Abstract
Background
Recently, malnutrition has been shown to be related to worse clinical outcomes in patients with heart failure. However, the association between nutritional status and clinical outcomes in patients with coronary artery disease (CAD) remains unclear. We investigated the prognostic value of malnutrition assessed by the Controlling Nutritional Status (CONUT; range 0–12, higher = worse, consisting of serum albumin, cholesterol and lymphocytes) score in patients with CAD.
Methods
The CONUT score was measured on admission in a total of 1987 patients with stable CAD who underwent elective percutaneous coronary intervention (PCI) between 2000 and 2011. Patients were divided into two groups according to their CONUT score (0–1 vs. ≥2). The incidence of major adverse cardiac events (MACE), including all-cause death and non-fatal myocardial infarction, was evaluated.
Results
The median CONUT score was 1 (interquartile range 0–2). During the median follow-up of 7.4 years, 342 MACE occurred (17.2%). Kaplan–Meier curves revealed that patients with high CONUT scores had higher rates of MACE (log-rank p < 0.0001). High CONUT scores showed a significant increase in the incidence of MACE compared with low CONUT scores, even after adjusting for confounding factors (hazard ratio: 1.64, 95% confidence interval 1.30–2.07, p < 0.0001). Adding CONUT scores to a baseline model with established risk factors improved the C-index (p = 0.02), net reclassification improvement (p = 0.004) and integrated discrimination improvement (p = 0.0003).
Conclusions
Nutritional status assessed by the CONUT score was significantly associated with long-term clinical outcomes in patients with CAD. Pre-PCI assessment of the CONUT score may provide useful prognostic information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malnutrition is a crucial issue for aged and hospitalized patients because of its high morbidity and mortality [1]. Several studies have shown that malnutrition is related to worse clinical outcomes in patients with end-stage renal failure, cancer, clinical limb ischemia or heart failure [2,3,4,5,6]. Nutritional assessment could be a useful predictor for patients with these diseases; however, there are several elements of malnutrition and nutritional status is difficult to assess. Nutritional assessment should be practical, easy to perform, noninvasive and not require the use of any devices. The Controlling Nutritional Status (CONUT) score is an efficient tool and helps evaluate protein reserves, calorie depletion and immune defenses [7]. Although previous studies showed that the CONUT score was associated with adverse clinical events among patients with heart failure [8, 9], the prognostic impact of CONUT scores in stable coronary artery disease (CAD) patients was not fully investigated. Therefore, the present study aimed to evaluate the prognostic value of malnutrition assessed by the CONUT score among CAD patients who underwent elective percutaneous coronary intervention (PCI).
Methods
Study population and data collection
The present investigation was a single-center, observational, retrospective cohort study. Among consecutive patients with CAD who underwent PCI for the first time at Juntendo University Hospital, Tokyo, Japan, between January 2000 and December 2011, only patients for whom pre-procedural CONUT score was available were included.
Baseline CONUT score was calculated from serum albumin levels, total cholesterol levels and total lymphocyte counts as previously reported (Table 1) [7]. Data of these components were collected in the morning of the day of PCI. CONUT scores range from 0 to 12. A person with normal nutritional status would have a CONUT score of 0, and the higher the score, the worse the nutritional status. We used the continuous variable CONUT score as the test variable and all-cause death and non-fatal myocardial infarction (MI) as the state variables. An investigation using the receiver operating characteristics curve showed the most appropriate cutoff value for the CONUT score to be 2 (area under the curve: 0.591; the sensitivity was 0.549 and the specificity was 0.593). Therefore, we set 2 as the cutoff value and divided patients into a low CONUT score (0–1) group and a high CONUT score (≥2) group.
Demographic data, coronary risk factors and medication use were collected from our institutional database. Blood samples were collected in the early morning after overnight fasting, and blood pressure (BP) was measured on admission. Patients with BP >140/90 mmHg or those receiving antihypertensive drugs were regarded as hypertensive. Dyslipidemia was defined as low-density lipoprotein cholesterol (LDL-C) ≥140 mg/dl, high-density lipoprotein cholesterol (HDL-C) ≤40 mg/dl, triglycerides ≥150 mg/dl or current treatment with statins and/or lipid-lowering agents [10]. Diabetes mellitus was defined as either hemoglobin A1c ≥6.5% or medication with insulin or oral hypoglycemic drugs. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate <60 ml/min/1.73 m2 as calculated using the modification of the diet in renal disease equation modified with a Japanese coefficient using baseline serum creatinine [11]. A current smoker was defined as a person who smoked at the time of PCI or who had quit smoking within 1 year before PCI. All patients had symptoms of effort angina, documented myocardial ischemia or both.
Written, informed consent was obtained from all patients prior to PCI. This study was performed in accordance with the Declaration of Helsinki and with the approval of our institutional review board.
Primary endpoints
The primary outcome was major adverse cardiac events (MACE), defined as a composite of all-cause death and non-fatal MI. Clinical follow-up included a review of medical charts, telephone contact and questionnaires sent to patients or their families. Mortality data were collected from the medical records of patients who died or who were treated at our institution, and details and causes of death were obtained from other hospitals to which patients had been admitted. Cardiac death was defined as death from CAD, cardiogenic shock or sudden death. Non-cardiac death was defined as death from other causes. MI was defined as evidence of myocardial necrosis in a clinical setting consistent with myocardial ischemia.
Statistical analysis
Quantitative data are presented as mean ± standard deviation (SD) or medians (interquartile range, IQR). Categorical variables are presented as frequencies. Continuous variables were compared using an unpaired t test or the Mann–Whitney U test. Categorical variables (presented as frequencies) were compared using the Chi-squared test or Fisher’s exact probability test. Unadjusted cumulative event rates between the two groups were compared using Kaplan–Meier curves and the log-rank test. Patients were also stratified by with or without CKD and age using 65 years as the cutoff in the Kaplan–Meier analysis. Effects of the CONUT score on clinical outcomes after PCI were determined using multivariate Cox proportional hazard regression analysis. Model 1 was adjusted for age and sex. Model 2 was adjusted for the variables in model 1 plus body mass index (BMI) and CKD. Model 3 was adjusted for the variables in model 2 plus established risk factors such as current smoking, diabetes, dyslipidemia, hypertension, left ventricular ejection fraction (LVEF), multivessel disease and statin use on admission. CONUT score levels were included in the multivariate modeling, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated.
To assess whether the accuracy of predicting adverse cardiac events would improve after adding CONUT scores into a baseline model with established risk factors (i.e., age, CKD, current smoking, diabetes, dyslipidemia, hypertension, LVEF, multivessel disease and use of statins), the C-index, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated. Differences were considered significant at p < 0.05. Statistical analyses were carried out using JMP version 12.0 (SAS Institute, Cary, NC, USA) and R version 3.2.3 (http://www.R-project.org/; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline and procedural characteristics
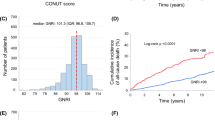
Of the 2092 patients who underwent elective PCI, pre-procedural CONUT data were available for 1987 patients (95.0%). For these patients, the median CONUT scores were 1 (IQR: 0, 2). Figure 1 shows the distribution of CONUT scores. Clinical and procedural characteristics of the patients are shown in Table 2. Patients in the high CONUT score group were significantly older and had a higher prevalence of hypertension, multivessel disease and CKD, as well as lower BMI, LDL-C, HDL-C, triglycerides and hemoglobin A1c levels on admission. Patients in this group were also less likely to be current smokers, have dyslipidemia and have lower LVEF.
Clinical outcomes
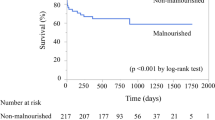
The median follow-up period was 7.4 years (IQR: 4.6–10.1 years). In total, 342 (frequency: 16.3%) cases of MACE were identified during follow-up, including 293 (14.0%) deaths and 49 (2.3%) non-fatal MIs. Among 293 all-cause deaths, 92 cardiac deaths [low CONUT group: 43 (3.8%), high CONUT group: 49 (5.7%)] and 201 non-cardiac deaths [low CONUT group: 86 (7.6%), high CONUT group: 115 (13.4%)] were occurred. Figure 2 shows the Kaplan–Meier curves for MACE for the two groups. The cumulative incidences of MACE were clearly higher in the high CONUT score group (log-rank p < 0.0001). Furthermore, Kaplan–Meier curves for MACE showed significant differences in the incidence of events between the two groups, even stratified by CKD and age of 65 years (Fig. 3).
Kaplan–Meier curves for MACE among patients aged <65 years (a); aged ≥65 years (b); without CKD (c); and with CKD (d). Kaplan–Meier curves for MACE show significant differences in the incidence of events between the two groups, even stratified by CKD or age of 65 years (log-rank test, all p < 0.001). CKD chronic kidney disease, CONUT Controlling Nutritional Status, MACE major adverse cardiac events, MI myocardial infarction
Table 3 shows the results of Cox proportional hazard regression analysis for MACE. The high CONUT score group was significantly associated with MACE compared with the low CONUT score group (HR: 1.64, 95% CI 1.30–2.07; p < 0.0001), even after adjustment for other risk factors (age, sex, BMI, CKD, current smoking, diabetes, dyslipidemia, hypertension, LVEF, multivessel disease and statin use). Continuous variables of the CONUT score were also related to the incidence of adverse cardiac events in all models (HR: 1.23 per 1 CONUT score increase, 95% CI 1.16–1.32; p < 0.0001).
Discrimination and reclassification of the CONUT score
Adding the CONUT score to a baseline model with established risk factors improved the prediction of MACE as shown by the significant increase in the C-index (Table 4). Furthermore, the addition of the CONUT score significantly improved the reclassification of patients beyond the baseline model with established risk factors (NRI: 0.17, 95% CI 0.05–0.29; p = 0.004). IDI also improved significantly compared to the baseline model after adding the CONUT score.
Discussion
The major findings of the present study are as follows: (1) Patients with high CONUT scores showed a significantly higher incidence of cardiac events than patients with low CONUT scores; (2) even after adjusting for important risk factors, an increase in CONUT score was associated with worse long-term outcomes in patients following PCI; and (3) the addition of the CONUT score to a conventional prediction model significantly improved risk prediction for major cardiac events.
The CONUT score was first reported by Ignacio de Ulíbarri et al. as an objective screening tool for identifying undernutrition in a hospital population [7]. Previous studies have reported the prognostic value of the CONUT score for several chronic diseases [12,13,14]. Nochioka et al. demonstrated that poor nutritional status assessed by the CONUT score was associated with an increased risk of adverse cardiac events for patients with chronic heart failure [9]. Malnutrition assessed by the CONUT score has also been reported to be an independent determinant of long-term outcomes in patients with acute heart failure [8, 15]. Recently, the CONUT score has been shown to be associated with clinical outcomes for patients with ischemic heart disease. Basta et al. evaluated 945 patients with ST-elevation MI for 2 years and showed that nutritional status evaluated by the CONUT score affected prognosis in elderly patients [16]. Kunikura et al. followed 1004 patients with stable CAD for 4.9 years and found a combined effect of the CONUT score and BMI for predicting adverse cardiac events [17]. In the present study, we assessed the usefulness of the CONUT score as a single predictor of long-term clinical outcomes among patients with stable CAD. Several studies have assessed the impact of malnutrition for elderly or CKD patients with heart failure, CAD or other chronic disease [2, 9, 16, 18]; however, in our study, patients in the high CONUT score group had a higher incidence of MACE, including the non-elderly and those without CKD. Our findings indicate that nutritional assessment using the CONUT score should be taken into consideration for stable CAD patients.
To date, several nutrition indicators have been reported, such as serum albumin, total cholesterol levels, the Mini-Nutritional Assessment (MNA) [19], the Subjective Global Assessment (SGA) [20] and the Geriatric Nutritional Risk Index (GNRI) [21]. The MNA and SGA require subjective data evaluated by medical staff. In addition, assessments using only one indicator of malnutrition may be affected by various factors and not provide adequate information. The CONUT score is appropriate for evaluating different aspects of various components of malnutrition. Malnutrition is a complex state involving depletion of the protein reserve, caloric consumption and decreasing immune defenses. Furthermore, it is simple to evaluate the CONUT score using inexpensive objective markers. The GNRI is also an objective nutrition assessment tool, using serum albumin, body weight and height. Further study is needed to assess which nutritional index is better for evaluating nutritional status and predicting clinical outcomes of CAD patients.
Albumin, which is a component of the CONUT score, is the major protein in human plasma and the most abundant protein in the extracellular component [22]. Albumin synthesis is regulated by stimuli including nutrient intake, insulin levels and oncotic pressure. Hypoalbuminemia is therefore thought to result from malnutrition, inflammation or cachexia [23]. Inflammation has been reported to play a major role in the initiation and progression of atherosclerosis and the triggering of cardiovascular disease events [24, 25]. In addition to nutritional and inflammatory factors, albumin has been reported to have several physiological functions related to atherothrombogenesis [26,27,28]. Low lymphocyte counts have also been associated with worse outcomes in patients with CAD [29, 30]. Lymphocytopenia is considered to be related to physiological stress due to corticosteroid release and reflect a poorly regulated immune response [31]. Although hypercholesterolemia is a well-known risk factor for cardiovascular disease in the general population, an association between low serum cholesterol and poor outcomes has been reported for patients with chronic heart failure and end-stage renal failure as well as the elderly [32,33,34]. This reverse epidemiology finding might occur in patients in which CAD has already been detected and treated. For these reasons, low serum albumin, total cholesterol and total lymphocyte count values could affect worse clinical outcomes after PCI.
Malnutrition is not a rare condition for patients with CAD. Of course, its rate among CAD patients is generally lower compared to those with heart failure or hospitalized patients. In our study, 49 and 24% of the patients were at a mild (CONUT score: 1–2) and moderate–severe risk (CONUT score: >3) [7] of malnutrition, respectively. Malnutrition is often present in cachexia, which is a complex metabolic syndrome characterized by anorexia, weight loss, insulin resistance and increased muscle protein breakdown [23]. These conditions carry a poor prognosis and are often found in patients with chronic heart disease. The association between malnutrition, inflammation and atherosclerosis has been reported in patients with end-stage renal disease [35]. Nakagomi et al. showed that a high CONUT score was significantly associated with inflammation and carotid atherosclerosis identified by carotid intima-media thickness in patients with chronic heart failure [36]. Thus, this malnutrition, inflammation and atherosclerosis (MIA) syndrome might be an important issue for not only CKD patients, but also patients with heart failure or CAD.
Some studies have shown that nutritional intake, such as supplementation, may be beneficial for patients with chronic heart failure [37, 38]. It may be possible that nutritional intervention is useful for CAD patients with undernutrition. Further investigations are required to evaluate whether nutritional interventions improve clinical outcomes in this population. In addition, previous studies demonstrated the association between frailty or physical activity and cardiovascular disease [39, 40]. Thus, it might be more useful to add or combine some information of this status such as frailty screening tools, cardiac pulmonary testing or muscle volume of patients, to the nutritional status assessed by the CONUT score in CAD patients.
We must acknowledge some limitations. First, as a single-center, observational study of a small patient cohort, unknown confounding factors might have affected the outcomes regardless of analytical adjustments. Second, the present study evaluated the CONUT score only once and did not assess changes over time. Third, a propensity-matched analysis would be a better way to assess the impact of CONUT score in clinical outcomes. However, sample size and clinical events of the present study were relatively small and the Cox hazard analysis or the discrimination analysis showed the usefulness of the CONUT score. Finally, the information about the compliance of cardiovascular drugs was not available. Poor compliance might affect the adverse outcomes.
Conclusions
In this study, nutritional status evaluated by the CONUT score was associated with long-term outcomes in patients with stable CAD after undergoing elective PCI. Pre-PCI assessment of the CONUT score may provide useful prognostic information in clinical practice.
References
Lew CC, Yandell R, Fraser RJ, Chua AP, Chong MF, Miller M (2016) Association between malnutrition and clinical outcomes in the intensive care unit: a systematic review. J Parenter Enteral Nutr. doi:10.1177/0148607115625638
Honda Y, Nagai T, Iwakami N, Sugano Y, Honda S, Okada A, Asaumi Y, Aiba T, Noguchi T, Kusano K, Ogawa H, Yasuda S, Anzai T, Na DEFI (2016) Usefulness of geriatric nutritional risk index for assessing nutritional status and its prognostic impact in patients aged ≥65 years with acute heart failure. Am J Cardiol 118(4):550–555. doi:10.1016/j.amjcard.2016.05.045
Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K (2013) Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 77(3):705–711
Luo H, Yang H, Huang B, Yuan D, Zhu J, Zhao J (2016) Geriatric nutritional risk index (GNRI) independently predicts amputation inchronic criticallimb ischemia (CLI). PLoS One 11(3):e0152111. doi:10.1371/journal.pone.0152111
Seo SH, Kim SE, Kang YK, Ryoo BY, Ryu MH, Jeong JH, Kang SS, Yang M, Lee JE, Sung MK (2016) Association of nutritional status-related indices and chemotherapy-induced adverse events in gastric cancer patients. BMC Cancer 16(1):900. doi:10.1186/s12885-016-2934-5
Takahashi H, Ito Y, Ishii H, Aoyama T, Kamoi D, Kasuga H, Yasuda K, Maruyama S, Matsuo S, Murohara T, Yuzawa Y (2014) Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J Cardiol 64(1):32–36. doi:10.1016/j.jjcc.2013.10.018
Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, Rodriguez F, Fernandez G (2005) CONUT: a tool for Controlling Nutritional Status. First validation in a hospital population. Nutr Hosp 20(1):38–45
Iwakami N, Nagai T, Furukawa TA, Sugano Y, Honda S, Okada A, Asaumi Y, Aiba T, Noguchi T, Kusano K, Ogawa H, Yasuda S, Anzai T, Na DEFI (2017) Prognostic value of malnutrition assessed by Controlling Nutritional Status score for long-term mortality in patients with acute heart failure. Int J Cardiol 230:529–536. doi:10.1016/j.ijcard.2016.12.064
Nochioka K, Sakata Y, Takahashi J, Miyata S, Miura M, Takada T, Fukumoto Y, Shiba N, Shimokawa H (2013) Prognostic impact of nutritional status in asymptomatic patients with cardiac diseases: a report from the CHART-2 Study. Circ J 77(9):2318–2326
Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K (2013) Diagnostic criteria for dyslipidemia. J Atheroscler Thromb 20(8):655–660
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53(6):982–992. doi:10.1053/j.ajkd.2008.12.034
Cabre M, Ferreiro C, Arus M, Roca M, Palomera E, Serra-Prat M (2015) Evaluation of CONUT for clinical malnutrition detection and short-term prognostic assessment in hospitalized elderly people. J Nutr Health Aging 19(7):729–733. doi:10.1007/s12603-015-0536-6
Iseki Y, Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, Ikeya T, Muguruma K, Tanaka H, Toyokawa T, Sakurai K, Hirakawa K (2015) Impact of the preoperative Controlling Nutritional Status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One 10(7):e0132488. doi:10.1371/journal.pone.0132488
Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, Muguruma K, Yashiro M, Hirakawa K, Ohira M (2016) The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer 16:722. doi:10.1186/s12885-016-2696-0
Agra Bermejo RM, Gonzalez Ferreiro R, Varela Roman A, Gomez Otero I, Kreidieh O, Conde Sabaris P, Rodriguez-Manero M, Moure Gonzalez M, Seoane Blanco A, Virgos Lamela A, Garcia Castelo A, Gonzalez Juanatey JR (2017) Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int J Cardiol 230:108–114. doi:10.1016/j.ijcard.2016.12.067
Basta G, Chatzianagnostou K, Paradossi U, Botto N, Del Turco S, Taddei A, Berti S, Mazzone A (2016) The prognostic impact of objective nutritional indices in elderly patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Int J Cardiol 221:987–992. doi:10.1016/j.ijcard.2016.07.039
Kunimura A, Ishii H, Uetani T, Aoki T, Harada K, Hirayama K, Negishi Y, Shibata Y, Sumi T, Kawashima K, Tatami Y, Kawamiya T, Yamamoto D, Suzuki S, Amano T, Murohara T (2017) Impact of nutritional assessment and body mass index on cardiovascular outcomes in patients with stable coronary artery disease. Int J Cardiol 230:653–658. doi:10.1016/j.ijcard.2017.01.008
Kobayashi I, Ishimura E, Kato Y, Okuno S, Yamamoto T, Yamakawa T, Mori K, Inaba M, Nishizawa Y (2010) Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant 25(10):3361–3365. doi:10.1093/ndt/gfq211
Guigoz Y, Vellas B, Garry PJ (1996) Assessing the nutritional status of the elderly: the mini nutritional assessment as part of the geriatric evaluation. Nutr Rev 54(1 Pt 2):S59–S65
Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN (1987) What is subjective global assessment of nutritional status? J Parenter Enteral Nutr 11(1):8–13
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C (2005) Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 82(4):777–783
Margarson MP, Soni N (1998) Serum albumin: touchstone or totem? Anaesthesia 53(8):789–803
von Haehling S, Doehner W, Anker SD (2007) Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc Res 73(2):298–309. doi:10.1016/j.cardiores.2006.08.018
Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH (1997) Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336(14):973–979. doi:10.1056/nejm199704033361401
Ross R (1999) Atherosclerosis–an inflammatory disease. N Engl J Med 340(2):115–126. doi:10.1056/nejm199901143400207
Kim SB, Chi HS, Park JS, Hong CD, Yang WS (1999) Effect of increasing serum albumin on plasma D-dimer, von Willebrand factor, and platelet aggregation in CAPD patients. Am J Kidney Dis 33(2):312–317
Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E (2008) The antioxidant properties of serum albumin. FEBS Lett 582(13):1783–1787. doi:10.1016/j.febslet.2008.04.057
Turell L, Carballal S, Botti H, Radi R, Alvarez B (2009) Oxidation of the albumin thiol to sulfenic acid and its implications in the intravascular compartment. Braz J Med Biol Res 42(4):305–311
Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, Renlund DG, Muhlestein JB (2005) Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 45(10):1638–1643. doi:10.1016/j.jacc.2005.02.054
Ommen SR, Gibbons RJ, Hodge DO, Thomson SP (1997) Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol 79(6):812–814
Onsrud M, Thorsby E (1981) Influence of in vivo hydrocortisone on some human blood lymphocyte subpopulations. I. Effect on natural killer cell activity. Scand J Immunol 13(6):573–579
Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC (2002) Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Cardiac Fail 8(4):216–224
Iseki K, Yamazato M, Tozawa M, Takishita S (2002) Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int 61(5):1887–1893. doi:10.1046/j.1523-1755.2002.00324.x
Volpato S, Zuliani G, Guralnik JM, Palmieri E, Fellin R (2001) The inverse association between age and cholesterol level among older patients: the role of poor health status. Gerontology 47(1):36–45. doi:10.1159/000052768
Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T (1999) Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55(5):1899–1911. doi:10.1046/j.1523-1755.1999.00422.x
Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, Atarashi H, Shimizu W (2016) Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb 23(6):713–727. doi:10.5551/jat.31526
Bourdel-Marchasson I, Emeriau JP (2001) Nutritional strategy in the management of heart failure in adults. Am J Cardiovasc Drugs Drugs Dev Interv 1(5):363–373
Broqvist M, Arnqvist H, Dahlstrom U, Larsson J, Nylander E, Permert J (1994) Nutritional assessment and muscle energy metabolism in severe chronic congestive heart failure–effects of long-term dietary supplementation. Eur Heart J 15(12):1641–1650
Salzwedel A, Reibis R, Wegscheider K, Eichler S, Buhlert H, Kaminski S, Voller H (2016) Cardiopulmonary exercise testing is predictive of return to work in cardiac patients after multicomponent rehabilitation. Clin Res Cardiol 105(3):257–267. doi:10.1007/s00392-015-0917-1
Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL (2017) Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol. doi:10.1007/s00392-017-1082-5
Acknowledgements
The authors are grateful to the staff of the Department of Cardiovascular Medicine at Juntendo University and the Department of Cardiology at Juntendo University Shizuoka Hospital. The authors also appreciate the secretarial assistance of Yumi Nozawa and Ayako Onodera.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Rights and permissions
About this article
Cite this article
Wada, H., Dohi, T., Miyauchi, K. et al. Prognostic impact of nutritional status assessed by the Controlling Nutritional Status score in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Clin Res Cardiol 106, 875–883 (2017). https://doi.org/10.1007/s00392-017-1132-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-017-1132-z