Abstract
Objectives
Transcatheter aortic valve implantation (TAVI) promises effective treatment for high-risk elderly patients with symptomatic severe aortic stenosis (AS). However, the adoption of TAVI must be justified and guarantee long-term performance. Systematic reviews are a core methodology in evidence-based health economics for judging medical effectiveness. In this work, the methodology was applied to provide objective evidence on the efficacy and safety of TAVI at 1-year follow-up and to assess whether TAVI confers a survival benefit compared with medical therapy.
Methods
In accordance with the toolkit of the “German Scientific Working Group Technology Assessment for Health Care” (GSWG), a systematic literature review on the safety and efficacy of TAVI procedures was conducted in major bibliographic databases to identify all relevant publications. Preestablished inclusion criteria were defined. An initial screening of identified articles regarding titles and abstracts was followed by a full-text screening. Data from eligible articles were extracted and evaluated according to GSWG checklists followed by a qualitative synthesis of information.
Results
The systematic literature search identified 12 primary publications (derived from 1,849 citations) for TAVI [number of patients (n) = 1,049] and 11 publications (derived from 189 citations) for medical therapy of AS (n = 946) that fulfilled the inclusion criteria.
Mean overall procedural success rate for included TAVI interventions was 93.3%. Mean combined procedural, post-procedural, and cumulative in-hospital/30-day mortality was 11.4% (n = 116; range 5.3–23%).
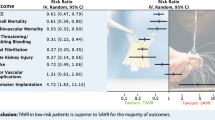
1 year after TAVI, the mean overall survival rate was 75.9% (range 64.1–87%) compared with 62.4% (range 40–84.8%) for medically treated patients (p value < 0.01). 1-year survival after TAVI for patients treated with transvascular (TV) procedures was higher than after transapical (TA) procedures (79.2 vs. 73.6%) (p value = 0.04). At 1-year follow-up, the improved valvular function remained stable, and there was a trend towards an improved ventricular function.
Conclusion
Based on the best available data, in patients with symptomatic severe AS, TAVI demonstrates an improved 1-year survival compared with medical treatment. The survival benefit of TV-TAVI over medical therapy elucidated from this systematic literature review is +16.8% and therefore, in good congruence with the recently published results from the randomized PARTNER US trial (+20%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aortic stenosis (AS) is the most common valvular heart disease in the elderly with an estimated prevalence of up to 5% in individuals older than 75 years [22, 23]. In the coming decades, there will be a tremendous aging of the population in developed countries with a unique increase of inhabitants older than 80 years [30]. Hence, AS will become more frequent and constitute a growing burden for public health.
The course of symptomatic severe AS under medical treatment is dismal with high mortality rates. After the onset of heart failure, median survival is only 11 months and after onset of syncope and angina 27 and 45 months, respectively, [17]. Because of the high risk of restenosis, balloon aortic valvuloplasty (BAV) can only be considered as a palliative treatment method for patients with a good quality of life (QoL) who are not eligible for surgical aortic valve replacement (AVR) [6, 8, 12, 28]. Although surgical AVR is regarded to be the mainstay for improved survival and symptom relief, not all patients, especially the elderly, are able to profit from this technique [5, 34]. The European Heart Survey of patients with valvular heart disease suggests that up to 33% of subjects over the age of 75 are not considered for surgical AVR because of age and comorbidities [18].
Transcatheter aortic valve implantation (TAVI) is a new, innovative medical procedure that promises effective treatment for high-risk patients not suitable for surgical AVR. As of today, TAVI is intended as a treatment alternative to “no intervention”. As of June 2010, approximately 20,000 procedures have been performed worldwide, and this number experiences exponential growth (according to TAVI device manufacturers).
However, the adoption of TAVI must be justified and guarantee long-term performance. Systematic reviews are core to formal decision making processes in evidence-based health economics [11]. They apply a series of methodological principles which aim at systematically identifying, evaluating, and summarizing all available data to provide objective evidence for judging medical effectiveness. To date, few systematic reviews on the safety and efficacy of TAVI procedures have been conducted, and none of them have focused on 1-year follow-up data or assessed whether TAVI confers a survival benefit in patients with symptomatic severe AS without valve intervention.
Methods
Inclusion criteria
This systematic review was based on published peer-reviewed clinical case series and cohort studies as well as published secondary literature, including systematic reviews and health technology assessments (HTA). In accordance with the toolkit of the “German Scientific Working Group Technology Assessment for Health Care” (GSWG) [14], preestablished inclusion criteria were defined. Publications had to fulfill these criteria listed in Table 1 to be eligible for consideration [12]. Similar criteria were chosen for the systematic literature review on medical therapy treatment of AS, adjusting the two criteria intervention and patient characteristics to “none or palliative BAV” and “patients with severe AS who either refused or were denied surgical AVR or TAVI, excluding asymptomatic patients” (Table 1).
Literature search
The literature search was performed in four bibliographic databases: MEDLINE, EMBASE, Centre for Reviews and Dissemination (CRD), and Cochrane Library. The search strategy consisted of controlled vocabulary, including The National Library of Medicine’s Medical Subject Headings (MeSH) terms, and free text keywords, such as “aortic valve” OR “aortic valve stenosis” AND “percutaneous” OR “transcatheter” OR “transvascular” OR “transfemoral” OR “transapical”. The search was not restricted to any publication time period but to English or German language and an adult patient population. Findings in EMBASE and MEDLINE were updated as of April 30, 2010. These searches were supplemented by handsearching the reference lists of key papers for further identification of potentially relevant studies. No limitation was placed on the study type. For secondary publications, the main search included CRD and International Network of Agencies for Health Technology Assessment (INAHTA) electronic databases and supplemental databases of major national HTA institutes [German “Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen” (IQWiG), and British “National Institute for Health and Clinical Excellence” (NICE)].
Data selection, extraction, and evaluation
Literature selection was conducted in two stages: an initial screening of titles and abstracts was followed by screening of the full-text publications. For all reports that were excluded based on the full-text screening, the rationale for exclusion was recorded. Then, the data from the included reports was extracted. A structured data extraction form was used including study and patient characteristics, primary outcome measures (procedural success rate, complications, mortality, and survival), and secondary outcome measures (post-procedural hemodynamic data, New York Heart Association (NYHA) class, length of hospital stay, QoL. The quality of all included publications was assessed along the checklist #2a of the GSWG [13].
Statistics
Metric variables were expressed as mean (range). Means were calculated based on all included studies. Where standard deviations (SD) were consistently reported, the overall SD was calculated as the square root of the mean of variances plus the variance of means. With the exception of number of patients, specified ranges referred to extreme values of reported means. Categorical or binary variables were expressed as percentages [absolute number of patients (n); range] and compared using Chi-square (χ 2) test. The estimation of 95% confidence intervals (CI) for survival rates relied on the normal approximation to the binomial distribution. Data collection and statistical analysis were performed using Microsoft Excel. All tests were two-sided. A value p < 0.05 was considered to indicate a statistically significant difference.
Results
Results of literature search
As illustrated with a process flow chart in Fig. 1, the original literature search identified a total of 2,038 citations (thereof 1,849 for TAVI and 189 for medical therapy of AS). Based on a screening of titles and abstracts, 60 potentially relevant publications were retrieved for full-text screening. Together with 21 publications identified through supplementary MEDLINE and EMBASE database alerts and handsearching the reference lists of key papers, 81 reports underwent a detailed full-text screening, yielding 12 [1, 15, 16, 19, 25–27, 29, 35, 36, 38, 40] primary publications for TAVI and 11 [2, 3, 7, 18–20, 24–26, 33, 34] for medical therapy of AS.
Quality and characteristics of included studies
Pending the finalization of this manuscript, no data from randomized controlled trials (RCT) was available. Therefore, all included data were based on observational clinical studies. The key study characteristics and patient demographics for each included study are described in Table 2.
Most included studies were recently published (median publication year for studies on TAVI and for medical therapy was 2009 and 2008, respectively). Three publications conducted small comparative cohort studies to assess TAVI patients versus control groups of patients referred for TAVI, but undergoing either alternative aortic valve interventions or medical therapy [19, 25, 26]. These three studies are included in both information syntheses on TAVI and medical therapy. Two studies conducted matched comparisons between TAVI and surgical AVR [35, 40].
The number of all patients (n) captured by this review totaled 1,049 for the TAVI group and 946 for the medical therapy group. There were three studies exclusively on the transvascular (TV) approach with the Medtronic CoreValve system (n = 213) [15, 25, 26]. The other studies reported on Cribier–Edwards/Edwards Sapien valve prostheses (n = 836). Of these, three series reported exclusively on transapical (TA) procedures (n = 147) [35, 39, 40] and six on patients who underwent either approach (TV n = 376, TA n = 295, approach not specified n = 18). All included studies reported 1-year follow-up data.
The demographics of included patients were comparable for TAVI and medical therapy. The mean age was high, with 82 years (TV 82.7/TA 81.6; range 80.1–85) for TAVI, and 79.9 years (range 73.3–86.2) for medically treated patients. Forty-five percent (TV 51.1%/TA 36.1%) of TAVI and 47% of medically treated patients were males. All patients were symptomatic and considered “inoperable” or at a “very high risk” for surgery, but the estimated operative risk of included TAVI patients as assessed by the logistic EuroSCORE method was higher than for medically treated patients (27.8 vs. 13.5%).
Safety of TAVI at 1-year follow-up
The mean overall procedural success rate was 93.3% (n = 948; range 86–100%) for all included TAVI interventions, with a lower procedural success rate of 89.2% (n = 382; range 85.7–97.3%) for TV procedures compared with 97.3% (n = 353; range 96.1–100%) for TA procedures (Table 3). The incidence of reported major adverse cardiac and cerebrovascular events (MACCE) is illustrated in Table 4. None of the studies observed any evidence of structural valve deterioration or other prosthetic valve dysfunction during follow-up.
The mean combined procedural, post-procedural, and cumulative in-hospital/30-day mortality was 11.4% (n = 116; range 5.3–23%). For TV and TA procedures, the mean in-hospital/30-day mortality was 9.5% (n = 53; range 5.3–13.3%) and 14% (n = 62; range 10–27%), respectively. One year after the TAVI procedure, the overall mean survival rate was 75.9% (range 64.1–87%). For TV and TA procedures, the reported 1-year survival rate was 79.2% (range 68.1–87%) and 73.6% (range 60–78%) (p value = 0.0.4), respectively. 30-day mortality and 1-year survival rates from each included study are provided in Table 3.
The 1-year survival rates and estimated 95%-CI overall and for both types of intervention are illustrated in Fig. 2. Three authors reported that the incidence of late procedure- or valve-related mortality was mostly noncardiac and due to comorbidities [15, 27, 36].
The mean 1-year survival rate of medically treated patients was 62.4% (range 40–84.8%; p value < 0.01 vs. TAVI). Fig. 3 compares the 1-year survival rates and estimated 95%-CI of TAVI and medical therapy. None of the studies provided details on causes of death to assess whether it was due to cardiovascular or other causes.
Efficacy of TAVI at 1-year follow-up
All included trials presented pre-procedural echocardiographic outcome measures that indicated severe AS [4]. Echocardiographic assessment revealed an aortic valve area (cm²) of 0.63 (range 0.50–0.67) and a transaortic mean gradient (mmHg) of 46.6 ± 15.6 (range 42–56). The echocardiographic baseline data did not differ significantly between TAVI and medically treated subgroups [aortic valve area (cm²): 0.68 (range 0.57–0.73); transaortic mean gradient (mmHg): 42.7 ± 17.8 (range 39–66)].
Patients in whom a TAVI was successfully performed were reported to experience an improved valvular function, left ventricular function, and functional status. The post-TAVI effect on echocardiographic and clinical outcomes is provided in Table 5. Even at 1-year follow-up, the improvement of the valvular function was sustained, and there was a trend towards an improved ventricular function.
Aortic regurgitation (AR) was present after TAVI in most patients to some degree as reported by seven studies (n = 594) [1, 15, 16, 27, 29, 36, 38]. Postoperatively, 77.8% (n = 462) of survivors had none to mild (grade 0/I) AR, and 19.0% (n = 113) had moderate (grade II) AR. Severe AR (grade III) occurred in 3.2% (n = 19) of patients. Four studies monitored AR during follow-up and consistently reported that the postoperative degree of AR remained unchanged until 1-year follow-up [29, 35, 36, 39].
Seven studies reported a mean length of hospital and intensive care unit (ICU) stay associated with TAVI: mean stay in hospital was 9.5 (range 5–19) days, and thereof, mean stay of 2.7 days in ICU. Hospital stay for TV patients was shorter than for TA patients (9 days (range 5–15) versus 10.6 days (range 7–19)) [1, 16, 19, 29, 36, 38, 40].
None of the included studies provided data on patients’ QoL before or after TAVI.
Discussion
This systematic review followed the methodology recommended by the toolkit of the GSWG [14] to objectively assess the safety and efficacy of TAVI as compared with medical therapy of AS. The results derived from the systematic review suggest that TAVI offers promising safety and lasting efficacy outcomes in a patient population with symptomatic severe AS not considered for surgical AVR.
With the population aging, AS will become a more prevalent public health issue over the following decades. As soon as symptoms develop, medical therapy is unlikely to modify the dismal course of this disease. Median survival after the onset of heart failure is 11 months, after syncope 27 months, and after angina 45 months [17]. Surgery is therefore indicated once patients develop symptoms [4, 31], but open heart surgery involves significant risks, in particular for elderly and frail patients. For this reason, many elderly patients are denied surgery [18]. In the past, these patients could only be managed conservatively with medical therapy or palliative BAV [9, 28]. With TAVI, an additional, less invasive option has emerged for these “inoperable” patients. Since the first TAVI performed by Cribier and colleagues in 2002 [10], many thousands of such devices have been implanted in high-risk patients worldwide.
The main finding of this review with respect to safety of TAVI is that based on current literature, high-risk, elderly patients undergoing TAVI appear to have a better outcome in terms of absolute 1-year survival compared with their medically treated counterparts. As illustrated by Fig. 3, the mean 1-year survival rate after TAVI is 75.9% (95% CI: 73.3–78.4%) [TV-TAVI: 79.2% (95% CI: 75.5–82.8%); TA-TAVI: 73.6% (95% CI: 69.2–77.9%)] versus 62.4% (95% CI: 59.3–65.5%) with medical therapy. Differences in 1-year survival between TV-TAVI and TA-TAVI procedures can at least partly be explained by the even poorer pre-procedural health status of TA patients; however, the pre-procedural health status of medically treated patients was significantly better than for TAVI patients (logistic EuroSCORE 13.5 vs. 27.8%). Consequently, the survival benefit of TAVI would probably be even greater if evaluated against medically treated patients with a comparably poor health status. This finding is important as it substantiates a potential survival benefit of TAVI of at least +16.8% for a group of patients in whom there was previously no effective treatment option. The magnitude of this result corresponds well to the 20% survival benefit reported by the PARTNER US trial for TV-TAVI procedures which randomized 358 patients from 21 centers considered not suitable for surgery: at 1 year, the rate of death from any cause was 30.7% with TAVI, as compared with 50.7% with medical therapy [21].
Consistent with previous secondary publications [32, 37], data on safety presented in this review demonstrate that TAVI is feasible with procedural success rates ranging from 86 to 100%; however, it remains a high-risk procedure. In the recent series included in this review, 30-day mortality rates, which most likely reflect procedure-related mortality, ranged from 5.3–23%.
With respect to the efficacy of TAVI, post-procedural improvements of echocardiographic measurements and NYHA functional class seem encouraging, irrespective of the chosen access route. Based on the available data, the improvements at 30-day follow-up are sustained until 1-year follow-up without significant functional deterioration. However, long-term outcomes, particularly with respect to device durability, are not yet available. In addition, none of the included studies provided evidence to what extent the QoL is improved by TAVI, an aspect that would be particularly interesting for a frail patient population with comorbidities.
To point out limitations of our results, the findings are based on merely observational studies. Until the data freeze for this manuscript on April 30, 2010, no RCTs have compared TAVI with surgical AVR, BAV, or medical treatment. The finalization of this manuscript in September 2010 coincided with the publication of the results from the first randomized PARTNER US trial comparing TV-TAVI versus medical therapy or BAV in patients at extreme surgical risk [21]. Therefore, the RCT data could not be considered as part of the formal information synthesis. Apart from the lack of randomization, the major shortcomings of the available published series summarized in this review are the lack of long-term data, selected and small patient groups, and, in some cases, the involvement of manufacturers. The above-discussed inconsistent patient selection criteria complicate the interpretation of outcomes from included studies. As we were unable to verify to what extent authors had potentially published trials with accumulating numbers of patients or increased lengths of follow-up, all publications meeting our inclusion criteria were considered for critical appraisal. Preceding secondary publications were evaluated but not included in the information synthesis because their results were to a large extent based on earlier experimental series published before 2009.
Conclusion
Applying a formal methodology used in evidence-based health economics, this systematic review aimed to objectively evaluate the safety and efficacy of TAVI. Based on the best available data set, in patients with inoperable AS, TAVI promises significantly improved 1-year survival when compared with sole medical treatment. Before the publication of the first RCT data in September 2010 which coincided with the finalization of this manuscript, our results represented the best available data set. As the TAVI survival benefit elucidated from the systematic literature review is in good congruence with the RCT data, we conclude that this methodology represents a powerful tool to confirm—or even anticipate—RCT outcomes.
References
Al-Attar N, Himbert D, Descoutures F, Iung B, Raffoul R, Messika-Zeitoun D, Brochet E, Francis F, Ibrahim H, Vahanian A, Nataf P (2009) Transcatheter aortic valve implantation: selection strategy is crucial for outcome. Ann Thorac Surg 87(6):1757–1762. doi:10.1016/j.athoracsur.2009.03.047 discussion 1762–1763
Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD, Gualano SK (2009) Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: The potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes 2(6):533–539
Bakaeen FG, Chu D, Ratcliffe M, Gopaldas RR, Blaustein AS, Venkat R, Huh J, LeMaire SA, Coselli JS, Carabello BA (2010) Severe aortic stenosis in a veteran population: treatment considerations and survival. Ann Thorac Surg 89(2):453–458
Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS (2006) ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 114(5):e84–231
Bouma BJ, van den Brink RB, van der Meulen JH, Verheul HA, Cheriex EC, Hamer HP, Dekker E, Lie KI, Tijssen JG (1999) To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart 82(2):143–148
Carabello BA, Paulus WJ (2009) Aortic stenosis. Lancet 373(9667):956–966
Charlson E, Legedza AT, Hamel MB (2006) Decision-making and outcomes in severe symptomatic aortic stenosis. J Heart Valve Dis 15(3):312–321
Conradi L, Reichenspurner H (2008) Review on balloon aortic valvuloplasty: a surgeon’s perspective in 2008. Clin Res Cardiol 97(5):285–287
Conradi L, Seiffert M, Franzen O, Baldus S, Schirmer J, Meinertz T, Reichenspurner H, Treede H (2010) First experience with transcatheter aortic valve implantation and concomitant percutaneous coronary intervention. http://dx.doi.org/10.1007/s00392-010-0243-6
Cribier A, Eltchaninoff H, Bash A, Borenstein N, Bauer F (2002) Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106:3006–3008
Drummond MF (2007) Methods for the economic evaluation of health care programmes, 3rd edn. Oxford University Press, Oxford reprinted
Figulla HR, Cremer J, Walther T, Gerckens U, Erbel R, Osterspey A, Zahn R (2009) Positionspapier zur kathetergeführten Aortenklappenintervention. Der Kardiologe 3(3):199–206
German Scientific Working Group for Health Care (2000) Checklist 2a: primary studies (RCTs/case-control studies/cohort studies/longitudinal studies/case series). In: German Scientific Working Group for Health Care (ed) Toolkit. Informationsmaterial für Verfasser von HTA-Berichten, Hannover
German Scientific Working Group for Health Care (ed) (2000) Toolkit. Informationsmaterial für Verfasser von HTA-Berichten, Hannover
Grube E, Buellesfeld L, Mueller R, Sauren B, Zickmann B, Nair D, Beucher H, Felderhoff T, Iversen S, Gerckens U (2008) Progress and current status of percutaneous aortic valve replacement: results of three device generations of the CoreValve Revalving System. Circ Cardiovasc Interv 1(3):167–175
Himbert D, Descoutures F, Al-Attar N, Iung B, Ducrocq G, Detaint D, Brochet E, Messika-Zeitoun D, Francis F, Ibrahim H, Nataf P, Vahanian A (2009) Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J Am Coll Cardiol 54(4):303–311. doi:10.1016/j.jacc.2009.04.032
Horstkotte D, Loogen F (1988) The natural history of aortic valve stenosis. Eur Heart J 9(Suppl E):57–64
Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P, Gohlke-Bärwolf C, Boersma E, Ravaud P, Vahanian A (2005) Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 26(24):2714–2720. doi:10.1093/eurheartj/ehi471
Kapadia SR, Goel SS, Svensson LG, Roselli EE, Savage RM, Wallace L, Sola S, Schoenhagen P, Shishehbor MH, Christofferson R, Halley C, Rodriguez LL, Stewart WJ, Kalahasti V, Tuzcu EM (2009) Characterization and outcome of patients with severe symptomatic aortic stenosis referred for percutaneous aortic valve replacement. J Thorac Cardiovasc Surg 137(6):1430–1435. doi:10.1016/j.jtcvs.2008.12.030
Kojodjojo P, Gohil N, Barker D, Youssefi P, Salukhe TV, Choong A, Koa-Wing M, Bayliss J, Hackett DR, Khan MA (2008) Outcomes of elderly patients aged 80 and over with symptomatic, severe aortic stenosis: impact of patient’s choice of refusing aortic valve replacement on survival. QJM 101(7):567–573
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. doi:10.1056/NEJMoa1008232. http://www.nejm.org/doi/abs/10.1056/NEJMoa1008232
Lindroos M, Kupari M, Heikkilä J, Tilvis R (1993) Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 21(5):1220–1225
Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M (2006) Burden of valvular heart diseases: a population-based study. Lancet 368(9540):1005–1011
O’Keefe JH Jr, Vlietstra RE, Bailey KR, Holmes DR (1987) Natural history of candidates for balloon aortic valvuloplasty. Mayo Clin Proc 62(11):986–991
Otten AM, van Domburg RT, van Gameren M, Kappetein AP, Takkenberg JJM, Bogers AJ, Serruys PW, De Jaegere P (2008) Population characteristics, treatment assignment and survival of patients with aortic stenosis referred for percutaneous valve replacement. EuroIntervention 4(2):250–255
Rajani R, Buxton W, Haworth P, Khawaja MZ, Sohal M, Brum RL, Hutchinson N, de Belder A, Trivedi U, Hildick-Smith D (2010) Prognostic benefit of transcatheter aortic valve implantation compared with medical therapy in patients with inoperable aortic stenosis. Catheter Cardiovasc Interv 75(7):1121–1126
Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, De Larochellière R, Teoh KH, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E (2010) Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 55(11):1080–1090
Sack S, Kahlert P, Khandanpour S, Naber C, Philipp S, Mohlenkamp S, Sievers B, Kalsch H, Erbel R (2008) Revival of an old method with new techniques: balloon aortic valvuloplasty of the calcified aortic stenosis in the elderly. Clin Res Cardiol 97(5):288–297. doi:10.1007/s00392-008-0650-0
Thielmann M, Wendt D, Eggebrecht H, Kahlert P, Massoudy P, Kamler M, Erbel R, Jakob H, Sack S (2009) Transcatheter aortic valve implantation in patients with very high risk for conventional aortic valve replacement. Ann Thorac Surg 88(5):1468–1474. doi:10.1016/j.athoracsur.2009.07.033
United Nations Population Division (2008) World population prospects: the 2008 revision population database. http://esa.un.org/unpp/. Accessed 06 Jul 2010
Vahanian A, Baumgartner H, Bax J, Butchart EG, Dion RA, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak JD, Nataf P, Tornos P, Torracca L, Wenink A, Priori SG, Blanc J, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Angelini A, Antunes MJ, Fernandez MAG, Gohlke-Bärwolf C, Habib G, McMurray J, Otto CM, Pierard L, Pomar JL, Prendergast B, Rosenhek RMD, Uva MS (2007) Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European Society of Cardiology. Eur Heart J 28(2):230–268
van Brabandt H, Neyt M (2009) Safety of percutaneous aortic valve insertion. A systematic review. BMC Cardiovasc Disord 9:45–51. doi:10.1186/1471-2261-9-45
van Geldorp MWA, van Gameren M, Kappetein AP, Arabkhani B, De Groot-de Laat LE, Takkenberg JJM, Bogers AJ (2009) Therapeutic decisions for patients with symptomatic severe aortic stenosis: room for improvement? Eur J Cardiothorac Surg 35(6):953–957
Varadarajan P, Kapoor N, Bansal RC, Pai RG (2006) Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: Results from a cohort of 277 patients aged > or = 80 years. Eur J Cardiothorac Surg 30(5):722–727
Walther T, Schuler G, Borger MA, Kempfert J, Seeburger J, Rückert Y, Ender J, Linke A, Scholz M, Falk V, Mohr FW (2010) Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity-matched conventional aortic valve replacement. Eur Heart J 31(11):1398–1403
Webb JG, Altwegg L, Boone RH, Cheung A, Ye J, Lichtenstein SV, Lee M, Masson J, Thompson CR, Moss RR, Carere RG, Munt B, Nietlispach F, Humphries K (2009) Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation 119(23):3009–3016. doi:10.1161/CIRCULATIONAHA.108.837807
Yan TD, Cao C, Martens-Nielsen J, Padang R, Ng M, Vallely MP, Bannon PG (2010) Transcatheter aortic valve implantation for high-risk patients with severe aortic stenosis: a systematic review. J Thorac Cardiovasc Surg 139(6):1519–1528
Ye J, Cheung A, Lichtenstein SV, Altwegg LA, Wong DR, Carere RG, Thompson CR, Moss RR, Munt B, Pasupati S, Boone RH, Masson J, Ali A, Webb JG (2009) Transapical transcatheter aortic valve implantation: 1-year outcome in 26 patients. J Thorac Cardiovasc Surg 137(1):167–173
Ye J, Cheung A, Lichtenstein SV, Nietlispach F, Albugami S, Masson J, Thompson CR, Munt B, Moss RR, Carere RG, Jamieson WRE, Webb JG (2010) Transapical transcatheter aortic valve implantation: follow-up to 3 years. J Thorac Cardiovasc Surg 139(5):1107–1113.e1
Zierer A, Wimmer-Greinecker G, Martens S, Moritz A, Doss M (2009) Is transapical aortic valve implantation really less invasive than minimally invasive aortic valve replacement? J Thorac Cardiovasc Surg 138(5):1067–1072. doi:10.1016/j.jtcvs.2009.04.057
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Figulla, L., Neumann, A., Figulla, H.R. et al. Transcatheter aortic valve implantation: evidence on safety and efficacy compared with medical therapy. A systematic review of current literature. Clin Res Cardiol 100, 265–276 (2011). https://doi.org/10.1007/s00392-010-0268-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-010-0268-x