Abstract
Objective
Transcatheter aortic valve implantation (TAVI) is a therapeutic option for old and multimorbid patients with severe aortic stenosis. When applying the groin first approach by transfemoral implantation, patients in the transapical group are highly selected with even higher morbidity. We report outcome of the transapical group.
Methods
Between April 2008 and May 2011, 267 patients underwent TAVI through either a transfemoral (n = 201 CoreValve, n = 33 Edwards Sapien prostheses; mean age 81 ± 6 years, logistic EuroSCORE 19.5 ± 12.6 %; 4–76, STS score 7.2 ± 4 %; 1.5–28.9) or transapical approach (n = 33 Edwards Sapien prostheses; mean age 80 ± 1 years, logistic EuroSCORE 31.6 ± 17.1 %; 9.4–69.1, STS score 12.8 ± 7.1 %; 2.5–28.8). The transapical access was chosen only when transfemoral implantation was not possible.
Results
EuroSCORE and STS score were significantly higher in the transapical group (p = 0.001, respectively). A 30-day survival was comparable with 87.9 % in the transapical versus 92 % in the transfemoral group (p = 0.52). In the transapical group, female gender was predominant (n = 23; 70 %). Eight patients underwent previous cardiac surgery. All transapical implantations were successful. No bleeding or neurological complications occurred. Six patients required postoperative pacemaker implantation. Cardiac decompensation with concomitant pneumonia was the underlying cause for early mortality, except for one patient with abdominal malperfusion. Follow-up (0–37 months) was complete in 100 %, nine patients died after 30 days postoperatively (6 cardiac and 3 non-cardiac related). Echocardiography revealed good valve function with not more than mild paravalvular incompetence.
Conclusions
Groin first approach is reasonable due to less invasive implantation technique. However, despite even higher predicted mortality, transapical aortic valve implantation is non-inferior to transfemoral approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Old age in conjunction with severe comorbidities increases the perioperative risk for all types of valvular surgery [1]. Transcatheter aortic valve implantation (TAVI) is a minimally invasive strategy reducing surgical trauma by avoiding conventional sternotomy and by performing off-pump beating heart aortic valve implantation. TAVI is now part of the daily clinical practice for the treatment of elderly high-risk patients with symptomatic aortic stenosis unfit for open surgery [2–6]. Retrograde transfemoral (TF-TAVI) and antegrade transapical (TA-TAVI) approaches are mostly used for implantation. The PARTNER Trial for the Cohort A demonstrated a significant increase in survival and improvement in functional performance after transfemoral TAVI in comparison to standard medical treatment [7]. This significant increase in survival after TAVI was confirmed in a recent review not differentiating between different access routes [8]. However, proper patient selection for these approaches is crucial for procedural success minimizing periprocedural risk. Obviously, transapical TAVI requires a left anterolateral minithoracotomy and transapical puncture of the heart, therefore being more invasive than the transfemoral approach. For these reasons, one may assume a higher incidence of mortality and morbidity in this patient cohort. In our institution, we follow as treatment protocol “a groin first” approach with transfemoral TAVI whenever possible. The transapical patients in our series are therefore highly selected and present with severe comorbidities. In this study, we report the outcome of transapical TAVI in this patient population according to the standardized endpoint definitions of the Valve Academic Research Consortium (VARC) criteria [9], as recent studies have shown the presence of paravalvular leakage after TAVI in up to 64.5 % and its impact on subsequent mortality [10].
Methods
Patient selection
Between April 2008 and May 2011, 267 TAVI procedures were performed at our institution with a “groin first” approach reflected by 234 transfemoral and 33 transapical approaches. The transapical access was chosen only when transfemoral implantation was not possible. All TA-AVI patients were screened by our “heart team” consisting of cardiologists and cardiac surgeons and found to be unfit for open surgery.
In addition to transthoracic and transesophageal echo (TEE), multi-detector computer tomography (Brillance iCT, Philips Medical Systems, Germany) of the chest, abdomen and pelvis was used for preoperative planning and assessment of feasibility. The anatomy of the entire aorta and the peripheral vessels were evaluated: extreme tortuosity of the arteries and peripheral vessel diameters less than 6.5 mm were contraindications for transfemoral access. The type of prosthesis was chosen according to the measurements of the aortic annulus, ascending aortic diameter and distance of the coronary ostia from the annulus. All patients gave written consent for treatment.
Patient population
Transfemoral TAVI
The mean age in the transfemoral TAVI group was 81 ± 6 years. The mean logistic EuroSCORE was 19.5 ± 12.6 % with a range of 4–76 %. The mean STS score was 7.2 ± 4 % with a range of 1.5–28.9 %. With transfemoral access in the majority of cases CoreValve prostheses (n = 201) were implanted, Edwards Sapien prostheses were used in 33 patients (Table 1).
Transapical TAVI
The mean age in the transapical TAVI group was similar with 80 ± 1 years. The prevalence of males, size, body surface area and body mass index were similar in both groups. The mean logistic EuroSCORE was 31.6 ± 17.1 % with a range of 9.4 to 69.1 %. The mean STS score was 12.8 ± 7.1 % with a range of 2.5–28.8 %. Edwards Sapien prostheses were used in 33 patients (Table 1).
The detailed comorbidities of the transapical group are listed in Table 2, only some are highlighted in the following section. According to our patient selection protocol, there was a high prevalence of peripheral artery disease or small vessel diameter or vessel tortuosity of the femoroiliac vessels, not suitable for transfemoral approach. Thirty percent of the patients had 3-vessel coronary artery disease. 45.5 % of the patients had previous PCI and 21 % of the patients underwent previous cardiac surgery (9 % CABG and 12 % mitral surgery). Eighteen percent of the patients had poor left ventricular function, and severe tricuspid regurgitation was present in 12 % of the patients. Nine percent of the patients presented with severe pulmonary hypertension. Atrial fibrillation was present in 75 % of the patients. Twelve percent of the patients had suffered a previous stroke. Nearly 40 % of the patients had COPD, and 30 % of the patients were cortisone dependent due to their COPD.
Implantation procedure
A detailed description of technical aspects of the transfemoral and transapical implantation is given previously [11]. Here, only a few aspects are stressed.
Technical aspects of transfemoral implantation
The procedure was performed in patient under analgosedation with local anesthesia. Thus, TEE was not performed during device implantation. First, a transjugular pacemaker was placed into the right ventricular apex. The right femoral artery was used as the main access site for the valve. A balloon valvuloplasty of the native stenotic valve was performed under rapid ventricular pacing. The CoreValve prostheses (Medtronic, Minneapolis, MN, USA) were released stepwise on the beating heart, whereas the Edwards Sapien prostheses (Edwards, Irvine, CA, USA) were deployed by balloon inflation under rapid ventricular pacing. The femoral access site was closed with the prepositioned ProStar™ XL sutures (Abbott Vascular, Abbott Park, IL, USA).
Technical aspects of transapical implantation
The procedures were initially done in a cath lab, later on TA-AVI was performed in a surgical hybrid suite by a multidisciplinary team consisting of cardiac surgeons, cardiologists and anaesthesiologists with extracorporeal circulation in stand-by for back-up. All patients were intubated and ventilated during the procedure. Patients were fully equipped for anaesthesiological monitoring as usual for open heart surgery. Short-acting intravenous medications were used to ensure early extubation, whenever possible. The left side of the chest was slightly elevated. Parallel to placement of a femoral venous guidewire and a 6-F femoral arterial sheath as access for emergency cardiopulmonary bypass, anterolateral minithoracotomy in the 5th or 6th intercostal space was performed to expose the left ventricular apex. After pericardial stay sutures and insertion of an epicardial ventricular pacing wire, two circular apical purse-string sutures with Teflon pledges as reinforcement were placed. The Edwards Sapien prostheses (Edwards, Irvine, CA, USA) were deployed by balloon inflation under rapid ventricular pacing (23 mm diameter n = 12; 26 mm diameter n = 21). After confirmation of optimal positioning with angiography and TEE, the sheath and wire were removed from the apex and the previously placed purse-string sutures were tied under rapid pacing to achieve optimal hemostasis. Possible apical bleeding was controlled with additional deep sutures with large-sized Teflon pledges, which were recently already routinely placed before apex puncture. After insertion of a chest tube, the minithoracotomy was closed in a routine fashion.
Data collection
Collection of follow-up data was approved by Institutional Ethics Committee. Follow-up was done by chart review, departmental follow-up questionnaire (6 months postoperatively) and telephone interviews with family doctors, patients or their relatives.
Statistics
Results are expressed in a standard fashion: continuous variables as mean values ± SD and categorical variables as proportions. For comparison of continuous variables between groups the t test was used. The survival function was illustrated by Kaplan–Meier curve.
Results
All transapical implantations were technically successful, no postdilatation or valve-in valve implantation was required. No procedural death occurred. In one patient with severe right heart failure support of femorally cannulated cardiopulmonary bypass was necessary for implantation (Table 3). No coronary obstruction during implantation and no perioperative myocardial infarction were observed. No severe bleeding complications occurred during transapical implantation and no re-exploration was required. There were no neurological events (Table 4). Female gender was predominant in the transapical group (n = 23; 70 %). Prostheses with 26 mm diameter were more often (n = 21) used than 23 mm (n = 12). Intraoperative echocardiography revealed good valve function with not more than mild paravalvular incompetence in 36.4 % of patients postoperatively and during follow-up. Mean ICU stay was 7 ± 10.2 days. Mean ventilation time was 26 ± 66.4 h, three patients had to be reintubated due to respiratory insufficiency. New onset of atrial fibrillation was observed in 6 (18.1 %) patients. Postoperative hemodialysis was necessary in 4 (12.1 %) patients. Six patients required postoperative pacemaker implantation due to higher grade AV block (18.1 %, Table 4).
30-Day mortality
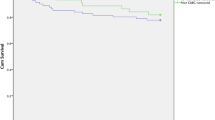
30-Day survival was comparable with 87.9 % in the transapical versus 92 % in the transfemoral group (p = 0.52). In contrast, predicted mortality by mean logistic EuroSCORE and STS score was significantly higher in the transapical group (p = 0.001 respectively, Fig. 1). Cardiac decompensation with concomitant pneumonia was the underlying cause for death in three patients, one patient died due to abdominal malperfusion. No death was related to aortic valvular function.
Late mortality
Follow-up (0–37 months) was complete in 100 %. Nine patients died after 30 days postoperatively (Table 4): underlying causes were cardiac in six cases (in 5 cases, the underlying cause was unknown, but classified as cardiac according to the VARC criteria) and non-cardiac in three cases. However, echocardiography revealed good valve function with not more than mild paravalvular incompetence at the last follow-up examination. No re-hospitalization (valve related), no prosthetic valve dysfunction (including endocarditis) and no repeat procedure for valve-related dysfunction were required (Table 4).
The mean age, mean EuroSCORE and mean STS scores were similar between survivors and patients with 30-day mortality. However, despite similar mean age and mean EuroSCORE, the mean STS score was elevated in patients with late mortality (no statistical comparison due to sample size, Table 4). They presented with a higher prevalence of tricuspid insufficiency. Interestingly, mean procedure time was even slightly shorter in cases of non-survivors (Table 5).
Survival was 78.8 % at 3 months, 69.7 % at 6 months and 66.7 % at 12 months. Actuarial survival analysis of all patients including 30-day mortality revealed 75.1 % at 3 months, 68 % at 6 months and 59.5 % at 12 months (Fig. 2). Among patients, who survived at least 30 days, actuarial survival was 85.5 % at 3 months, 77.4 % at 6 months and 67.7 % at 12 months.
Discussion
The outcome in the highly selected TA-TAVI group was similar to that in the TF-TAVI group with regard to 30-day mortality, despite significant higher predicted mortality by EuroSCORE and STS score in the TA-AVI group due to higher morbidity. These results match the reported 30-day mortality of 8–23 % after TA-AVI with series of 26–59 patients [5, 12–16]. Absolute 6-month survival after TA-AVI was 69.7 % in our series and is similar to another study with groin first approach reporting the 6-month survival of 73.4 % [16]. When 30-day mortality was excluded, actuarial survival after 6 months was 77.4 % in our series. Absolute 1-year survival was 66.7 % in our series and is in accordance to the literature (65 %) [14]. Actuarial survival after 1 year was 59.5 % in our series and similar to 64.8 % in the above-mentioned study [14]. The SOURCE Registry and another single-center study recently reported the actuarial 1-year survival of 72.1 and 72 % after TA-TAVI [17, 18]. However, it has to be kept in mind, that in both studies “all comers” were included, reflected by 29.8 % of the patients with a logistic EuroSCORE <20 % [17], whereas in our study collective TA-TAVI patients were highly selected with severe comorbidities.
It is well-known that the currently available scoring systems (especially the logistic EuroSCORE) tend to overestimate the predicted surgical mortality rates for aortic valve replacement [19]. However, both scoring systems (logistic EuroSCORE and STS score) are not including additional risk factors for adverse outcomes after cardiac surgery such as frailty, chronic liver disease, cancer, porcelain aorta, end stage lung disease, severe vasculopathy and nutritional deficiencies [20–22]. Especially frailty was associated with an incremental risk for mortality in patients with cardiovascular disease [20]. Available frailty scoring models include defined criteria such as gait velocity, grip strength, cognitive impairment, weight loss, inactivity and incontinence to predict cardiovascular mortality independent of cardiac risk factors and other comorbidities [23, 24]. So far frailty is mostly only assessed by clinical judgement prior to TAVI, as in the present study. The integration of frailty risk scores into a surgical model and/or an interventional model would be helpful for better patient selection, decision-making [25] and as benchmark performance measure. This may be even mandatory in the future to justify TAVI in patients with logistic EuroSCORE of less than 20 % and to ensure reimbursement for this rapidly growing treatment concept.
A recent study analyzed predictors for 90-day mortality in TAVI patients [26]. Only the preoperative patient characteristics such as advanced NYHA class and tricuspid regurgitation more than moderate were independent risk factors for increased mortality in this study. However, significantly higher STS score in non-survivors in the univariate analysis (not differentiating between different access routes) was no independent risk factor. Intraprocedural independent variables for adverse outcome after 90 days were intraprocedural resuscitation, residual aortic insufficiency more than grade two and overall procedure time (probably indicating that something was going wrong) [26]. In our series, the STS score was elevated in patients with late mortality, however, no statistical comparison was carried out due to small sample size. Interestingly, in our series, the mean procedure time was slightly shorter in patients with early and late mortality. Nevertheless, recompensation of patients prior to TAVI seems mandatory in the light of these findings to optimize outcome. A recent risk-factor analysis of transapical TAVI (no groin first approach) identified reduced vital capacity and concomitant preoperative mitral regurgitation >1+ as the only independent predictors of 30-day mortality, whereas classic variables such as age, logistic EuroSCORE >30 % and STS score >15 % failed to predict mortality [27]. In our series, non-survivors presented with a higher degree of tricuspid regurgitation. The observed 1-year survival of 66.7 % in our series may be related to the low incidence of paravalvular leaks resulting in no valve-related re-hospitalization or repeat intervention. New transapical devices with the possibility to reposition a valve may further reduce the incidence of paravalvular leaks, so far the Achilles’ heel of TAVI [11].
Transapical TAVI may be on the first look more invasive than transfemoral TAVI with only “percutaneous” access. However, despite a significant higher risk profile in the TA-AVI group (reflecting the high prevalence of peripheral, carotid and cerebrovascular disease), the survival rates after 1 and 2 years were comparable to TF-AVI patients in a Canadian multicenter study [28]. In case of transapical access, there is less manipulation on the aorta and the aortic arch, explaining the lower incidence of perioperative strokes despite more severe atherosclerosis in most studies [5, 17]. The PARTNER Trial recently reported a quite high incidence of major strokes after TAVI (3.8 %), which was similar for both transfemoral and transapical implantation techniques [29]. These findings are in contrast to our series and may be partly explained by the small case load and limited experience of some PARTNER participants. By transapical implantation, peripheral vascular complications can be avoided and the introduction of apical closure devices may further limit the risk for access complications due to apical bleeding or pseudoaneurysm formation.
In conclusion, the “groin-first” approach is reasonable due to less invasive percutaneous implantation technique. In the light of the significant survival benefit and the gain of functional performance after transfemoral TAVI (PARTNER Trial Cohort A) [7], patients with even severe comorbidities benefit from this treatment. Therefore, transapical aortic valve implantation is justified despite even higher predicted mortality in these selected high-risk patients with generalized atherosclerosis, while outcome is non-inferior to transfemoral approach. However, careful individual assessment is pivotal in every single patient for TA-TAVI, especially when a groin first strategy is followed.
References
Rao V, Christakis GT, Weisel RD et al (1996) Changing pattern of valve surgery. Circulation 94:113–120
Lichtenstein SV, Cheung A, Ye J et al (2006) Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 114:591–596
Beyersdorf F (2007) Transapical transcatheter aortic valve implantation. Eur J Cardiothorac Surg 31:7–8
Walther T, Falk V, Borger MA et al (2007) Minimally invasive transapical beating heart aortic valve implantation—proof of the concept. Eur J Cardiothorac Surg 31:9–15
Walter T, Simon P, Dewey T et al (2007) Transapical minimally invasive aortic valve implantation: multi-center experience. Circulation 116(11 Suppl):I240–I245
Ye J, Cheung A, Lichtenstein V et al (2007) Six-month outcome of transapical transcatheter aortic valve implantation in the initial seven patients. Eur J Cardiothorac Surg 31:16–21
Leon MB, Smith CR, Mack M et al (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363:1597–1607
Figulla L, Neumann A, Figulla HR et al (2011) Transcatheter aortic valve implantation: evidence on safety and efficacy compared with medical therapy. A systematic review of current literature. Clin Res Cardiol 100:265–276
Leon MB, Piazza N, Nikolsky E et al (2011) Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J 32:205–217
Gilard M, Eltchaninoff H, Iung B, FRANCE 2 Investigators et al (2012) Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 366:1705–1715
Beller CJ, Bekeredjian R, Krumsdorf U et al (2011) Transcatheter aortic valve implantation after previous mechanical mitral valve replacement: expanding indications? Heart Surg Forum 14:E166–E170
Svesson LG, Dewey T, Kapadia S et al (2008) United States feasibility study of transcatheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 86:46–54
Walther T, Falk V, Kempfert J et al (2008) Transapical minimally invasive aortic valve implantation: the initial 50 patients. Eur J Cardiothorac Surg 33:983–988
Ye C, Cheung A, Lichtenstein SV et al (2009) Transapical transcatheter aortic valve implantation: 1-year outcome in 26 patients. J Thorac Cardiovasc Surg 137:167–173
Zierer A, Wimmer-Greinecker G, Martens S, Moritz A, Doss M (2008) The transapical approach for aortic valve implantation. J Thorac Cardiovasc Surg 136:948–953
Bleiziffer S, Ruge H, Mazzitelli D et al (2009) Survival after transapical and transfemoral aortic valve implantation: talking about two different patient populations. J Thorac Cardiovasc Surg 138:1073–1080
Thomas M, Schymik G, Walther T et al (2011) One-year outcomes of cohort 1 in the Edwards SAPIEN aortic bioprosthesis European Outcome (SOURCE) Registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 124:425–433
Puls M, Viel T, Danner BC et al (2012) The risk-to-benefit ratio of transcatheter aortic valve implantation in specific patient cohorts: a single-centre experience. Clin Res Cardiol. doi:10.1007/s00392-012-0426-4
Piazza N, Wenaweser P, van Gameren M et al (2010) Relationship between the logistic EuroSCORE and the Society of Thoracic Surgeons predicted risk of mortality score in patients implanted with the CoreValve revalving system—a Bern-Rotterdam study. Am Heart J 159:323–329
Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H (2009) Role of frailty in patients with cardiovascular disease. Am J Cardiol 103:1616–1621
Engelman DT, Adams DH, Byrne JG et al (1999) Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg 118:866–873
Higgins TL (1998) Quantifying risk and assessing outcome in cardiac surgery. J Cardiothorac Vasc Anesth 12:330–340
Cigolle CT, Ofstedal MB, Tian Z, Blaum CS (2009) Comparing models of frailty: the health and retirement study. J Am Geriatr Soc 57:830–839
Hogan DB, MacKnight C, Bergman H (2003) Models, definitions, and criteria of frailty. Aging Clin Exp Res 15:1–29
Osten MD, Feindel C, Greutmann M et al (2010) Transcatheter aortic valve implantation for high risk patients with severe aortic stenosis using the Edwards Sapien balloon-expandable bioprosthesis: a single centre study with immediate and medium-term outcomes. Catheter Cardiovasc Interv 75:475–485
Bauernschmitt R (2011) Transcatheter aortic valve replacement (TAVI)—risk factors for 90-day mortality. Intervent Med Appl Sci 3:128–133
Kempfert J, Rastan A, Holzhey D et al (2011) Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 124(Suppl 1):S124–S129
Rodes-Cabau J, Webb JG, Cheung A et al (2010) Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 55:1080–1090
Smith CR, Leon MB, Mack MJ et al (2011) PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364:2187–2198
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. J. Beller and B. Schmack contributed equally to this work.
Rights and permissions
About this article
Cite this article
Beller, C.J., Schmack, B., Seppelt, P. et al. The groin first approach for transcatheter aortic valve implantation: are we pushing the limits for transapical implantation?. Clin Res Cardiol 102, 111–117 (2013). https://doi.org/10.1007/s00392-012-0502-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-012-0502-9