Abstract
Background
Galectin-3 (Gal-3) is a recently discovered marker for myocardial fibrosis and elevated levels are associated with an impaired outcome after short-term follow-up in heart failure (HF) patients. However, whether Gal-3 is related to cardiac remodeling and outcome after long-term follow-up is unknown. Therefore, we determined the utility of Gal-3 as a novel biomarker for left ventricular remodeling and long-term outcome in patients with severe chronic HF.
Methods and results
A total of 240 HF patients with New York Heart Association (NYHA) Class III and IV were included. Patients were followed for 8.7 ± 1 years, had a mean age of 71 ± 0.6 years and 73 % of the study population was male. Circulating levels of NT-proBNP and Gal-3 were measured. Serial echocardiography was performed at baseline and at 3 months. At baseline median left ventricular end-diastolic volume (LVEDV) was 267 mL [interquartile range 232–322]. Patients were divided into three groups according to the change in LVEDV. Patients in whom the LVEDV decreased over time had significant lower levels of Gal-3 at entry compared to patients in whom the LVEDV was stable or increased (14.7 vs. 17.9 vs. 19.0 ng/mL; p = 0.004 for trend), whereas no significant differences were seen in levels of NT-proBNP (p = 0.33). Multivariate linear regression analyses revealed that Gal-3 levels were positively correlated to change in LVEDV (p = 0.007). In addition, Gal-3 was a significant predictor of mortality after long-term follow-up (p = 0.001).
Conclusion
Gal-3 is associated with left ventricular remodeling determined by serial echocardiography and predicts long-term mortality in patients with severe chronic HF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is one of the most frequent and challenging medical disorders characterized by cardiac remodeling and ventricular dysfunction [1]. With the acknowledgement of remodeling as a determinant of disease progression and poor prognosis, it has become imperative to identify those patients with the highest risk of adverse outcome. Natriuretic peptides (NPs) can help selecting patients at high risk for future events such as rehospitalization for worsening HF or death [2, 3]. However, NPs only indicate ventricular loading conditions and do not reveal other important mechanisms in HF. In acute HF, sensitivity and specificity of the NPs are low [4]. The use of novel markers, such as Gal-3, could add information about relevant structural changes in the heart, including inflammation, fibrosis, remodeling [5, 6] and a possible guide for treatment [7].
Galectin-3 (Gal-3) is a member of the galectin family that binds β-galactosides. In the failing heart, Gal-3 is produced by macrophages and cardiac fibroblasts [8–10]. An increase of Gal-3 stimulates the release of various mediators, such as TGF-β1, and promotes cardiac fibroblast proliferation, collagen deposition and ventricular dysfunction [8]. In failure-prone hypertrophied rat and human hearts [8] as well as in patients with acute [11, 12] and chronic HF [13–15], increased plasma levels of Gal-3 were detected. Taken together, these observations suggest that circulating Gal-3 is useful in identifying patients at risk for developing cardiac remodeling and consequently, poor prognosis.
We hypothesized that Gal-3 levels are especially elevated in those patients with HF and remodeling of the left ventricle, determined by serial echocardiography, compared to Gal-3 levels of HF patients without remodeling.
Methods and materials
Study population
Our study material consisted of data obtained from the Deventer-Alkmaar Heart Failure (DEAL-HF) [16, 17]. Briefly, 240 patients with typical signs and symptoms of HF were included, combined with echocardiographic or radionuclide ventriculographic findings of a reduced left ventricular systolic function (LVEF ≤ 45 %) or diastolic dysfunction [18]. The main exclusion criteria were an expected survival of less than 1 year and planned hospitalization. Almost all patients (97 %) had a left ventricular systolic dysfunction with a reduced ejection fraction (HFrEF) with a mean ejection fraction of 31 %. The study was approved by the local Medical Ethics Committees and complied with the Declaration of Helsinki. All patients gave written informed consent.
Echocardiography
Of the initial 240 patients, 12 patients (5 %) died in the first 3 months, before a second echocardiography could be performed. It was impossible to measure a left ventricular end-diastolic volume (LVEDV) in 38 patients (16 %) due to poor image quality and in 8 patients (3 %) either Gal-3 levels and/or NT-proBNP were missing or were lost to follow-up. A total of 182 patients with a complete dataset of serial echocardiography was analysed. Standard parasternal, apical three-chamber and apical four-chamber views were performed at baseline and after 3 months of follow-up. A Philips Sonos 5500 or a Philips NZE28 Sonos 7500-Live 3D echo machine was used. Each patient was analysed with the same echo machine to limit variability. Two investigators performed and analysed the echocardiographic data. Calculation of LVEDV, left ventricular end-systolic volume and ejection fraction (EF) was performed by using the Teichholz-method [19]. Two investigators blinded to the Gal-3 data and outcome analysed all data. The degree of LV remodeling was assessed according to the change in LVEDV between baseline and after 3 months of follow-up, expressed as a percentage of the baseline measurement. According to previous published studies [20, 21], we divided patients into three categories according to the change in LVEDV: decrease in LVEDV <8 %, stable LVEDV −8–8 %) and an increase in LVEDV >8 %.
Laboratory assessment
A complete dataset of 232 patients was available for evaluation of plasma NT-proBNP and Gal-3 levels. Plasma was collected in chilled in disposable tubes, containing aprotinin. After centrifugation at 4 °C, plasma samples were stored at −70 °C. NT-proBNP levels were determined by an immunoelectrochemiluminesence method (Elecsys; Roche Diagnostics, Basel, Switzerland). Plasma Gal-3 levels were determined using an optimized ELISA kit (BG Medicine, Waltham, USA) and were measured on a Bio-tekELx800 microplate reader (Biotek Instruments, Winooski, USA). Calibration of the assay was performed according to the manufacturer recommendations and values were normalized to a standard curve [22].
Glomerular filtration rate (GFR) was estimated from serum creatinine using a formula that accounts for the influence of age and body weight (the Cockroft Gault equation). This formula is validated in chronic HF patients [23].
Statistical analyses
Data are given as mean ± SEM when normally distributed, as median and interquartile range (IQR) when skewed, and as frequencies for categorical variables. We compared differences between groups with the Mann–Whitney U test, student’s t test, Kruskal–Wallis test or ANOVA with LSD post hoc testing where appropriate. Non-normally distributed continuous variables were log-transformed and normal distribution was checked thereafter. Gal-3 values were categorized into quartiles based upon their distribution among all patients and a Kaplan–Meier product limit analysis was performed. For mortality analysis, we used the complete cohort of 232 patients, since this increased the power and did not compromise the Cox-regression analysis. The log-rank test was used to test equality of estimated survival distribution functions across quartiles of Gal-3. In addition, the association between Gal-3 levels and the instantaneous relative risk of death from any cause was analysed using a Cox proportional hazards regression analysis and Kaplan–Meier product limit estimation. An univariate Cox proportional hazards regression model with Gal-3 as the predictor variable was used to estimate the hazard ratio (HR) and 95 % confidence interval (CI) associated with baseline Gal-3 value and death from any cause. Gal-3 values were examined continuously [expressed per standard deviation (SD)]. Furthermore, we assessed the association between change in Gal-3 levels between 3 months and baseline and 12 months and baseline. The variables Gal-3, age, sex, baseline LVEDV, NYHA class, eGFR, NT-proBNP, etiology of HF and duration of HF were assessed for univariate linear association with the change in LVEDV. Variables that showed a significant (p < 0.15) univariate association were included stepwise in a multivariable linear regression model on the basis of the strength of the univariate association. Receiver operating characteristic (ROC) curve analysis was performed. We considered sensitivity and specificity of equal importance, in ROC analyses, the best prognosticators for the primary endpoints were considered to be those parameters that gave the highest product of sensitivity and specificity for predicting remodeling. All reported probability values were two-tailed, and a p value <0.05 was considered statistically significant. For all statistical analysis, SPSS version 16.0 was used.
Results
Baseline characteristics of the total cohort have been published previously [16]. In short, the mean age of the total cohort was 71 ± 0.6 years, 73 % of the study population was male, 30 % had diabetes mellitus and in 63 % of the patients ischemia was the primary cause of HF (Table 1). The mean follow-up was 8.7 ± 1 year. Data of the echocardiography sub-population (n = 182) are shown in Table 2.
LV remodeling and Gal-3
Forty-six patients (25 %) showed a regression of their LVEDV of more than 8 %, whereas 108 patients (59 %) had a stable LVEDV and 28 patients (15 %) had an increase of more than 8 % in LVEDV. Variables associated with the degree of remodeling are shown in Table 1. In order to investigate whether Gal-3 levels were related to baseline LVEDV, we divided the total patient cohort into quartiles divided by the LVEDV (Table 3). No differences in Gal-3 levels could be observed between the different quartiles (p = 0.60). In addition, LVEDV did not correlate to Gal-3 (r = −0.08, p = 0.28).
Figure 1 demonstrates that patients, in whom the LVEDV decreased over time, had significant lower levels of Gal-3 compared to patients with a stable LVEDV or an increase in LVEDV of >8 %: 14.7 [12.8–18.2] (median and IQR) versus 17.9 [13.7–22] versus 19.0 ng/mL [14.9–24.4] (p = 0.004 for trend). No significant differences were seen in levels of NT-proBNP between these three groups: 1,827 [770–3,036] versus 2,148 [922–4,905] versus 2,190 pg/mL [1,015–5,886] (p = 0.33).
Gal-3 and NT-proBNP levels according to cardiac remodeling. Decrease in LVEDV (>8 % decrease in LVEDV compared to baseline), stable LVEDV (change in LVEDV between −8 and 8 % compared to baseline), increase LVEDV (>8 % increase in LVEDV compared to baseline). Data are presented as median and interquartile range (IQR)
Receiver operating characteristic curve analysis revealed that Gal-3 was able to predict regression of the LVEDV with an area under the curve (AUC) of 0.66 [0.57–0.74] (p = 0.002). In contrast, NT-proBNP did not predict remodeling with an AUC of 0.572 [0.48–0.66] (p = 0.14; data not shown).
Factors with univariable association (p < 0.15) with change in LVEDV including baseline LVEDV, Gal-3 levels, sex, etiology of HF, NYHA-class and duration of HF are presented in Table 4. Interestingly, after adjustment of these variables, Gal-3 levels remained a significant predictor of remodeling (p = 0.004), whereas NT-proBNP did not.
Temporal profile of Gal-3
To assess whether serial measurements of Gal-3 could predict regression of the LVEDV, ROC curves with both Gal-3 at 3 months and 1 year were calculated. The ROC analysis for Gal-3 at 3 months revealed an AUC of 0.62 (p = 0.03), while the AUC of Gal-3 at 1-year follow-up was 0.61 (p = 0.05; data not shown). This indicates that a single measurement at baseline was at least as good as serial measurements. In addition, there was no correlation between change in Gal-3 levels (3 months-baseline and 1 year-baseline) and change in LVEDV (r = −0.044, p = 0.56 and r = −0.057, p = 0.48, respectively).
Clinical endpoints
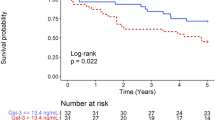
During follow-up of the total cohort (n = 240), 162 patients died (68 %). The mean follow-up of the surviving patients was 8.7 ± 1.0 years; three patients were lost to follow-up. As expected, Kaplan–Meier analysis revealed that an increase of the LVEDV was associated with a higher mortality rate (log-rank, p = 0.01; Fig. 2). Baseline Gal-3 levels were associated with mortality during long-term follow-up. Figure 3 shows the Kaplan–Meier survival curve according to the four quartiles. Mortality rate increased across the Gal-3 quartiles (log-rank, p = 0.001). This remained statistically significant in the multivariate analysis when we adjusted for age, gender, NT-proBNP and renal function.
The HR associated with Gal-3 per SD was: 1.17, 95 % CI [1.03–1.33] (p = 0.01). Change in Gal-3 levels between baseline versus 3 months and baseline versus 12 months was not associated with survival (HR 0.997, 95 % CI [0.987–1.007], p = 0.537 and HR 0.998, 95 % CI [0.988–1.008], p = 0.729, respectively).
Discussion
To our best knowledge, this is the first study that demonstrates an independent relation between the novel cardiac biomarker Gal-3 and remodeling of the left ventricle as determined by serial echocardiography in patients with severe chronic HF. Baseline Gal-3 levels are associated with remodeling, whilst NT-proBNP levels do not. Moreover, we established an independent association between Gal-3 levels and long-term mortality in chronic HF patients.
After an initial insult to the myocardium, cardiac remodeling occurs as a compensatory mechanism, which ultimately leads to left ventricular dysfunction and HF [1, 2]. This complex process with an increase of left ventricular wall thickness, dilatation and reshaping of the left ventricle leads to lengthening and/or hypertrophy of cardiomyocytes. Insufficient angiogenesis, together with an increase in the size of cardiomyocytes, leads to a mismatch between metabolic demand and supply [24]. Furthermore, activation and proliferation of fibroblasts with subsequent collagen synthesis play an important role in the process of cardiac remodeling.
Circulating Gal-3 levels predict future events in patients with acute and chronic HF. Van Kimmenade et al. [11] found that an elevated Gal-3 level was the best independent predictor of 60-day mortality in patients with acute decompensated HF. Recently, we showed that in patients with moderate to severe HF predominately due to left ventricular systolic dysfunction, Gal-3 was an independent predictor of mortality. Increased levels of Gal-3 were associated with increasing age, progressive renal dysfunction and severity of HF as assessed by increasing levels of NT-proBNP [25]. Moreover, the combination of high baseline levels of Gal-3 and NT-proBNP were observed to have incremental power with respect to prognostication. Patients having both markers elevated at levels in the highest quartile had an approximately 1.5- to 2-fold higher mortality compared to patients in other categories. The current study showed that Gal-3 levels were not only related to outcome, but also correlated significantly to remodeling of the left ventricle. In addition, remodeling and elevation of Gal-3 levels were more prominent in patients with a modest enlargement of the LVEDV as measured in the first echocardiogram compared to patients with larger ventricles at baseline, who showed more frequently reverse remodeling. No correlation between baseline LVEDV and Gal-3 levels was observed. Nevertheless, changes in LVEDV were correlated to Gal-3 levels at baseline. Although baseline NT-proBNP levels predicted mortality, it did not predict remodeling of the left ventricle, probably because NPs are loading markers, whereas Gal-3 is a marker of inflammation and (subsequent) fibrosis. Production of these NPs is stimulated by wall stress of the ventricle and is dependent on the volume and pressure states of the heart [26]. Gal-3 production, however, seems to be independent of the loading status.
The study of de Boer et al. [27], consisting of 592 patients with chronic HF, confirmed the value of Gal-3 as an independent prognostic marker. Patients with elevated levels of Gal-3 in the highest quartile had a three times higher risk of HF hospitalization and/or death. Moreover, the predictive value of Gal-3 was much stronger in patients with HF with preserved ejection fraction (HFpEF) compared to patients with HF with reduced ejection fraction (HFrEF). As has been mentioned in the study of de Boer, we confirmed that repeated measurements of Gal-3 levels over time is not superior in predicting survival compared to one single measurement.
Limitations
There are several limitations related to our study. Major limitations are its relatively small size and the lack of other endpoints besides all-cause mortality. However, since all patients were at least in NYHA III, it is expected that in the vast majority of the patients died of a cardiovascular cause. Another limitation is that almost (97 %) patients had a reduced ejection fraction (HFrEF) and were in NYHA class III. Therefore, our data cannot be extrapolated to patients with HFpEF and NYHA class II. Furthermore, we performed echocardiography at baseline and 3 months. This is a rather short interval, however, since this is a severe HF population, extending this period would lead to the loss of follow-up of a larger proportion of patients, reducing the power of our analysis. In this 3-month period, already 12 patients (5 %) died before the second echocardiography could be performed.
Due to these limitations, we consider our study mainly as hypothesis generating and our results need to be confirmed in a larger cohort of patients. Moreover, the effect of aldosterone antagonist treatment in relation to Gal-3 levels and subsequent remodeling would be valuable to evaluate in a prospective trial.
Conclusions
We demonstrate that Gal-3 predicts remodeling of the left ventricle in patients with severe chronic HF, whereas NT-proBNP does not. This relation is independent to other known risk factors for LV remodeling. In addition we show that Gal-3 predicts mortality after long-term follow-up.
Abbreviations
- DEAL-HF:
-
Deventer Alkmaar Heart Failure study
- EF:
-
Ejection fraction
- Gal-3:
-
Galectin-3
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with a preserved ejection fraction
- HFrEF:
-
Heart failure with a reduced ejection fraction
- LVEDV:
-
Left ventricle end-diastolic volume
- LVESV:
-
Left ventricular end-systolic volume
- NPs:
-
Natriuretic peptides
- NT-proBNP:
-
N-terminal part pro brain natriuretic peptide
- ROC:
-
Receiver operating characteristic
References
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 35:569–582
Dickstein K et al (2008) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 29:2388–2442
Franke J et al (2011) Is there an additional benefit of serial NT-proBNP measurements in patients with stable chronic heart failure receiving individually optimized therapy? Clin Res Cardiol 100:1059–1067
Binder A et al (2010) Limited value of brain natriuretic peptide as a prognostic marker in acute heart failure—a meta-analysis. Int J Cardiol 145:540–541
Emdin M, Vittorini S, Passino C, Clerico A (2009) Old and new biomarkers of heart failure. Eur J Heart Fail 11:331–335
Battistoni A, Rubattu S, Volpe M (2012) Circulating biomarkers with preventive, diagnostic and prognostic implications in cardiovascular diseases. Int J Cardiol 157(2):160–168
Böhm M et al (2011) Biomarkers: optimizing treatment guidance in heart failure. Clin Res Cardiol 100:973–981
Sharma UC et al (2004) Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110:3121–3128
de Boer RA, Yu L, van Veldhuisen DJ (2010) Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep 7:1–8
Liu FT et al (1995) Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol 147:1016–1028
van Kimmenade RR et al (2006) Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol 48:1217–1224
Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RRJ, Januzzi JL (2010) Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail 12:826–832
van Veldhuisen DJ et al (2009) Clinical and prognostic value of galectin-3, a novel fibrosis-associated biomarker, in patients with chronic heart failure. J Card Fail 15:814
Milting H et al (2008) Plasma biomarkers of myocardial fibrosis and remodeling in terminal heart failure patients supported by mechanical circulatory support devices. J Heart Lung Transplant 27:589–596
Ueland T et al (2011) Galectin-3 in heart failure: high levels are associated with all-cause mortality. Int J Cardiol 150:361–364
Bruggink-André de la Porte PWF et al (2005) Heart failure programmes in countries with a primary care-based health care system. Are additional trials necessary? Design of the DEAL-HF study. Eur J Heart Fail 7:910–920
de la Porte PW et al (2007) Added value of a physician-and-nurse-directed heart failure clinic: results from the Deventer-Alkmaar heart failure study. Heart 93:819–825
Remme W (2001) Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 22:1527–1560
Teichholz LE, Kreulen T, Herman MV, Gorlin R (1976) Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 37:7–11
van Bommel RJ et al (2009) Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J 30:2470–2477
Nicolosi GL et al (2009) Effects of perindopril on cardiac remodelling and prognostic value of pre-discharge quantitative echocardiographic parameters in elderly patients after acute myocardial infarction: the PREAMI echo sub-study. Eur Heart J 30:1656–1665
Christenson RH et al (2010) Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin Biochem 43:683–690
Smilde TDJ, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL (2006) Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation 114:1572–1580
de Boer RA, Pinto YM, van Veldhuisen DJ (2003) The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Microcirculation 10:113–126
Lok DJA et al (2010) Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 99:323–328
Clerico A et al (1998) Circulating levels of cardiac natriuretic peptides (ANP and BNP) measured by highly sensitive and specific immunoradiometric assays in normal subjects and in patients with different degrees of heart failure. J Endocrinol Invest 21:170–179
de Boer RA et al (2011) Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 43:60–68
Acknowledgments
BG Medicine, Inc. has certain rights related to Galectin-3 measurements. BG Medicine, Inc. provided an unrestricted research grant to the Department of Cardiology of the University Medical Center Groningen, that employs dr. van der Meer, Lipsic, de Boer and van Veldhuisen. Dr. van Veldhuisen and de Boer have received consultancy and speaker’s fees from BG Medicine, Inc. The Deventer Cardiology Research department received an unrestricted research grant from BG Medicine Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lok, D.J., Lok, S.I., Bruggink-André de la Porte, P.W. et al. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol 102, 103–110 (2013). https://doi.org/10.1007/s00392-012-0500-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-012-0500-y