Abstract
Aims
The wearable cardioverter defibrillator (WCD) is used for temporary protection of patients deemed to be at high risk for sudden death. There is limited experience regarding the clinical development of patients with tachymyopathy. We aimed to evaluate the clinical development of tachymyopathy patients protected with a WCD in a single-center non-randomized patient cohort.
Methods and results
We fitted 130 consecutive patients deemed to be at high risk for ventricular tachyarrhythmias with the WCD. Of these, 20 patients (15%) presenting with newly diagnosed heart failure in the setting of rapidly conducted atrial fibrillation/flutter were suspected to suffer from tachymyopathy. The control group consisted of the remaining 110 patients with other indications for WCD therapy. LVEF increased by more than 10% in 13/20 (65%) tachymyopathy patients compared to 40/110 (36%) patients in the control population (p = 0.01). Similarly, BNP levels decreased in 15/20 (75%) tachymyopathy patients compared to 41/110 (37%) in the control group (p = 0.05). ICD implantation rates were lower in the tachymyopathy group (3/20) compared to the control population (40/110; p = 0.04). On further follow-up (mean 12 ± 8 months), patients with suspected tachymyopathy had no sustained ventricular arrhythmias. Compared to 5/110 patients in the control group, no tachymyopathy patient died.
Conclusion
Most of the patients with suspected tachymyopathy have a favorable clinical outcome. The WCD is useful for temporary protection while LV function recovers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tachycardia-induced cardiomyopathy or tachymyopathy is characterized by a potentially reversible impairment of LV function [1]. Animal models revealed that chronic rapid pacing produces a severe reversible cardiomyopathy sustained by transient functional and structural changes of myocytes [2, 3]. Similarly, RA and LV diameters increased up to 150% along with elevated ANP-levels confirming the development of heart failure due to AV-pacing in a goat model [4]. Compared to other patients with chronic atrial fibrillation, patients with tachymyopathy due to atrial fibrillation or flutter are reported to be younger, to have more often a short duration of tachyarrhythmia, and to demonstrate significant improvement of LV function and better survival after successful rhythm control [5, 6]. However, there is conflicting data whether patients with tachymyopathy have an increased risk for ventricular arrhythmias and sudden cardiac death [7], and hence deserve protection by means of an implantable defibrillator. The time period during which heart failure treatment is attempted and during which recovery of LV function may take place leaves patients unprotected [8,9,10,11, 17, 18]. Current guidelines therefore recommend use of the WCD in such patients [12].
To the best of our knowledge, this is the first study to specifically evaluate the effectiveness of the WCD in patients with clinically suspected tachymyopathy due to atrial fibrillation or flutter.
Methods
Patient population
This prospective observational cohort study is based on data of 130 consecutive patients deemed to be at high risk for life-threatening ventricular arrhythmias receiving a WCD at the J. W. Goethe University Hospital of Frankfurt. All patients were fitted with a Life Vest™ system (Zoll, Pittsburgh, USA). Patients with newly diagnosed heart failure in the setting of rapidly conducted atrial fibrillation/flutter were suspected to suffer from tachymyopathy. Coronary artery disease was ruled out invasively in 18/20 patients and non-invasively in two patients. The control group consisted of the remaining 110 patients with other indications for WCD therapy (i.e., ischemic heart disease/non-ischemic heart disease/myocarditis/congenital heart disease). The study was approved by the IRB of the J. W. Goethe University and conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
WCD (wearable cardioverter-defibrillator)
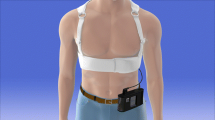
Commercially available WCD devices were used. The WCD consists of a garment containing three self-gelling defibrillation patch electrodes, two on the back and one in the front, and four nonadhesive ECG electrodes connected to a monitoring unit. Worn around the chest like a vest, the WCD provides continuous ECG monitoring and can automatically deliver up to five posterior–anterior defibrillation shocks. Once an arrhythmia is detected, an alarm sequence starts with a silent vibration and is followed by escalating audible siren alarms. The device detection algorithm incorporates three inputs: heart rate, template matching, and persistence of the event. The default ventricular tachycardia (VT) and ventricular fibrillation (VF) detection rate thresholds are 150 and 200 beats/min, respectively. The algorithm also includes a pair of response buttons that allows a conscious patient to respond to the alarm by pressing down on the button preventing an unnecessary WCD shock. The device uses a biphasic shock waveform with programmable energy levels of up to 150 J. The duration of the patient responsiveness test is at least 25 s but may last longer if the response buttons are activated or if ECG signal interference is detected. The WCD broadcasts an asystole alarm (including voice alerts to call for help and perform cardiopulmonary resuscitation) and starts ECG recording when there is a severe bradycardia detected.
Data collection and follow-up
All patients were clinically followed for at least 12 months after initiation of WCD therapy. Data were prospectively collected from the index hospitalization at the time of WCD fitting. In addition to preventive WCD therapy, patients received optimal pharmacological treatment adhering to contemporary guidelines. Follow-up visits were performed after 1, 3 and 12 months. Data collection included patient characteristics, initial indication for WCD therapy, left ventricular function at the time of WCD fitting and during follow-up, and relevant comorbid conditions. Pertinent medication use (beta blockers and antiarrhythmic therapy) was documented and checked during follow-up. Data were also collected from the ZOLL® LifeVest Network™. For missing data, particularly in case of missed follow-up visits, family members, treating physicians, or other hospitals were contacted to retrieve the missing information.
Statistics
Statistical analysis was performed using SPSS version 24 program (IBM, USA). Baseline characteristics were compared by the Wilcoxon Mann–Whitney U test or H Test of Kruskal and Wallis for continuous variables and the Chi2 test or Fisher’s exact test for categorial variables. Survival analysis was performed using Kaplan–Meier analysis. Survival curves were compared using the log-rank test. Only two-sided tests were used and p values p < 0.05 were considered statistically significant.
Results
Patient population
This report is based on data from 130 consecutive patients fitted with a WCD and followed for 12 ± 8 months. The most common clinical indication for WCD prescription was new symptomatic congestive heart failure with impaired left ventricular function (mean LVEF = 28 ± 11%; mean NYHA-class = 2.5 ± 0.9), either related to ischemic (35%) or non-ischemic cardiomyopathy (35%), followed by patients with clinically suspected tachymyopathy (15%) (Fig. 1). Patient characteristics are detailed in Table 1. All patients with tachymyopathy had recently diagnosed heart failure in the setting of rapidly conducted atrial fibrillation (p < 0.001) or atrial flutter (p = 0.06). In the control group, 15 patients (14%) were survivors of a prior event of sudden cardiac arrest, whereas no tachymyopathy patient had experienced prior episodes of life-threatening ventricular arrhythmia.
Clinical development during WCD use
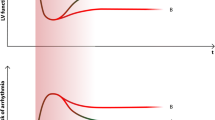
Median WCD use was 42 days (1–166 days; mean wear time of 23 h per day). Over this time period, 13 tachymyopathy patients (65%) improved their LV function by ≥ 10% over baseline compared to 40 patients (36%) of the control group (p = 0.01) (Table 2; Fig. 2a). Similarily, tachymyopathy patients showed more often a lowering of their BNP levels during WCD use (p = 0.02) (Table 2; Fig. 2b).
Restoration and maintenance of sinus rhythm
At start of WCD use, five tachymyopathy patients had already had sinus rhythm restored, whereas 13 patients were still in atrial fibrillation and two patients in atrial flutter. All 20 patients with suspected tachymyopathy received beta blocker therapy for ventricular rate control, six patients received amiodarone in addition. Three patients had documented LAA thrombi on TOE, hence rhythm control strategies could not be immediately pursued. During WCD use, 12 patients were electrically cardioverted and a total of seven patients (35%) underwent an ablative therapy (Table 2).
Arrhythmic events during WCD use
During WCD use, none of the tachymyopathy patients suffered from sustained ventricular arrhythmias (log rank for p = 0.19). Only one tachymyopathy patient experienced one episode of a non-sustained VT (Table 3).
In the control group, four patients had sustained ventricular arrhythmias requiring WCD shock therapy in two patients for ventricular fibrillation. Two patients aborted WCD shock therapy by activation of the response button. Episodes of asystole/long pauses were rare and occurred in three patients. Two patients in the control group experienced inappropriate WCD shock therapy, both for rapidly conducted supraventricular tachycardia.
ICD implantation
Following WCD therapy, 43/130 patients (33%) were implanted with an ICD since their LVEF did not improve beyond 35% despite optimal medication or since there was a secondary preventive indication (15/130; 12%) (Table 2). Only three patients with tachymyopathy received an ICD because LVEF did not improve.
Follow-up post-WCD use
After WCD therapy, all patients were further followed for up to one year. A total of 43 patients (33%) received an ICD. There were six ventricular arrhythmia episodes in ICD recipients, of which four required ICD shock therapies. None of the non-ICD recipients suffered from a clinical episode of VT/VF. During WCD use, none of the patients died. After WCD use, five patients of the control group died, mainly from progressive heart failure, whereas none of the tachymyopathy patients died (Table 4; appendix).
Discussion
Main findings
The present study is the first to prospectively evaluate the usefulness of the WCD in patients presenting with suspected tachymyopathy due new-onset heart failure in the setting of atrial fibrillation or atrial flutter. WCD therapy proved to be useful in identifying and temporarily protecting individuals who did not improve their LV function, and hence were implanted with an ICD. In contrast to patients in the control group, however, the majority of subjects with tachymyopathy had a favorable clinical outcome.
Prior findings on tachymyopathy
Little is known about the causal relationship why some patients without structural heart disease are more susceptible to develop heart failure in the setting of an incessant supraventricular or ventricular arrhythmia compared to other patients with chronic arrhythmias [1, 14]. This clinical phenomenon called tachymyopathy has been studied in experimental models [2,3,4, 16] and in humans [5,6,7, 15]. Patients with clinically suspected cardiomyopathy due to atrial fibrillation are more likely to be male [5, 15] and to have a shorter history of persistent atrial fibrillation [5]. Acute tachyarrhythmia induces immediate hemodynamic changes such as loss of atrial contraction contributing to the left ventricular filling period, resulting in reduced cardiac output and oxygen uptake [14]. Chronically elevated filling pressures provoking atrial stretch and dilation have been described in several pertaining animal models [2,3,4]. Similar observations have been made in clinical studies [1, 5, 16]. For instance, a study of 659 patients undergoing pulmonary vein isolation for atrial fibrillation comparing heart failure patients with tachymyopathy patients and controls demonstrated a significantly worse LVEF, larger left atrial diameters (LAD) and larger LVEDD in patients with tachymyopathy [5]. Following ablation, significant improvement of LVEF, LAD and LVEDD was noted in tachymyopathy after 6 months [5]. Our findings are consisted with these observations. During WCD use, tachymyopathy patients were more likely to improve their LVEF than patients with other causes of heart failure. In addition, neurohumoral activation, electromechanic changes [16] and fibrosis due to tachymyopathy lead to progressive heart failure associated with increased blood levels of ANP in an experimental goat model [4]. This is further emphasized by the observation that elevated NT-pro BNP levels in tachymyopathy patients showed a strong trend to normalize during a relatively short follow-up. The present study also confirms prior findings indicating particularly effective therapy of catheter ablation of atrial fibrillation [5, 15].
WCD as a means of risk stratification
The WCD is used as a shock device to temporarily protect patients deemed to be at an increased risk for sudden cardiac death and to help to avoid unnecessary ICD implantations [8, 17]. Although patients are protected against life-threatening arrhythmias, the treating physician can optimize pharmacological and non-pharmacological (i.e., catheter ablation) therapies. Once this is achieved, changes in the clinical status allow risk assessment to decide whether the patient is in need of permanent ICD therapy. To the best of our knowledge, this is the first study to describe WCD use in tachymyopathy patients. During WCD use, four patients in the control group had documented sustained ventricular arrhythmia requiring WCD shock therapy in two patients. None of the tachymyopathy patients had sustained ventricular arrhythmias; only one patient had a documented non- sustained VT. After WCD use, significantly more ICDs were implanted in the control group. Only three tachymyopathy patients continued to have a primary prevention ICD indication of whom two patients had persistent atrial fibrillation despite all efforts to establish rhythm control. Nerheim and colleagues studied 24 patients with tachymyopathy with a similar LVEF at baseline as our patients [7]. During a total follow-up time of 12 months, three patients died despite improvement of LV function. In these patients, the tachyarrhythmia preceding heart failure recurred prior to their demise [7]. Our study is in agreement with these observations and suggests that the majority of afflicted individuals with tachymyopathy improve their LV function, and hence do not have to undergo defibrillator implantation. During subsequent follow-up over 1 year, none of these patients died or suffered from VT or VF.
Conclusions
Patients with tachymyopathy due to atrial fibrillation or flutter showed a more favorable outcome compared to patients with other indications for WCD therapy and needed less often permanent ICD therapy.
References
Packer DL, Bardy GH, Worley SJ, Smith MS, Cobb FR, Coleman RE, Gallagher JJ, German LD (1986). Tachycardia induced cardiomyopathy: a reversible form of left ventricular dysfunction. Am J Cardiol 57:563–70
Zellner JL, Spinale FG, Eble DM, Hewett KW, Crawford FA Jr (1991) Alterations in myocyte shape and basement membrane attachment with tachycardia-induced heart failure. Circ Res 69:590–600
Everett TH, Li H, Mangrum JM, McRury ID, Mitchell MA, Redick JA (2000) Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation 102:1454–1460
Schoonderwoerd BA, Crijns HJ, van Veldhuisen DJ, Boomsma F, van den Berg MP, Bel KJ, van Gelder IC (2004). Atrial natriuretic peptides during experimental atrial tachycardia: role of developing tachycardiomyopathy. J Cardiovasc Electrophysiol 15:927–32
Calvo N, Bisbal F, Guiu E, Ramos P, Nadal M, Tolosana JM, Arbelo E, Berruezo A, Sitges M, Brugada J, Mont L (2013) Impact of atrial fibrillation induced tachycardiomyopathy in patients undergoing pulmonary vein isolation. Int J Cardiol 168:4093–4097
Brembilla-Perrot B, Ferreira JP, Manenti V, Sellal JM, Olivier A, Villemin T, Beurrier D, De Chillou C, Louis P, Brembilla A, Juillière Y, Girerd N (2016) Predictors and prognostic significance of tachycardiomyopathy: insights from a cohort of 1269 patients undergoing atrial flutter ablation. Eur J Heart Fail 18:394–401
Nerheim P, Birger-Botkin S, Piracha L, Olshansky B (2004). Heart failure and death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation 110:247–52
Klein HU, Goldenberg I, Moss AJ (2013) Risk stratification for implantable cardioverter defibrillator therapy: the role of the wearable cardioverter-defibrillator. Eur Heart J 34:2230–2242
Feldman AM, Klein H, Tchou P, Murali S, Hall WJ, Mancini D, Boehmer J, Harvey M, Heilman MS, Szymkiewicz SJ, Moss AJ. WEARIT investigators and coordinators; BIROAD investigators and coordinators (2004). Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol 27:4–9
Kutiyfa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, Zareba W, Goldenberg I (2015) Use of the wearable cardioverter defibrillator in high-risk cardiac patients. Data from the prospective registry of patients using the wearable cardioverter defibrillator (WEARIT-II Registry). Circulation 132:1613–1619
Wäßnig NK, Günther M, Quick S, Pfluecke C, Rottstädt F, Szymkiewicz SJ, Ringquist S, Strasser RH, Speiser U (2016) Experience with the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circulation 134:635–43
Priori SG, Blomström-Lundquist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliot PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ (2015). 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology. Eur Heart J 36:2793–867
Hinkle LE Jr, Thaler HAT (1982). Clinical classification of cardiac deaths. Circulation 65:457–64
Grönefeld GC, Hohnloser SHH (2003) Heart failure complicated by atrial fibrillation: mechanistic, prognostic, and therapeutic implications. J Cardiovasc Pharmacol Ther 8:107–13
Potpara TS, Marinkovic JM, Polovina MM, Stankovic GR, Seferovic PM, Ostojic MC, Lip GY (2012) Gender-related differences in presentation, treatment and long-term outcome in patients with first-diagnosed atrial fibrillation and structurally normal heart: the Belgrade atrial fibrillation study. Int J Cardiol 161:39–44
Schotten U, Greiser M, Benke D, Buerkel K, Ehrenteidt B, Stellbrink C, Vazquez-Jimenez JF, Schoendube F, Hanrath P, Allessie M (2002) Atrial fibrillation induced contractile dysfunction: a tachycardiomyopathy of a different sort. Cardiovasc Res 53:192–201
Erath JW, Vamos M, Sirat AS, Hohnloser SHH (2017) The wearable-cardioverter defibrillator in a real-world clinical setting: experience in 102 consecutive patients. Clin Res Cardiol 106:300–306
Duncker D, Westenfeld R, Konrad T, Pfeffer T, Correia de Freitas CA, Pfister R, Thomas D, Fürnkranz A, Andrié RP, Napp A, Schmitt J, Karolyi L, Wakili R, Hilfiker-Kleiner D, Bauersachs J, Veltmann C (2017) Risk for life-threatening arrhythmia in newly diagnosed peripartum cardiomyopathy with low ejection fraction: a German multi-centre analysis. Clin Res Cardiol 106:582–589
Acknowledgements
The assistance in data requisition of Antje Steidl, R.N., is greatly appreciated by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no funding for this study.
Conflict of interest
Dr. Julia W. Erath reports receiving travel support and lecture fees from ZOLL Medical and Servier and is a fellow of the Boston Scientific heart rhythm fellowship program, outside the submitted work. Dr. Mate Vamos reports receiving lecture fees from Pfizer, Bayer and Medtronic, outside the submitted work. Dr. Alexander P. Benz reports receiving travel support from St. Jude Medical, outside the submitted work. Professor Dr. Stefan H. Hohnloser reports receiving consulting fees from Bayer Healthcare, Boehringer Ingelheim, Gilead, J&J, Medtronic, Pfizer, St. Jude Medical, Sanofi-Aventis, Zoll Medical; and lecture fees from Boehringer Ingelheim, Bayer Healthcare, Bristol-Myers Squibb, Pfizer, St. Jude Medical, Sanofi-Aventis, and Cardiome, outside the submitted work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Erath, J.W., Vamos, M., Benz, A.P. et al. Usefulness of the WCD in patients with suspected tachymyopathy. Clin Res Cardiol 107, 70–75 (2018). https://doi.org/10.1007/s00392-017-1159-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-017-1159-1