Abstract
Objective
The purpose of this study was to assess the incidence, predictors, and prognostic clinical impact of atrial fibrillation (AF) over time after cavotricuspid isthmus (CTI) ablation of typical atrial flutter (AFL).
Methods
This was a follow-up observational study using 408 patients who underwent CTI AFL ablation between 1998 and 2010. The relationships between the different predictors and the outcomes (AF, stroke, and death) were modeled by means of multistate Cox model analyses.
Results
The incident rate of AF per 100 person-years during follow-up was 10.2 (95 % CI 8.7–11.8). Prior AF and chronic obstructive pulmonary disease (COPD) were the only independent variables to predict AF occurrence in the population. Their hazard ratios (HRs) were 2.55 (95 % CI 1.84–3.52) and 1.56 (95 % CI 1.08–2.27), respectively. Patients who transitioned to AF had an increased risk of death by an HR of 2.82 (95 % CI 1.88–4.70) and an increased risk of stroke by an HR of 2.93 (95 % CI 1.12–8.90). Age, COPD, and heart failure (HF) were predictive factors of death by HRs of 1.05 (95 % CI 1.00–1.08), 2.85 (95 % CI 1.39–5.83), and 2.72 (95 % CI 1.15–6.40), respectively. Age, smoking, COPD, and HF were predictive factors of death in the group of patients with AF during follow-up. HRs were 1.07 (95 % CI 1.02–1.12), 2.55 (95 % CI 1.55–4.21), 7.60 (95 % CI 3.01–19.16), and 3.07 (95 % CI 1.18–7.95), respectively.
Conclusions
The transition to AF after CTI AFL ablation was high during a long-term follow-up period and maintained over time. Prior AF and COPD were the primary predictors of transition to AF after CTI AFL ablation. Patients who transitioned to AF had an increased risk of stroke and a more than twofold mortality rate. These clinical implications make it necessary to investigate AF after CTI ablation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial flutter (AFL) and atrial fibrillation (AF) are frequently seen together in clinical practice. This association generally reflects a similar arrhythmogenic substrate, though each is caused by different electrophysiological mechanisms [1, 2].
Treatment with catheter ablation targeting the cavotricuspid isthmus (CTI) is successful in the long-term for around 90 % of patients [3]. However, long-term success of CTI AFL ablation may be weakened by the occurrence of AF. Several studies have evaluated the incidence of AF after successful CTI AFL ablation [4–7]. Most of these studies had small numbers of patients, and the duration of follow-up was often short.
To get a better understanding of the clinical consequences of ablation, it is important to take both conditions, AFL and AF, into account and to differentiate more specifically between the various factors that may have influence on long-term survival. Transitions from AFL ablation to AF or to death could very well differ depending on the risk factors. These transitions between different states (i.e., from ablation to death or from AF to death) can be analyzed using multistate models. Multistate models provide a rich framework for handling complex situations that involve more than two states and a number of possible transitions among them [8].
In this study, we evaluated the onset and predictors of AF during long-term follow-up in a large cohort of patients with typical AFL successfully treated with CTI ablation, together with long-term clinical outcomes in terms of death, recurrence of AFL, and ischemic stroke. The relationships between the predictors and outcomes were modeled using multistate Cox models analysis, a technique that, as far as we know, has not previously been employed in this context.
Methods
Baseline population characteristics
Four hundred and twelve patients with typical AFL who successfully underwent radiofrequency catheter ablation at our hospital between November 1998 and May 2010 were included in this retrospective study. Patients with intra-atrial re-entrant tachycardia following reparative surgery for complex congenital heart disease were excluded. In addition, those with a prior history of AF ablation were excluded. Four patients were lost during follow-up and were excluded from the analysis, resulting in a population of 408 patients. All patients had had spontaneous AFL episodes documented by a 12-lead surface electrocardiogram (ECG).
For each patient, we performed an initial clinical evaluation that included history, physical examination, 12-lead surface ECG, a minimum of 24 h of continuous ECG monitoring, X-ray examination, blood chemistry tests, and two-dimensional echocardiography with color flow Doppler measurements. The use of medications, including beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, anti-arrhythmic drugs, antithrombotic drugs, and warfarin, was determined according to the discretion of the responsible clinician.
The sample population ranged in age between 19 and 91 years, averaging 64.6 ± 11.2 years, and was 344 (84.3 %) male. Clinical characteristics of the patients are shown in Table 1.
At the time of electrophysiological study, 268 patients (65.7 %) were in AFL and 140 (34.3 %) presented with sinus rhythm. In patients with spontaneous AFL (87.4 % counter-clockwise and 12.6 % clockwise), the cycle length was 244 ms. The interquartile range was 225–263.
All patients were discharged with at least 4 weeks of oral anticoagulant (OAC) therapy or enoxaparin. In 241 patients (59.1 %), OAC therapy was maintained. In 110 patients (26.9 %), acetylsalicylic acid was prescribed after discontinuation of OAC therapy.
Patients with AF during follow-up had a higher CHA2DS2-VASc score than those without AF. They were also more likely to be on antiarrhythmic drug treatment (AAD) prior to CTI ablation (Table 1).
Electrophysiological study and CTI ablation
Three electrode catheters were inserted into one or both femoral veins. A standard quadripolar catheter was used to map the His bundle region, a 10-pole catheter was used to locate the coronary sinus, and a 12-pole Halo XP catheter was used to record activation in the anterolateral aspect of the right atrium. An 8-mm tip catheter was used in 98 % of the patients, while an irrigated-tip catheter was used in the remainder. Radiofrequency was delivered for 60 s at each location at a maximum power output of 90 W and maximum temperature of 55 °C. CTI dependence was confirmed by concealed entrainment if AFL rhythm was either present at the beginning of the electrophysiological analysis or induced in the laboratory. If the patient was in sinus rhythm, bidirectional CTI conduction was determined before ablation. The objective of the ablation procedure was to effect a bidirectional conduction block across the CTI.
Post-ablation management and follow-up
After catheter ablation, all patients underwent continuous ECG monitoring for at least 24 h before hospital discharge. Patients were seen at our clinic every 6 months for follow-up, and every 12 months a 7-day Holter monitor was used for assessing asymptomatic arrhythmia episodes. Each visit to the Emergency Room or General Practitioner generated a report in the patient’s electronic history and an ECG was performed. The occurrence of AF was defined as at least 30 s of AF during any ECG, or Holter monitor.
Statistical analysis
Incidence rates are expressed as the number of events per 100 person-years. Descriptive analysis using χ 2 and t tests was conducted to examine the characteristics of the population.
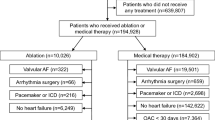
Progression from CTI ablation was studied using a multistate model. In this approach, transitions were assessed during the course of the disease, and prognostic factors for each transition were studied. A patient may be in one of the six outcomes, or states, at any time (see Fig. 1).
The arrows indicate transitions between the six states that are clinically relevant. Transition 1 indicates a change from CTI ablation to AF, transition 2 indicates a change from CTI ablation to death, transition 3 indicates a change from CTI ablation to stroke, transition 4 indicates a change from AF to death, transition 5 indicates a change from AF to stroke, and transition 6 indicates a change from stroke to death.
The following characteristics were considered for each transition: age at ablation, tobacco and alcohol consumption, overweight (body mass index >25 kg/m2), diabetes, chronic obstructive pulmonary disease (COPD), heart failure (HF), left atrial diameter (LAD) and left ventricular ejection fraction (LVEF) <45 %. Follow-up began at the time of CTI ablation and ended at the last date of follow-up (death or the end of the study).
To estimate the adjusted hazard ratios (HRs) for all the transitions, multivariate Cox regression models were performed. We previously verified the assumption that the transition rates were not affected by the time spent in the previous state (P > 0.05), verifying that the Markov model was satisfactory for this dataset.
All statistical analyses were carried out in R using the survival package (for fitting parametric Cox models), splines package (for fitting nonparametric Cox models), tdc.msm package (for checking the Markov assumption), and Epi package (for performing the multistate models). These packages are freely available at http://cran.r-project.org [9, 10].
This study was carried out in accordance with the Principles of the Declaration of Helsinki (1975) and approved by the ethical committee of Clinical Investigation in Galicia. All enrolled patients gave their written informed consent.
Results
Study population
A flow chart showing transition states in patients after CTI AFL ablation is shown in Fig. 1. Incidence rates of AF, stroke, and death among patients and HR for transitions between AF, stroke, and death are shown in Table 2.
Occurrence of AF after CTI AFL ablation
Patients were followed up for 1.6–13.5 years, averaging 5.9 ± 3.1 years. Atrial fibrillation was documented in 168 patients with an incidence rate of 10.2 per 100 person-years (95 % CI 8.7–11.8) (Table 2). Of these, 72 (42.8 %) had a prior history of AF and 96 (57.2 %) developed AF for the first time. Eleven patients (2.7 %) developed atypical AFL during follow-up.
Of the 408 patient population, 121 had prior AF (29.6 %), of whom 72 (59.5 %) continued to experience AF after the procedure. Of the remaining 287 patients who had no prior AF, 96 (33.4 %) went on to manifest AF.
The cumulative incidence of post-ablation AF is shown in Fig. 2, and was significantly higher in patients with prior AF than in those without prior AF. These differences were maintained throughout the follow-up period (Fig. 3).
Those patients who developed AF during follow-up were more likely to be smokers and had larger LADs than those who did not. Other baseline characteristics between these two groups were not significantly different (Table 1).
Prior AF and COPD were the only variables that predicted the occurrence of post-ablation AF by HRs of 2.55 (95 % CI 1.84–3.52) and 1.56 (95 % CI 1.08–2.27), respectively (Table 3).
AFL recurrence
During follow-up, recurrences of typical AFL occurred in 56 patients (13.7 %), of which 52 underwent a repeat CTI ablation while the others were treated by electrical cardioversion according to the cardiologist’s decision. In 12 patients (2.9 %), atypical AFL appeared. The AFL recurrence rate in the first year was 5.6 % while in the first 2 years it was 9.0 %, and was similar between patients with and without post-ablation AF (15.7 vs. 12.3 %, P = 0.323).
Death
Death occurred in 75 patients (18.5 %). The rate of death among those who did not make a transition to another state was 2.2 per 100 person-years (95 % CI 1.5–3.0), whereas among those who transitioned to AF, it was 5.0 per 100 person-years (95 % CI 3.4–7.0). Among those who transitioned to stroke, it was 7.6 per 100 person-years (95 % CI 2.8–16.6). Transition to AF increased the risk of death nearly threefold: HR was 2.82 (95 % CI 1.88–4.70). By comparison, transition to stroke increased the risk of death by nearly twofold and transition to AF and then stroke increased the risk of death by more than threefold. The HRs were 1.83 (95 % CI 0.75–4.49) and 3.24 (95 % CI 1.25–8.36), respectively (Table 2). The causes of death were exacerbation of COPD with respiratory failure (24 patients, 32 %), cancer (15 patients, 21 %), heart failure (14 patients, 19 %), coronary artery disease (5 patients, 7 %), cerebrovascular disease (3 patients, 4 %), sudden cardiac death (2 patients, 3 %), valvular heart disease (2 patients, 3 %), severe peripheral artery disease (2 patients, 3 %) and other causes (7 patients, 9 %). Of those who died of cancer, four patients died of lung cancer.
Age, COPD, and heart failure were predictive factors for death among those who did not transition to AF or stroke by HRs of 1.05 (95 % CI 1.01–1.09), 2.85 (95 % CI 1.39–5.83), and 2.72 (95 % CI 1.15–6.40). In the cohort of patients who transitioned to AF, the predictive variables for death were age, smoking, COPD, and HF by HRs of 1.07 (95 % CI 1.02–1.12), 2.55 (95 % CI 1.55–4.21), 7.60 (95 % CI 3.01–19.16), and 3.07 (95 % CI 1.18–7.96), respectively. Overweight was a protective variable by an HR of 0.29 (95 % CI 0.11–0.75) (Tables 3, 4). No predictive variables were found for death following stroke (transition 6), probably because of the small sample (six patients).
Stroke
Eighteen patients (4.4 %) transitioned to stroke. The etiology of stroke was attributed to ischemia in all of the patients and was classified by a neurology specialist as cardioembolic (seven patients, 39 %), atherothrombotic (four patients, 22 %), lacunar (three patients, 17 %), and indeterminate (four patients, 22 %). The median time from ablation to stroke was 5.1 ± 3.1 years with in interquartile range of 3.4–7.7 years (Fig. 4). Seven of the patients had AF prior to stroke while the remaining 11 had a stroke without known prior AF (Fig. 1). Of this group, four patients developed AF after the stroke in an average of 3.4 years.
Seven patients were on OAC therapy, three with acetylsalicylic acid therapy and eight did not receive any antithrombotic therapy as a treatment for stroke prevention.
The international normalized ratio ranged from 2 to 3 in the latter monitoring before the stroke in 4 of 7 patients (57.1 %). The four patients who developed AF after stroke did not receive OAC therapy. At the end of the follow-up, 11 patients were on OAC, 4 on acetylsalicylic acid, and 3 were without antithrombotic therapy. Mean CHA2DS2VASC score was 2.56 in the sample that transitioned to stroke and 2.45 in the sample that transitioned to AF and from AF to stroke. It was of note that none of the 11 patients with AF during follow-up had a CHA2DS2VASC of 0.
The incidence rate of stroke in the sample that did not transition to AF or death was 0.7 per 100 person-years (95 % CI 0.3–1.2). The incidence rate of stroke in patients who transitioned to AF was 1.1 per 100 person-years (95 % CI 0.4–1.2). The HR for stroke in patients who transitioned to AF was 2.93 (95 % CI 1.12–8.90) (Table 2; Fig. 5).
We did not identify any predictive variables for stroke in the sample that did not transition to AF or death. When analyzing the sample that transitioned to AF, age was associated with a significant risk of stroke by an HR of 1.17 (95 % CI 1.02–1.33) (Table 4).
Discussion
The primary findings in this follow-up observational multistate model study were (1) the occurrence of AF after CTI AFL ablation was high, and increased continuously to 60 % after 10 years of follow-up, (2) prior AF and COPD were the only independent variables that predicted AF occurrence in the study population, (3) post-ablation AF was an independent risk factor for the occurrence of stroke in the follow-up, and (4) patients with AF after CTI AFL ablation had a nearly threefold increase in mortality rate compared to those without AF during follow-up.
Although the occurrence of AF after CTI AFL ablation has been previously reported, to the best of our knowledge, this is the longest follow-up study to evaluate the clinical outcomes after successful CTI AFL ablation using a multi-state model such as the Cox proportional hazards model, for analyzing the HR for AF transition, stroke, and death.
Occurrence of AF and death after CTI ablation
The occurrence of AF is still the major problem after successful ablation of CTI [11, 12]. This was highlighted by a recently published meta-analysis, which showed that up to one-third of patients have AF after CTI AFL ablation [3], and the AF incidence was between 53 % in patients with prior AF and 23 % in those without prior AF. However, in that study, after 5 years of follow-up, the incidence was similar in both groups. In another series, the incidence of AF after CTI AFL ablation was 15.2 per 100 person-years, 25 times higher than the incidence of AF in the general population of the same age. If the focus was shifted to only those patients with isolated AFL, the incidence was 20 times higher than that in the general population [13]. In contrast, we analyzed a large population over an appropriately long follow-up period (ranging from 1.6 to 13 years). Our results are consistent with those found in these studies [4–7, 12], yielding an AF incidence of 10.2 per 100 person-years. Because of the retrospective nature of this study, it is possible that some cases of AF were not identified, and the true rate of AF higher. For example, in a recent study of patients with a history of hypertension but without clinical AF, after a procedure for a pacemaker or defibrillator, 10 % of the patients developed subclinical AF after 3 months and 35 % after 2.5 years of follow-up. Episodes of subclinical atrial tachyarrhythmia were almost eight times as common as episodes of clinical AF [14]. Furthermore, in our series, 48 (20 %) patients who had not developed AF during follow-up were on AAD treatment contributing to underestimate the true AF incidence.
The incident rate of AF in our study is slightly higher than that found by Halligan et al. [11] in patients with lone AFL, where 56 % of CTI AFL not undergoing ablation therapy developed AF within a mean of 5 years, and lower than that found by Moubarak et al. [15] where 73 % of CTI AFL ablation patients with a mean age of 72 years developed AF during a 7-year follow-up. These differences can probably be accounted for by characteristics of the population, such as the percentage of prior AF and age of patients at the time of procedure. Nonetheless, what makes the current series unique is that the transition to AF in patients with prior AF was higher than those without prior AF during late follow-up. This observation is at odds with other series [3] in which the development of AF was similar among patients with and without prior AF in the late follow-up. In our series, a continuous trickle of patients with AF occurrence was observed which, while more pronounced during the first 5 years, remained steady during the entire follow-up.
None of the basal structural variables, including LAD and LVEF <45 %, was predictive for AF transition in multivariate analysis, suggesting that AF has mainly an electrical substrate in patients’ post-CTI AFL ablation. Our data did not confirm a previous report of LVEF <45 % as a predictive variable of transition to AF after CTI AFL ablation [4]. Paroxysmal AFL was correlated with AF during follow-up in the univariate analysis, as in other series [13], but this association was not confirmed in the multivariate analysis.
An independent variable for transition to AF and death was COPD, which has been associated with higher rates of cardiovascular disease, especially myocardial infarction, lung cancer, diabetes, hip fracture, and depression [16]. The most probable explanation for the high cardiovascular morbidity in COPD patients is the high prevalence of smoking and other known factors for cardiovascular heart disease. However, several large-scale population-based studies have shown that airflow limitation is an independent risk factor for cardiovascular disease. Therefore, there may be a “COPD effect” characterized by systemic inflammation, oxidative stress and hypoxia that leads to a high cardiovascular risk [17]. To the best of our knowledge, there has not yet been an association between COPD and AF described. However, recent studies have shown a pronounced association between sleep apnea and AF, and the mechanisms of this association include increased sympathetic tone, systemic and pulmonary hypertension, hypoxia, and inflammation. These mechanisms are shared by patients with COPD [18]. The association between COPD and AF could be clinically relevant as well, because COPD has been recently described as a factor associated with major bleeding events in non-valvular AF [19].
The recurrence rate of AFL at the end of the follow-up was 13.7 %, which is a slightly higher than that reported in the majority of other series [3–7, 12, 13]. One potential partial explanation is that an 8-mm tip catheter was used in the majority of patients (98 %) versus an irrigated-tip catheter, and it is known that an irrigated-tip catheter produces more profound lesions than an 8-mm tip catheter.
An interesting finding is that the mortality rate in patients who developed AF during the follow-up was nearly three times higher than in patients who did not develop AF during follow-up. This is in spite of the fact that the global mortality rate was lower (2.2 per 100 person-years) than in other series (3.3 %) [3]. Our data support the increased mortality rate observed in different global population series using patients with AF [20–23].
Stroke
Ischemic stroke is known to occur at an annual rate of 10–15 % among patients with AF/AFL, depending on the presence of concomitant risk factors. In reports of stroke of unknown etiology, intermittent AF has been found in up to 23 % depending on the length of time the patients have had their rhythm monitored [24]. The proportion of patients with AF is higher in patients with implantable devices. Thereby, in a series assessing the 17-year baseline data of 128 ischemic stroke patients who also had an implanted pacemaker or defibrillator, AF was found in 71 %. In 13 % of AF patients, AF was unrecognized [25, 26].
In our study, the incidence rate of stroke in patients who did not transition to other states (death or AF) was 0.6 per 100 person-years. To put this number into perspective, the overall incidence of stroke in the Framingham Heart Study was 0.53 per 100 person-years. Thus, the stroke rate in the CTI AFL population was very similar to that of the general population, but significantly lower than what would have been expected from the natural history of AFL [11].
The incidence rate of stroke in patients who developed AF during the follow-up was 1.1 per 100 person-years. This incidence is lower than the 2.1 per 100 person-years reported in another series [27] of CTI AFL ablation, in spite of the fact that the incidence of AF during follow-up was nearly identical to ours. In another series [15], a stroke rate of 7 % was reported in 135 CTI AFL ablation patients with a mean follow-up of 7 years. This incidence was likely higher due to the older population at baseline and more AF occurrence within the follow-up period. On top of this is the issue of asymptomatic AF. In a recent study that enrolled patients with a pacemaker or defibrillator and hypertension without a history of AF, the occurrence of subclinical atrial tachyarrhythmia was associated with an increased risk of clinical AF and an increased risk of ischemic stroke [14]. In our study, 4 patients out of 18 (22.4 %) who had a stroke during follow-up had clinical AF after suffering the stroke. It is possible that a component of subclinical atrial tachycardia or asymptomatic AF was a factor in the development of stroke.
We could identify only AF and age (among those who transitioned to AF) as predictive variables of stroke, and no predictive variables of death in those who transitioned to stroke. Because there was a small sample of stroke patients in the multi-state analysis, the data were unsuitable for a multivariate analysis.
Limitations
Our study represented a single center’s experience and was limited by its retrospective design. The exact incidence of arrhythmia episodes, especially those which were asymptomatic, is not known and is difficult to estimate. Before ablation of AFL, patients cannot differentiate between AFL and AF symptoms, so the incidence of prior AF may be underestimated. After ablation of AFL, we performed only Holter monitoring to detect asymptomatic AF. Thus, the true incidence of asymptomatic AF may be underestimated.
Conclusions
The development of AF after CTI AFL ablation was high during a long follow-up and was maintained over time. Prior AF and COPD were the main predictors of transition to AF after CTI AFL ablation. Patients who transitioned to AF during follow-up had a more than twofold mortality rate than those without AF. AF significantly increased the risk of stroke. These clinical implications make it necessary to investigate AF after CTI ablation.
Abbreviations
- AFL:
-
Atrial flutter
- AF:
-
Atrial fibrillation
- CTI:
-
Cavotricuspid isthmus
References
Waldo AL, Feld GK (2008) Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol 51:779–786
Moreira W, Timmermans C, Wellens HJ et al (2007) Can common-type atrial flutter be a sign of an arrhythmogenic substrate in paroxysmal atrial fibrillation? Clinical and ablative consequences in patients with coexistent paroxysmal atrial fibrillation/atrial flutter. Circulation 116(24):2786–2792
Pérez FJ, Schubert CM, Parvez B et al (2009) Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ Arrhythm Electrophysiol 2(4):393–401
Paydak H, Kall JG, Burke MC et al (1998) Atrial fibrillation after radiofrequency ablation of type I atrial flutter: time to onset, determinants and clinical course. Circulation 98:315–322
Schmieder S, Ndrepepa G, Dong J et al (2003) Acute and long-term results of radiofrequency ablation of common atrial flutter and the influence of the right atrial isthmus ablation on the occurrence of atrial fibrillation. Eur Heart J 24(10):956–962
Bertaglia E, Zoppo F, Bonso A et al (2004) Northeastern Italian study on atrial flutter ablation investigators. Long term follow up of radiofrequency catheter ablation of atrial flutter: clinical course and predictors of atrial fibrillation occurrence. Heart 90(1):59–63
Chinitz JS, Grestenfeld EP, Marchlinski FE et al (2007) Atrial fibrillation is common after ablation of isolated atrial flutter during long term follow up. Heart Rhythm 4:1029–1033
Meira-Machado L, Cadarso-Suárez C, de Uña-Álvarez J et al (2009) Multi-state models for the analysis of time to event data. Stat Methods Med Res 18:195–222
Carstensen B, Plummer M, Hills M et al (2010) Epi: a package for statistical analysis in epidemiology. R package version 1.15.2. http://CRAN.R-project.org/package=Epi
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org. Accessed 8th January 2013
Halligan SC, Gersh BJ, Brown RD et al (2004) The natural history of lone atrial flutter. Ann Intern Med 140:265–268
Ellis K, Wazni O, Marrouche N et al (2007) Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol 18(8):799–802
Laurent V, Fauchier L, Pierre B et al (2009) Incidence and predictive factors of atrial fibrillation after ablation of typical atrial flutter. J Interv Card Electrophysiol 24:119–125
Healey J, Connolly S, Gold M et al (2012) Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 366:120–129
Moubarak G, Pavin D, Donal E et al (2011) Ischemic strokes after ablation of typical atrial flutter. Int J Cardiol 147(1):183–184
Sode B, Dahl M, Nordestgaard B (2011) Myocardial infarction and other co-morbidites in patients with chronic obstructive pulmonary disease: a Danish nationwide study of 7.4 million individuals. Eur Heart J 32:2365–2375
Maclay J, McAllister D, McNee W (2007) Cardiovascular risk in chronic obstructive pulmonary disease. Respirology 12:634–641
Arnaudis B, Lairez O, Escamilla R et al (2013) Impact of chronic obstructive pulmonary disease severity on symptoms and prognosis in patients with systolic heart failure. Clin Res Cardiol 101:717–726
Goodman SG, Wojdyla D, Piccini J et al (2013) Factors associated with major bleeding events: insights from the rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (rocket AF). J Am Coll Cardiol. doi:10.1016/j.jacc.2013.11.013 (pii):S0735-1097(13)06208-6, (Epub ahead of print)
Niewlaat R, Prins MH, Le Heuzey JY et al (2008) Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J 29:1181–1189
Benjamim E, Wolf P, D’Agostin R et al (1998) Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation 98:946–952
Krahn AD, Manfreda J, Tate RB et al (1995) The natural history of atrial fibrillation: incidence, risk factors and prognosis in the Manitoba follow-up study. Am J Med 98:476–484
Conen D, Chae C, Glynn R et al (2011) Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. J Am Med Assoc 305:2080–2087
The Stroke Risk in Atrial Fibrillation Working Group (2007) Independent predictors of stroke in patients with atrial fibrillation. A systematic review. Neurology 69:546–554
Haft JI (2012) The importance of atrial fibrillation/flutter as a cause of ischemic stroke. Int J Cardiol 158:143–144
Providència R, Barra S, Paiva L (2013) Atrial fibrillation, elevated troponins, ischemic stroke, and adverse outcomes: understanding the connection. Clin Res Cardiol 102:701–711
Tomson T, Kapa S, Bala R et al (2012) Risk of stroke and atrial fibrillation after radiofrequency catheter ablation of typical atrial flutter. Heart Rhythm 9:1779–1784
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seara, J.G., Roubin, S.R., Gude Sampedro, F. et al. Risk of atrial fibrillation, stroke, and death after radiofrequency catheter ablation of typical atrial flutter. Clin Res Cardiol 103, 543–552 (2014). https://doi.org/10.1007/s00392-014-0682-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-014-0682-6