Abstract
The aim of the study is to evaluate the use of beta-blockers in chronic heart failure (CHF) and the extent of heart rate reduction achieved in clinical practice and to determine differences in outcome of patients who fulfilled select inclusion criteria of the SHIFT study according to resting heart rate modulated by beta-blocker therapy. We evaluated an all-comer population of our dedicated CHF outpatient clinic between 2006 and 2010. For inclusion, individually optimized doses of guideline-recommended pharmacotherapy including beta-blockers had to be maintained for at least 3 months and routine follow-up performed at our outpatient CHF-clinic thereafter. Treatment dosages of beta-blockers, and demographic and clinical profiles including resting heart rate were assessed. The outcome of patients who fulfilled select inclusion criteria of the SHIFT study (left-ventricular ejection fraction (LVEF) ≤35 %, sinus rhythm, NYHA II–IV) and were followed-up for at least 1 year was stratified according to resting heart rates: ≥75 versus <75 bpm and ≥70 versus <70 bpm. The composite primary endpoint was defined as all-cause death or hospital admission for worsening heart failure during 12-month follow-up. In total, 3,181 patients were assessed in regard to treatment dosages of beta-blockers, and demographic and clinical profiles including resting heart rate. Of the overall studied population, 443 patients fulfilled all inclusion criteria and entered outcome analysis. Median observation time of survivors was 27.5 months with 1,039.7 observation-years in total. Up-titration to at least half the evidence-based target dose of beta-blockers was achieved in 69 % and full up-titration in 29 % of these patients. Patients with increased heart rates were younger, more often male, exhibited a higher NYHA functional class and lower LVEF. The primary endpoint occurred in 21 % of patients in the ≥70 bpm group versus 9 % of patients in the group with heart rates <70 bpm (p <0.01). Likewise, comparing the groups ≥75 and <75 bpm, the primary endpoint was significantly increased in the group of patients with heart rates ≥75 bpm 27 vs. 12.2 %; p < 0.01). 5-year event-free survival was significantly lower among patients with heart rates ≥70 bpm as compared to those with <70 bpm (log-rank test p < 0.05) and among patients in the ≥75 bpm group versus <75 bpm group (log-rank test p < 0.01). In conclusion, in clinical practice, 53 % of CHF patients have inadequate heart rate control (heart rates ≥75 bpm) despite concomitant beta-blocker therapy. In this non-randomized cohort, adequate heart rate control under individually optimized beta-blocker therapy was associated with improved mid- and long-term clinical outcome up to 5 years. As further up titration of beta-blockers is not achievable in many patients, the administration of a selective heart rate lowering agent, such as ivabradine adjuvant to beta-blockers may pose an opportunity to further modulate outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of beneficial qualities have been attributed to beta-blockers in the treatment of chronic heart failure (CHF). These include the important role of beta-blockers in the reduction of left-ventricular wall stress, deceleration of adrenergic drive and improvement of myocardial remodeling [1, 2]. The positive effects of beta-blockers on pathomechanisms are mirrored in their independent effect on cardiovascular morbidity and mortality next to therapy with renin–angiotensin–aldosteron system antagonists [3–7]. Consequently, beta-blockers have entered international treatment guidelines with a class I recommendation for high-risk patients after acute myocardial infarction more than a decade ago and shortly afterwards became established pharmacotherapy for non-ischemic etiologies of CHF as well [8–14].
A considerable amount of experimental and clinical data suggests that heart rate reduction in general, positively influences cardiovascular disease [15, 16]. In 2010, the ‘Systolic Heart failure treatment with the If inhibitor Ivabradine Trial’ (SHIFT) demonstrated that heart rate reduction by the selective sinus-node inhibitor ivabradine is likewise beneficial for outcome in CHF [17, 18]. Patients were randomized to ivabradine or placebo if they fulfilled the following criteria: symptomatic heart failure, left-ventricular ejection fraction (LVEF) ≤35 %, sinus rhythm with heart rate ≥70 beats per minute (bpm), admission to hospital for heart failure within the previous year, and guideline-recommended background pharmacotherapy including a beta-blocker, if tolerated. Post hoc subgroup analysis have demonstrated the greatest risk reduction versus placebo in regard to the primary outcome (composite of cardiovascular death or hospital admission for worsening heart failure) and secondary endpoints including all-cause death and cardiovascular death in patients with baseline heart rates ≥75 bpm [18]. As a consequence of this observance, the European Medicines Agency approved a license extension of ivabradine to include the treatment of symptomatic, systolic CHF in patients in sinus rhythm with resting heart rates ≥75 bpm, in combination with guideline-adherent pharmacotherapy [19].
In light of the increasing amount of data in favor of rigorous heart rate reduction and the just recently expanded indication for ivabradine, the question arises as to which proportion of CHF patients have increased heart rates despite individually optimized beta-blocker therapy and may potentially benefit from ivabradine therapy adjuvant to beta-blockers. The aim of this study was first to evaluate the use of beta-blockers in CHF and the extent of heart rate reduction achieved in clinical practice of a specialized outpatient CHF-clinic of a university hospital. Secondly, in accordance to the threshold heart rates detected in the SHIFT study, to determine differences in outcome of patients who fulfilled select inclusion criteria of the SHIFT study with resting heart rates ≥75 bpm compared to those <75 and ≥70 bpm compared to those <70 bpm.
Methods
Study population
In this present analysis, we evaluated an all-comer population of our dedicated CHF outpatient clinic between 2006 and 2010. For inclusion, individually optimized doses of guideline-recommended pharmacotherapy including beta-blockers had to be maintained for at least 3 months and routine follow-up performed at our outpatient CHF-clinic thereafter. Individually optimized therapy implied up titration of drug classes to target doses according to published guidelines as far as tolerated by the patient. Patients with acute coronary syndrome and those receiving ivabradine during initial evaluation were excluded from the analysis. As this was an otherwise all-comer CHF population, further co-morbidities or concomitant medical therapies did not constitute exclusion criteria.
Patients were assessed in regard to treatment dosages of beta-blockers, and demographic and clinical profiles including resting heart rate. Outcome of patients who fulfilled select inclusion criteria of the SHIFT study (LVEF ≤35 %, sinus rhythm, NYHA II–IV) and were followed-up for at least 1 year was stratified according to resting heart rates: ≥75 versus <75 bpm and ≥70 versus <70 bpm.
The study conformed to the principles outlined in the 1964 Declaration of Helsinki. Ethics committee approval for the study and patient informed consent were achieved before inclusion in the study.
Data collection
Data were collected prospectively with the diagnosis of CHF based on European Society for Cardiology guidelines. The etiology of CHF was categorized as ischemic on the basis of a history of MI or the findings in coronary angiography. Other etiologies were defined according to the WHO-Definition of Cardiomyopathies [20]. The stage of clinical symptoms was determined according to NYHA classification. Pharmacotherapy and the respective daily doses recorded were those prescribed to the patient after individual optimization of therapy over a period of at least 6 weeks. Heart rate was measured by using a 12-lead-ECG with respect to the guidelines in ECG diagnostics. General information about patient characteristics, cardiac history, as well as specific data deriving from current investigations (ECG, echocardiography and others) and laboratory measurements were collected during the index visit. Venous blood samples were taken in the morning after overnight fasting. Left-ventricular systolic function was determined by the LVEF through the biplane disc summation method (Simpson rule) in echocardiography. The enrolled patients were followed prospectively for at least 1 year, then generally for an unlimited time period. Follow-up information on vital status, clinical events and medical treatment was obtained using standardized case report forms mostly during re-examinations at our clinic or at least by telephone calls including standardized questionnaires on a yearly basis. Endpoint information about hospitalization and death were obtained from treating physicians or hospital medical records.
Statistical analysis and outcome measures
Patients’ characteristics are presented as number of patients and percentages, median and quartiles, or mean and standard deviation, as appropriate. Descriptive data of patients who fulfilled the above mentioned select criteria of the SHIFT trial were stratified by heart rate (1) ≥75 versus <75 bpm and (2) ≥70 versus <70 bpm. The respective groups were compared using Chi-square test or Fisher’s exact test, and Student′s t test or Mann–Whitney U test, as appropriate. The composite of death from any cause or hospital admission for worsening heart failure during 12-month follow-up constituted the primary endpoint of this analysis. All-cause death and heart transplantation or all-cause death after the index visit were defined as secondary endpoints. Unadjusted 5-year cumulative event-free survival of death of any cause or hospital admission for worsening heart failure of the prespecified cohort were estimated by the Kaplan–Meier method and compared by the log-rank test. For all endpoint analyses, all patients receiving cardiac transplantation (independent of their urgency status) were followed until their surgical procedure and then censored. All tests were two-tailed and a p < 5 % was considered to indicate statistical significance. Statistical analysis was performed with IBM SPSS version 20 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics according to resting heart rate
In total, 3,181 patients were assessed in regard to treatment dosages of beta-blockers, and demographic and clinical profiles including resting heart rate. Of the overall studied population, 622 (20 %) fulfilled select criteria adopted from the randomized SHIFT trial (LVEF ≤35 %, sinus rhythm, NYHA II–IV). Of these patients, 443 patients entered outcome analyses with complete follow-up of at least 1 year. Table 1 illustrates characteristics of the overall cohort of patients receiving individually optimized pharmacotherapy for heart failure including beta-blockers. Detailed characteristics of study patients who fulfilled inclusion criteria for outcome analysis are displayed in Table 2 according to categories of heart rate. The distribution of resting heart rate is presented in Fig. 1. Patients with heart rates ≥70 bpm were younger and more often male. They exhibited a significantly higher NYHA functional class and lower LVEF. There was a trend to a higher percentage of significant renal insufficiency (4 % in the group with heart rates ≥70 bpm vs. 1 % in the group with heart rates <70 bpm) which narrowly missed the significance threshold (p = 0.06). When compared with patients with heart rates ≥70 bpm, more patients with lower heart rates were up titrated to at least half the evidence-based target dose of beta-blockers. Further, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers were less often and diuretics more often prescribed in this cohort. Characteristics of patients and guideline-adherent medical therapy in the ≥75 bpm group were similarly distributed, with a significantly higher proportion of patients with concomitant renal insufficiency compared to those in the <75 bpm group.
Outcome of patients fulfilling select criteria of the SHIFT study
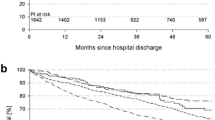
Median observation time of survivors was 27.5 months (quartiles 17.4–41.9 months) with 1,039.7 observation-years in total. For the composite primary endpoint of all-cause death or hospital admission for worsening heart failure during 12-month follow-up, a significant difference between patients with lower and higher heart rates was observed. The primary endpoint occurred in 21 % of patients the ≥70 bpm group versus 9 % of patients in the group with heart rates <70 bpm (p < 0.01). Likewise, comparing the groups ≥75 and <75 bpm, the primary endpoint was significantly increased in the group of patients with heart rates ≥75 bpm 27 % versus 12 %; p < 0.01). Further details of primary and secondary outcome variables are summarized in Table 3. During the observation period of 5 years, 44 patients (9.9 %) were censored because of cardiac transplantation. A 5-year unadjusted cumulative event-free survival of death of any cause or hospital admission for worsening heart failure was closely related to patients′ resting heart rate. Long-term survival was significantly lower among patients with heart rates ≥70 bpm as compared to those with <70 bpm (log-rank test p < 0.05; Fig. 2a) and among patients in the ≥75 bpm group versus <75 bpm group (log-rank test p < 0.01; Fig. 2b).
a, b Kaplan–Meier 5-year event-free survival curves according to resting heart rate. a Heart rate ≥70 bpm versus <70 bpm. Endpoint all-cause death any cause or hospital admission for worsening heart failure. Survival curves not adjusted. 5-year event-free survival was significantly lower among patients with heart rates ≥70 bpm (log-rank test p < 0.05). b Heart rate ≥75 versus <75 bpm. Endpoint all-cause death any cause or hospital admission for worsening heart failure. Survival curves not adjusted. 5-year event-free survival was significantly lower among patients with heart rates rate ≥75 bpm (log-rank test p < 0.01)
Discussion
Apart from demonstrating the beneficial impact of ivabradine therapy in CHF, the results of the randomized SHIFT trial have lead to a greater understanding of variances in cardiovascular risk of CHF patients according to resting heart rate [18]. Prior to this, the advantage of heart rate reduction by beta-blockers had been brought forward [21, 22]. In 2009, McAlister et al. [23] performed a meta-analysis of randomized, placebo-controlled heart failure trials that examined survival benefits of beta-blockers in CHF. They concluded that the extent to which heart rate reduction is achieved by beta blockade is significantly associated with the survival benefit of beta-blockers in CHF. Moreover, the impact of adequate heart rate reduction seems to be more prominent than the effect achieved by beta-blocker dose [23–25]. Although these and other analyses have revealed a convincing linkage between heart rate lowering drugs, primarily beta-blockers, and their evident impact on clinical outcome, surveys and observational studies have shown that outside of randomized drug trials, guideline-recommended prescription and up titration of beta-blockers to target dose as well as the consideration of their heart rate modifying effect seem to represent a challenge in daily clinical practice [26–29]. Exemplarily, one of the main topics studied in the Heart Failure Pilot Survey was the overall use of guideline-adherent pharmacological therapy between 2009 and 2010 across Europe. Beta-blockers were prescribed in a relatively high percentage of CHF patients (87 %). The increase of beta-blocker prescription in comparison to data of the earlier EuroHeart Failure survey, in which the rate of prescribed beta-blockers was only 37 %, represents a major step forward. However, only 21–37 % of patients were able to achieve the respective target dose of beta-blocker [26, 29]. Even in landmark beta-blocker studies, which demonstrated tolerability of target dose in up to 80 % of study patients, a substantial percentage of these patients were unable to maintain the recommended beta-blocker dose over a long period of time [5, 30–32]. Based on the current state of knowledge, whether up titration to target dose in an all-comer population of CHF unconditionally translates into a significant survival benefit over low-to-moderate doses remains debatable.
Our present study provides information on the resting heart rate achieved with individualized optimized CHF medication including beta-blockers in clinical practice of a specialized outpatient CHF-clinic of a university hospital by revealing the significant proportion of CHF patients in sinus rhythm with insufficient heart rate control.
Despite treatment at our dedicated CHF outpatient clinic, less than a third of all patients (29 %) who fulfilled select inclusion criteria of the SHIFT trial achieved full beta-blocker target doses. Though the percentage of high beta-blocker doses prescribed in our study is relatively high as compared to earlier surveys and community-based samples [26, 29, 33, 34], in more than half (53 %) the patients in this prespecified cohort, heart rates remained increased, despite the circumstance that almost 70 % of all studied patients tolerated at least 50 % of target beta-blocker dose. This observation underlines the urgent need for complementary therapeutic strategies to reduce heart rate even in a dedicated environment such as a specialized heart failure clinic.
When compared with patients with lower heart rates, patients with increased heart rates more often achieved only low doses of beta-blockers. Though it may seem intuitive to at least partially explain the association between heart rate and outcome simply by lower beta-blocker doses in those with higher heart rates, large randomized trials on metoprolol and bisoprolol have failed to prove superiority of high versus moderate to low beta-blocker doses [25, 35]. In the specific case of the metoprolol CR/XL Randomized Intervention Trial (MERIT-HF) heart rate reduction ≤70 bpm was even more quickly and more often achieved in patients who did not tolerate the targeted daily dose of 100 mg of metoprolol succinate. Indeed, these results support the notion that our observations cannot simply be linked to general dose–effect relationships alone. Instead, a certain proportion of the effect is most likely driven by individual dose-related adverse effects, patients′ co-morbidities and their genetic disposition to beta-blocker response. We found a lower event rate of primary and secondary endpoints as well as a profound survival benefit in five-year outcome in patients with lower heart rates, both when dividing the cohort at a threshold heart rate of 70 or 75 bpm. Despite the non-randomized nature of our data, these results affirm the evidence of previously published studies showing that increased heart rate is a true risk factor in CHF which seems to be modifiable through beta-blockers to some extent. Our analyses underline the necessity of rigorous heart rate control, even in patients considered to be under optimal pharmacotherapy.
Limitations
This present cohort study carries the characteristic shortcomings of a registry. The value of our registry approach lies its all-comer population of CHF patients, including those who for diverse reasons, would have been excluded by conventional trial protocols. However, to simplify comparisons, for this analysis we only studied outcome in patients who fulfilled select inclusion criteria of SHIFT trial but were not receiving ivabradine. In line with these criteria, we did not exclude patients receiving other drugs with heart rate lowering effects such as digitalis or non-dihydropyridine calcium channel blockers. The applied registry approach only considered the use of cardiac resynchronization therapy (CRT) without further details on serial CRT device interrogations. Thus, this strategy allows no evaluation of the percentage of atrial pacing in the respective patients. High percentages of atrial pacing would limit the validity of our results in patients with CRT devices. Furthermore, we present crude survival curves, without adjustments for differences found in baseline characteristics which may have had effects on outcome. Before determining resting heart rates, we allowed for a relatively long period of up titration and maintained of therapy to guarantee individual optimization. By this, we attempted to achieve the maximal tolerated beta-blocker dose, including patients in whom up titration was particularly complicated. Overlooking available data on heart failure management, concomitant use of telemedical monitoring during the phase of beta-blocker up titration may have been beneficial to ensure that further dose escalation was not achievable. Similar to the majority of trials assessing the clinical effect of drug therapy, we analyzed the doses of pharmacotherapy prescribed by physicians at our institution. The fact that we were unable to take patients′ compliance to therapy or consistency of intake into account and did not review individual reasons for not taking beta-blocker target doses may constitute potential limitations.
Conclusion
In conclusion, this single-center experience of a specialized outpatient CHF clinic demonstrates that the extent of beta-blocker induced heart rate reduction in clinical practice is not satisfactory. Currently, a substantial proportion of patients who fulfill select inclusion criteria of the SHIFT study (LVEF ≤35 %, sinus rhythm, NYHA II–IV) have inadequate heart rate control despite maximal tolerated beta-blocker doses. In this non-randomized cohort, adequate heart rate control under individually optimized beta-blocker therapy was associated with improved mid- and long-term clinical outcome up to 5 years. As further up titration of beta-blockers is most likely not achievable in many patients, the administration of a selective heart rate lowering agent such as ivabradine adjuvant to beta-blockers may pose an opportunity to further modulate outcome in CHF.
References
Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR (2000) Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol 36(7):2072–2080 (pii:S0735-1097(00)01006-8)
Eichhorn EJ, Heesch CM, Barnett JH, Alvarez LG, Fass SM, Grayburn PA, Hatfield BA, Marcoux LG, Malloy CR (1994) Effect of metoprolol on myocardial function and energetics in patients with nonischemic dilated cardiomyopathy: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 24(5):1310–1320 (pii:0735-1097(94)90114-7)
Zugck C, Haunstetter A, Kruger C, Kell R, Schellberg D, Kubler W, Haass M (2002) Impact of beta-blocker treatment on the prognostic value of currently used risk predictors in congestive heart failure. J Am Coll Cardiol 39(10):1615–1622 (pii:S0735109702018405)
Sin DD, McAlister FA (2002) The effects of beta-blockers on morbidity and mortality in a population-based cohort of 11,942 elderly patients with heart failure. Am J Med 113(8):650–656 (pii:S0002934302013463)
Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 106(17):2194–2199
Jost A, Rauch B, Hochadel M, Winkler R, Schneider S, Jacobs M, Kilkowski C, Kilkowski A, Lorenz H, Muth K, Zugck C, Remppis A, Haass M, Senges J (2005) Beta-blocker treatment of chronic systolic heart failure improves prognosis even in patients meeting one or more exclusion criteria of the MERIT-HF study. Eur Heart J 26(24):2689–2697. doi:10.1093/eurheartj/ehi4733
Foody JM, Farrell MH, Krumholz HM (2002) beta-Blocker therapy in heart failure: scientific review. JAMA 287(7):883–889 (pii:jsr10002)
Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Levy S, Linde C, Lopez-Sendon JL, Nieminen MS, Pierard L, Remme WJ (2005) Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 26(11):1115–1140. doi:10.1093/eurheartj/ehi204
Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B (2005) ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 112(12):e154–e235. doi:10.1161/CIRCULATIONAHA.105.167586
Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW (2009) 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 53(15):e1–e90. doi:10.1016/j.jacc.2008.11.013
Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL (2008) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 29(19):2388–2442. doi:10.1093/eurheartj/ehn309
Komajda M, Lapuerta P, Hermans N, Gonzalez-Juanatey JR, van Veldhuisen DJ, Erdmann E, Tavazzi L, Poole-Wilson P, Le Pen C (2005) Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J 26(16):1653–1659. doi:10.1093/eurheartj/ehi251
Stork S, Hense HW, Zentgraf C, Uebelacker I, Jahns R, Ertl G, Angermann CE (2008) Pharmacotherapy according to treatment guidelines is associated with lower mortality in a community-based sample of patients with chronic heart failure: a prospective cohort study. Eur J Heart Fail 10(12):1236–1245. doi:10.1016/j.ejheart.2008.09.008
Franke J, Zugck C, Wolter JS, Frankenstein L, Hochadel M, Ehlermann P, Winkler R, Nelles M, Zahn R, Katus HA, Senges J (2012) A decade of developments in chronic heart failure treatment: a comparison of therapy and outcome in a secondary and tertiary hospital setting. Clin Res Cardiol 101(1):1–10. doi:10.1007/s00392-011-0348-6
Reil JC, Custodis F, Swedberg K, Komajda M, Borer JS, Ford I, Tavazzi L, Laufs U, Bohm M (2011) Heart rate reduction in cardiovascular disease and therapy. Clin Res Cardiol 100(1):11–19. doi:10.1007/s00392-010-0207-x
Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M (2007) Resting heart rate in cardiovascular disease. J Am Coll Cardiol 50(9):823–830. doi:10.1016/j.jacc.2007.04.079
Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L (2010) Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L (2010) Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 376(9744):886–894. doi:10.1016/S0140-6736(10)61259-7
Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 93(5):841–842
Metra M, Torp-Pedersen C, Swedberg K, Cleland JG, Di Lenarda A, Komajda M, Remme WJ, Lutiger B, Scherhag A, Lukas MA, Charlesworth A, Poole-Wilson PA (2005) Influence of heart rate, blood pressure, and beta-blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J 26(21):2259–2268. doi:10.1093/eurheartj/ehi386
Lechat P, Hulot JS, Escolano S, Mallet A, Leizorovicz A, Werhlen-Grandjean M, Pochmalicki G, Dargie H (2001) Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation 103(10):1428–1433
McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW (2009) Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med 150(11):784–794 (pii:150/11/784)
Porapakkham P, Krum H (2010) Is target dose of beta-blocker more important than achieved heart rate or heart rate change in patients with systolic chronic heart failure? Cardiovasc Ther 28(2):93–100. doi:10.1111/j.1755-5922.2010.00136.x
Wikstrand J, Hjalmarson A, Waagstein F, Fagerberg B, Goldstein S, Kjekshus J, Wedel H (2002) Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure (MERIT-HF). J Am Coll Cardiol 40(3):491–498 (pii:S0735109702019708)
Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors A, Nielsen OW, Zannad F, Tavazzi L (2010) EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail 12(10):1076–1084. doi:10.1093/eurjhf/hfq154
de Groote P, Isnard R, Assyag P, Clerson P, Ducardonnet A, Galinier M, Jondeau G, Leurs I, Thebaut JF, Komajda M (2007) Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur J Heart Fail 9(12):1205–1211. doi:10.1016/j.ejheart.2007.09.008
Fonarow GC, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, Mehra M, O’Connor CM, Reynolds D, Walsh MN (2007) Improving the use of evidence-based heart failure therapies in the outpatient setting: the IMPROVE HF performance improvement registry. Am Heart J 154(1):12–38. doi:10.1016/j.ahj.2007.03.030
Komajda M, Follath F, Swedberg K, Cleland J, Aguilar JC, Cohen-Solal A, Dietz R, Gavazzi A, Van Gilst WH, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, Widimsky J, Freemantle N, Eastaugh J, Mason J (2003) The EuroHeart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J 24(5):464–474 (pii:S0195668X02007005)
Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) (1999). Lancet 353 (9169):2001–2007 (pii:S0140673699044402)
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial (1999). Lancet 353 (9146):9–13 (pii:S0140673698111819)
Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler–Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker SD, Thompson SG, Poole-Wilson PA (2005) Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 26(3):215–225. doi:10.1093/eurheartj/ehi115
Gheorghiade M, Albert NM, Curtis AB, Thomas Heywood J, McBride ML, Inge PJ, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW, Fonarow GC (2012) Medication dosing in outpatients with heart failure after implementation of a practice-based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail 18(1):9–17. doi:10.1111/j.1751-7133.2011.00250.x
Asghar H, Rahko PS (2010) Quality of heart failure management: a comparison of care between a comprehensive heart failure program and a general cardiology practice. Congest Heart Fail 16(2):65–70. doi:10.1111/j.1751-7133.2009.00136.x
Simon T, Mary-Krause M, Funck-Brentano C, Lechat P, Jaillon P (2003) Bisoprolol dose-response relationship in patients with congestive heart failure: a subgroup analysis in the cardiac insufficiency bisoprolol study(CIBIS II). Eur Heart J 24(6):552–559 (pii:S0195668X02007431)
Conflict of interest
C.Z. has received speaker′s honoraria from SHL Telemedizin, Servier, Novartis, Pfizer and AstraZenica. All other authors have declared no conflicts related to this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Franke, J., Wolter, J.S., Meme, L. et al. Optimization of pharmacotherapy in chronic heart failure: is heart rate adequately addressed?. Clin Res Cardiol 102, 23–31 (2013). https://doi.org/10.1007/s00392-012-0489-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-012-0489-2