavallaro F, Antonelli M (2007) In–hospital cardiac arrest: Incidence, prognosis and possible measures to improve survival. Intensive Care Med 33:237–245
Link MS, Berkow LC, Kudenchuk PJ et al (2015) Part 7: advanced cardiovascular life support—2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 132(Suppl 2):S444–S464
Karam N, Marijon E, Dumas F et al (2017) Characteristics and outcomes of out-of-hospital sudden cardiac arrest according to the time of occurrence. Resuscitation 116:16–21
Soar J, Nolan JP, Böttiger BW et al (2015) European Resuscitation Council Guidelines for Resuscitation 2015 Sect. 3. Adult advanced life support. Resuscitation 95:100–147
Kim SJ, Kim HJ, Lee HY et al (2016) Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: a meta-analysis. Resuscitation 103:106–116
Vdovin N, Günther SPW, de Waha S et al (2017) Early risk stratification in patients with cardiogenic shock complicating acute myocardial infarction treated with extracorporeal life support and primary percutaneous coronary intervention. JACC Cardiovasc Interv 10(23):2469–2471
Chen YS, Lin JW, Yu HY et al (2008) Cardiopulmonary resuscitation with assisted extracorporeal life support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 372:554–561
Wang CH, Chou NK, Becker LB et al (2014) Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest—a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation 85(9):1219–1224
Fagnoul D, Combes A, De Backer D (2014) Extracorporeal cardiopulmonary resuscitation. Curr Opin Crit Care 20(3):259–265
Blumenstein J, Leick J, Liebetrau C et al (2016) Extracorporeal life support in cardiovascular patients with observed refractory in-hospital cardiac arrest is associated with favourable short and long-term outcomes: a propensity-matched analysis. Eur Heart J Acute Cardiovasc Care 5(7):13–22
Spangenberg T, Meincke F, Brooks S et al (2016) “Shock and Go?” extracorporeal cardio-pulmonary resuscitation in the golden-hour of ROSC. Catheter Cardiovasc Interv 88(5):691–696
Combes A, Brodie D, Chen YS et al (2017) The ICM research agenda on extracorporeal life support. Intensive Care Med 43(9):1306–1318
Debaty G, Babaz V, Durand M et al (2017) Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation 112:1–10
Conrad SA, Rycus PT (2017) Extracorporeal membrane oxygenation for refractory cardiac arrest. Ann Card Anaesth 20(Suppl):S4–S10
Richardson AS, Schmidt M, Bailey M et al (2017) ECMO cardio-pulmonary resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation 112:34–40
Haas NL, Coute RA, Hsu CH et al (2017) Descriptive analysis of extracorporeal cardiopulmonary resuscitation following out-of-hospital cardiac arrest—an ELSO registry study. Resuscitation 119:56–62
Ouweneel DM, Schotborgh JV, Limpens J et al (2016) Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med 42:1922–1934
Trummer G, Bein B, Buerke M et al (2011) Standardized terminology of mechanical heart, lung and circulatory assist devices: a recommendation of the Section “Heart and Circulation” of the German Interdisciplinary Association of Critical Care Medicine. Appl Cardiopulm Pathophysiol 15:181–182
Michels G, Thiele H, Kluge S, Pfister R (2017) Are there any prognostic predictors for extracorporeal cardiopulmonary resuscitation (ECPR) in case of out-of-hospital cardiac arrest?. Med Klin Intensivmed Notfmed 112(7):634–636
ELSO ECPR Supplement to the ELSO General Guidelines (2013) Version 1.3. https://www.elso.org/Portals/0/IGD/Archive/FileManager/6713186745cusersshyerdocumentselsoguidelinesforecprcases1.3.pdf
Günther S, Born F, Buchholz S et al (2018) Patienten unter Reanimation: Kandidaten für “Extracorporeal Life Support”? Z Herz Thorax-Gefäßchir 32:133–140
Wengenmayer T, Rombach S, Ramshorn F et al (2017) Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care 21(1):157
Kuroki N, Abe D, Iwama T et al (2017) Association between delay to coronary reperfusion and outcome in patients with acute coronary syndrome undergoing extracorporeal cardiopulmonary resuscitation. Resuscitation 114:1–6
Abrams D, Garan AR, Abdelbary A et al (2018) Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. https://doi.org/10.1007/s00134-018-5064-5
Yannopoulos D, Bartos JA, Raveendran G et al (2017) Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol 70(9):1109–1117
Hayashida K, Suzuki M, Yonemoto N et al (2017) Early lactate clearance is associated with improved outcomes in patients with postcardiac arrest syndrome: a prospective, multicenter observational study (SOS-KANTO 2012 Study). Crit Care Med 45(6):e559–e566
Slottosch I, Liakopoulos O, Kuhn E et al (2017) Lactate and lactate clearance as valuable tool to evaluate ECMO therapy in cardiogenic shock. J Crit Care 42:35–41
Momiyama Y, Yamada W, Miyata K et al (2017) Prognostic values of blood pH and lactate levels in patients resuscitated from out-of-hospital cardiac arrest. Acute Med Surg 4(1):25–30
Zeserson E, Goodgame B, Hess JD et al (2018) Correlation of venous blood gas and pulse oximetry with arterial blood gas in the undifferentiated critically ill patient. J Intensive Care Med 33(3):176–181
Siegenthaler W (2005) Differentialdiagnose, vol 19. Thieme, Auflage
Geisler AC, Söffker G, Breunig F et al (2014) Der besondere Fall-Optimale Rettungskette. Hamb Arztebl Bd 68(10):44–46
Hohmann C, Pfister R, Michels G (2018) Are the initial pH and the lactate values after cardiopulmonary resuscitation always crucial?. Med Klin Intensivmed Notfmed. https://doi.org/10.1007/s00063-018-0432-z
Shin J, Lim YS, Kim K et al (2017) Initial blood pH during cardiopulmonary resuscitation in out-of-hospital cardiac arrest patients: a multicenter observational registry-based study. Crit Care 21(1):322
Gil E, Na SJ, Ryu JA et al (2017) Association of body mass index with clinical outcomes for in-hospital cardiac arrest adult patients following extracorporeal cardiopulmonary resuscitation. PLoS One 12(4):e0176143
Kang SB, Kim KS, Suh GJ et al (2017) Long-term survival of out-of-hospital cardiac arrest patients with malignancy. Am J Emerg Med 35(10):1457–1461
Sulzgruber P, Sterz F, Poppe M et al (2017) Age-specific prognostication after out-of-hospital cardiac arrest—the ethical dilemma between ‘life-sustaining treatment’ and ‘the right to die’ in the elderly. Eur Heart J Acute Cardiovasc Care 6(2):112–120
Pabst D, El-Banayosy A, Soleimani B, Brehm CE (2018) Predictors of survival for nonhighly selected patients undergoing resuscitation with extracorporeal membrane oxygenation after cardiac arrest. ASAIO J 64(3):368–374
Lorusso R, Gelsomino S, Parise O et al (2017) Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock in elderly patients: trends in application and outcome from the Extracorporeal Life Support Organization (ELSO) Registry. Ann Thorac Surg 104(1):62–69
Riggs KR, Becker LB, Sugarman J (2015) Ethics in the use of extracorporeal cardiopulmonary resuscitation in adults. Resuscitation 91:73–75
Mancini ME, Diekema DS, Hoadley TA et al (2015) Part 3: Ethical Issues: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 132(18 Suppl 2):S383–S396
Meltzer EC, Ivascu NS, Stark M et al (2016) A survey of physicians’ attitudes toward decision-making authority for initiating and withdrawing VA-ECMO: results and ethical implications for shared decision making. J Clin Ethics 27(4):281–289
Makdisi T, Makdisi G (2017) Extra-corporeal membrane oxygenation support: ethical dilemmas. Ann Transl Med 5(5):112
Niecke A, Schneider G, Hartog CS, Michels G (2017) Traumatized relatives of intensive care patients. Med Klin Intensivmed Notfmed 112(7):612–617
Stiell IG, Nichol G, Leroux BG et al (2011) Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. N Engl J Med 365(9):787–797
Goslar T, Knafelj R, Radsel P et al (2016) Emergency percutaneous implantation of veno-arterial extracorporeal membrane oxygenation in the catheterisation laboratory. EuroIntervention 12(12):1465–1472
Kashiura M, Sugiyama K, Tanabe T, Akashi A, Hamabe Y (2017) Effect of ultrasonography and fluoroscopic guidance on the incidence of complications of cannulation in extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a retrospective observational study. BMC Anesthesiol 17(1):4
Voicu S, Henry P, Malissin I et al (2018) Improving cannulation time for extracorporeal life support in refractory cardiac arrest of presumed cardiac cause—comparison of two percutaneous cannulation techniques in the catheterization laboratory in a center without on-site cardiovascular surgery. Resuscitation 122:69–75
Reynolds JC, Callaway CW, El Khoudary SR et al (2009) Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Intensive Care Med 24(3):179–186
Dumas F, Cariou A, Manzo-Silberman S et al (2010) Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 3(3):200–207
Kragholm K, Malta Hansen C, Dupre ME et al (2017) Direct transport to a percutaneous cardiac intervention center and outcomes in patients with out-of-hospital cardiac arrest. Circ Cardiovasc Qual Outcomes 10(6):e003414. https://doi.org/10.1161/CIRCOUTCOMES.116.003414
Scholz KH, Andresen D, Böttiger BW et al (2017) Quality indicators and structural requirements for Cardiac Arrest Centers-German Resuscitation Council (GRC). Kardiologe 11:205–208
Jarosz A, Darocha T, Kosiński S et al (2017) Profound accidental hypothermia: systematic approach to active recognition and treatment. ASAIO J 63(3):e26–e30
Beckmann A, Benk C, Beyersdorf F et al (2011) Position article for the use of extracorporeal life support in adult patients. Eur J Cardiothorac Surg 40(3):676–680
Combes A, Brodie D, Bartlett R et al (2014) Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 190(5):488–496
Dirnberger D, Fiser R, Harvey C et al (2015) Extracorporeal Life Support Organization (ELSO), Guidelines for ECMO Transport. https://www.elso.org/resources/guidelines.aspx
Broman LM, Frenckner B (2016) Transportation of Critically ill patients on extracorporeal membrane oxygenation. Front Pediatr 4:63
Michels G, Zinke H, Möckel M et al (2017) Recommendations for education in ultrasound in medical intensive care and emergency medicine: position paper of DGIIN, DEGUM and DGK. Med Klin Intensivmed Notfmed 112(4):314–319
Zhang Z (2017) Echocardiography for patients undergoing extracorporeal cardiopulmonary resuscitation: a primer for intensive care physicians. J Intensive Care 5:15
Price S, Platz E, Cullen L et al (2017) Expert consensus document: echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol 14(7):427–440
Nolan JP, Hazinski MF, Aickin R et al (2015) Part 1: Executive summary: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 95:e1–e31
Olasveengen TM, de Caen AR, Mancini ME et al (2017) 2017 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations summary. Circulation 136(23):e424–e440
Perkins GD, Olasveengen TM, Maconochie I et al (2018) European Resuscitation Council guidelines for resuscitation: 2017 update. Resuscitation 123:43–50
Swol J, Belohlávek J, Haft JW et al (2016) Conditions and procedures for in-hospital extracorporeal life support (ECLS) in cardiopulmonary resuscitation (CPR) of adult patients. Perfusion 31(3):182–188
Leick J, Liebetrau C, Szardien S et al (2013) Door-to-implantation time of extracorporeal life support systems predicts mortality in patients with out-of-hospital cardiac arrest. Clin Res Cardiol 102(9):661–669
Nanjayya VB, Murphy D (2015) Ultrasound guidance for extra-corporeal membrane oxygenation general guidelines. https://www.elso.org/Portals/0/Files/elso_Ultrasoundguideance_ecmogeneral_guidelines_May2015.pdf
Ahn HJ, Lee JW, Joo KH et al (2018) Point-of-care ultrasound-guided percutaneous cannulation of extracorporeal membrane oxygenation: make it simple. J Emerg Med 54(4):507–513
Beck L, Burg MC, Heindel W, Schülke C (2017) Extracorporeal membrane oxygenation in adults—variants, complications during therapy, and the role of radiological imaging. Rofo 189(2):119–127
Pang PY, Wee GH, Hoo AE et al (2016) Therapeutic hypothermia in adult patients receiving extracorporeal life support: early results of a randomized controlled study. J Cardiothorac Surg 11:43
Spartera M, Jabbour RJ, Chiarito M, De Bonis M, Pappalardo F (2017) Stepwise use of circulatory support devices in a patient refractory to cardiopulmonary resuscitation. Cardiovasc Revasc Med 18(6):447–449
Tepper S, Masood MF, Baltazar Garcia M et al (2017) Left ventricular unloading by impella device versus surgical vent during extracorporeal life support. Ann Thorac Surg 104(3):861–867
Ibanez B, James S, Agewall S et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177
Johnson B, Runyon M, Weekes A, Pearson D (2017) Team-focused cardiopulmonary resuscitation: prehospital principles adapted for emergency department cardiac arrest resuscitation. J Emerg Med S0736-4679(17):30771–30770
Barbaro RP, Odetola FO, Kidwell KM et al (2015) Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med 191(8):894–901
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Michels G is a member of the scientific advisory board of the German Society of Medical Intensive Care and Emergency Medicine (Deutsche Gesellschaft für Internistische Intensivmedizin und Notfallmedizin, DGIIN), commissionery leader of the working group of “Cardiopulmonary Resuscitation” of the German Society for Cardiology (Deutsche Gesellschaft für Kardiologie, DGK) and member of the working group “ECMO / eCPR” of the German Resuscitation Council (GRC) and received lecture fees from Pfizer, Novartis, Servier, ZOLL and Orion Pharma. Bauersachs J is the leader of the DFG-funded Clinical Research Group (KFO) 311 “(Pre) terminal heart and lung failure: Relief and Repair” and received lecture fees and research support from Abiomed and ZOLL. Böttiger BW is European Resuscitation Council (ERC) Board Director Science and Research, chairman of the German Resuscitation Council (GRC), member of the “Advanced Cardiac Life Support” (ALS) Task Force of the International Liaison Committee on Resuscitation (ILCOR), member of the presidium of the German Interdisciplinary Association for Intensive Care and Emergency Medicine (Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin, DIVI), associated editor of the European Journal of Anaesthesiology (EJA), co-editor of the journal Resuscitation, editor of the journal Notfall + Rettungsmedizin. For lectures he has received fees from the following companies: Medupdate, Forum for Medical Education (FoMF), Baxalta, Bayer Vita, ZOLL, BARD. Ghanem A has received lecture fees from Getinge. Markewitz A received a lecture fee from TÜV Süd, München. Gräsner JT is the leader of the scientific working group “emergency medicine” of the German Society of Anaesthesiology and Intensive Care Medicine (Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin, DGAI), speaker of the organizing committee of the “German Reanimation Registry”, Chair of the European Registry of Cardiac Arrest, member of the executive committee of the German Council for Resuscitation (GRC), member of the presidium of the professional association of german anaesthesiologists. Kreimeier U is leader of the working group “Advanced Life Support” at the German Resuscitation Council (GRC). Wengenmayer T, Hagl C, Dohmen C, Bauer A, Pfister R, Busch HJ, Beckmann A, Fischer M, Kill C, Janssens U, Kluge S, Born F, Hoffmeister HM, Preusch M, Boeken U, Riessen R and Thiele H declare, that there is no conflict of interest with respect to the subject publication.
Additional information
This consensus statement was also published in Medizinische Klinik—Intensivmedizin und Notfallmedizin, Der Kardiologe, Der Anaesthesist, Zeitschrift für Herz-, Thorax- und Gefäßchirurgie und Anästhesiologie and Intensivmedizin. The consensus paper is currently available in German only. This paper is the first English version of the eCPR German consensus statement.
Rights and permissions
About this article
Cite this article
Michels, G., Wengenmayer, T., Hagl, C. et al. Recommendations for extracorporeal cardiopulmonary resuscitation (eCPR): consensus statement of DGIIN, DGK, DGTHG, DGfK, DGNI, DGAI, DIVI and GRC. Clin Res Cardiol 108, 455–464 (2019). https://doi.org/10.1007/s00392-018-1366-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1366-4