Abstract
Background
Buerger’s disease often shows poor collateral artery generation (i.e. neovascularization) in the ischemic limbs. However, the etiology has not yet been clarified. Circulating endothelial progenitor cells (EPCs) derived from bone marrow contribute to neovascularization in the multi-step process which includes the following capacities; mobilization, differentiation, adhesion, migration, invasion and secretion.
Materials and methods
We assessed EPCs capacities in vitro and ex vivo in age- and sex-matched controls (n = 12) and patients with Buerger’s disease (n = 12), derived from peripheral blood-derived mononuclear cells (PB-MNCs).
Results
In the flow cytometry analysis, the numbers of circulating EPC (CD34+/KDR+ or CD133+/KDR+ PB-MNC) were similar between controls and patients with Buerger’s disease. Next, we cultured PB-MNC to obtain EPCs. The number of early outgrowth EPCs was significantly decreased in patients with Buerger’s disease (p < 0.005), indicating the reduced generation of early outgrowth EPCs in Buerger’s disease. However, adhesion, migration, invasion and secretion capacities were not impaired in patients with Buerger’s disease.
Conclusions
The early outgrowth EPCs generation is reduced in patients with Buerger’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Buerger’s disease, also known as thromboangiitis obliterans, is a non-atherosclerotic segmental inflammatory disease that typically occurs in young male smokers [1]. It was first described in 1879 [2], and its detailed pathological findings were initially reported by Leo Buerger in 1908 [3]. Occlusive change of the peripheral arteries in the fore- and hindlimbs induces chill and intermittent claudication as the initial symptoms, and thereafter ischemic rest pain and skin ulcers often additionally occur. Although the generation of collateral arteries (i.e. neovascularization) is a physiological process to salvage critical tissue ischemia, neovascularization in Buerger’s disease is generally poor. The progression of Buerger’s disease often leads to limb amputation, and therefore the quality of life in the patient remarkably decreases. It is generally known that exposure to tobacco induces the progression of Buerger’s disease; however, the pathological etiology has not yet been clarified.
Asahara et al. previously reported that postnatal neovascularization is induced by not only the sprouting from pre-existing vessels (i.e. angiogenesis) [4–6], but also by the generation of new vessels by the recruitment of endothelial progenitor cells (EPCs) that are derived from bone marrow (i.e. vasculogenesis) [7–9]. Circulating EPCs in the peripheral blood accumulate in ischemic tissue, and work in concert with the existing mature endothelial cells (ECs), thereby generating new arteries in ischemic tissues. The neovascularization process is considered to progress by the following steps: (1) mobilization from the bone marrow to the circulation and then to ischemic tissues, (2) chemotaxis and adhesion to mature ECs, (3) (trans-) migration and invasion of the intracellular space in adjacent ECs, (4) in situ differentiation to ECs and (5) the secretion of neovascularization-related cytokines to stimulate neovascularization by sprouting new capillaries from pre-existing arteries [10].
The number of circulating EPCs and the function have been reportedly decreased in patients having atherosclerotic risk factors [11–13] and patients with cardiovascular diseases [14–16], suggesting that the malfunction of EPCs contributes to impaired neovascularization in patients with atherosclerotic obliterance. In this study, we assessed the neovascularization-related capacities (mobilization, adhesion, migration, invasion and secretion) of EPCs in patients with Buerger’s disease.
Materials and methods
Study population
We collected peripheral blood via venous puncture from age- and sex-matched control subjects (n = 12) and patients with Buerger’s disease (n = 12; 6 in Fontaine class II and 6 in class IV). Buerger’s disease was diagnosed by the diagnostic criteria proposed by the committee for Buerger’s disease of Japanese Ministry of Health and welfare, which was similar to that of Shionoya [17] and Olin [1]. All non-smokers in healthy controls and in patients with Buerger’s disease quitted smoking more than 1 month before the collections of blood samples. This study was approved by the Committees on the Ethics Review Board of the Kurume University School of Medicine, and written informed consent was obtained from all subjects.
Cell culture
Peripheral blood-derived mononuclear cells (PB-MNCs) were isolated from a total of 30 ml peripheral blood by density gradient centrifugation with Ficoll (Ficoll-Paque PLUS; GE Healthcare Bio-Sciences AB, Sweden). PB-MNCs were re-suspended in endothelial basal medium (EBM-2; Clonetics, San Diego, CA, USA) with supplements (0.1% hEGF, 0.4% hFGF-B, 0.1% VEGF, 0.1% ascorbic acid, 0.04% hydrocortisone, 0.1% long R3-IGF-1, 0.1% heparin, 0.1% gentamicin, 0.1% amphotericin and 20% FBS) and seeded onto a six-well tissue plate coated with fibronectin (Sigma, St. Louis, MO, USA) to culture them for the following 4 days at 37°C, 5% CO2, in a humidified incubator.
Flow cytometric analysis
Because circulating EPCs are mobilized from the bone marrow into the peripheral circulation [8, 9], we calculated the ratio of circulating EPCs in PB-MNCs to evaluate the mobilization capacity of EPC [8, 9]. To identify circulating EPCs in peripheral blood, we adopted the characteristic cell-surface antigens, CD34 (PE-labeled mouse antibody; BD Biosciences Pharmingen, San Jose, CA, USA), CD133 (PE-labeled mouse antibody; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), KDR (VEGFR2) (biotin-conjugated mouse antibody; Sigma, St. Louis, MO, USA, SAv-FITC conjugate; BD Biosciences, San Jose, CA, USA) [8, 10] that are recognized as the specific cell-surface markers of circulating EPC. After the incubation of PB-MNCs with the above antibodies, samples were analyzed by fluorescence-activated cell sorting (FACS) (Becton-Dickinson, San Diego, CA, USA). CD34+KDR+ or CD133+KDR+ PB-MNCs were defined as circulating EPCs [8, 10, 18]. Then to identify whether the cultured cells were EPCs, we checked the expressions of endothelial lineage markers on the surface of the cells. We used healthy human umbilical vein ECs as positive controls. After detaching the cells with trypsin (TrypLE™ Express, GIBCO Invitrogen, Carlsbad, CA, USA) from the culture plates, we incubated them with the monoclonal antibodies of CD31 (PE-labeled mouse antibody; BD Biosciences, San Jose, CA, USA) and KDR (biotin-conjugated mouse antibody; Sigma, St. Louis, MO, USA, SAv-FITC conjugate; BD Biosciences, San Jose, CA, USA) and analyzed the expressions with FACS.
Generation of early outgrowth EPCs
After 4 days of PB-MNCs culture, we washed the adherent cells on the culture plate with PBS and incubated them with Dil-AcLDL (Biomedical Technologies Inc., Stoughton, MA, USA) for 1 h. Then, we fixed them with 2% paraformaldehyde and counterstained with FITC-labeled lectin from Ulex europaeus (VECTOR Laboratories Inc., Burlingame, CA, USA) and DAPI (Dojindo Molecular Technologies Inc., Kumamoto, Japan). The adherent cells that were stained by all dyes of Dil-AcLDL, lectin and DAPI were defined as early outgrowth EPCs [19], which were generated from PB-MNCs. After detaching the adherent cells with trypsin, we counted them and calculated the generation ratio by dividing the number of early outgrowth EPCs with the number of PB-MNCs that were seeded at the first time.
Adhesion assay
We performed cell-matrix adhesion assay [20]. In brief, after washing and detaching of early outgrowth EPCs on the culture plate at day 4 of the culture, the cells were labeled with fluorescent green reagent (CellTracker™ Green CNFDA; Molecular probes Inc., Eugene, Oregon, USA) and plated on a fibronectin (10 μg/ml)-coated dish. After 40 min of the incubation at 37°C, 5% CO2, in a humidified incubator, we washed out the non-adherent cells and manually counted the adherent cells in three randomly selected high-power fields under a fluorescent microscope.
Migration and invasion assay
We used modified-Boyden chamber assay to evaluate the migration and invasion capacities of early outgrowth EPCs. After washing and detaching of the EPCs at day 4 of the culture, a total of 1 × 105 EPCs were re-suspended in 500 μl EBM-2 medium and placed onto the upper chamber whose bottom was made with 8-μm pore size PET membrane coated with an extracellular matrix, Matrigel (BD BioCoat™ Matrigel™ invasion chamber, BD Biosciences, San Jose, CA, USA) to test the invasion capacity. In migration assay, we used another upper chamber in absence of Matrigel coating. Each upper chamber was placed onto a 24-well culture dish that was filled by 500 μl EBM-2 medium with 20% fatal bovine serum and VEGF (50 ng/ml rhVEGF; R&D Systems Inc., Minneapolis, MN, USA). After 24 h of the incubation at 37°C, the transmigrated cells into the lower chamber via PET membrane of the upper chamber were fixed with 2% paraformaldehyde and counterstained with DAPI. Then, we counted the transmigrated cell number in three randomly selected high-power fields under fluorescent microscope.
Cytokines in culture medium and peripheral blood
To evaluate secretion capacities of early outgrowth EPCs, we measured the concentration of neovascularization-related cytokines [21, 22] that were secreted from cultured EPCs into the culture medium. The concentration of VEGF, b-FGF, PDGF-BB, IGF-1, angiopoietin 1, angiopietin 2 and SDF-1α in the supernatant of culture medium without supplements were measured after 4 days of the cell culture using a commercially available high-sensitivity enzyme-linked immunosorbent assay (ELISA) system (Amersham Pharmacia Biotech, Little Chalfont, UK). In the peripheral blood, serum levels of VEGF, interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-8 (IL-8), angiopoietin 1, angiopietin 2, SDF-1α, TGF-1α, VCAM-1 and MCP-1 were also measured by ELISA according to the instructions of the manufacturer.
Statistical analysis
Continuous variables are presented as mean ± SEM. Statistical comparisons between control subjects and patients with Buerger’s disease were analyzed by t test (two-sided). The comparisons of categorical variables were generated by the Pearson χ2 test. Statistical correlation between the percentage of circulating EPC and the concentration of high-sensitivity C-reactive protein (hs-CRP) or IL-6 in peripheral blood were assessed by univariate analysis. The serum concentrations of hs-CRP and IL-6 were further assessed by a multiple linear and stepwise regression analyses. Statistical significance was assumed at a value of p < 0.05. All analyses were performed with SPSS 14.0 software.
Results
The baseline characteristics of control subjects and patients with Buerger’s disease are shown in Table 1. The baseline characteristics were similar between control subjects and patients with Buerger’s disease except hs-CRP, which was significantly elevated in patients with Buerger’s disease (p < 0.005). Serum concentrations of neovascularization-related cytokines are shown in Table 2. IL-6 was significantly elevated in patients with Buerger’s disease (p < 0.05).
Number of circulating EPCs and capacities of EPCs
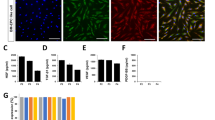
In the flowcytometric analysis, the percentage of CD133+/KDR+ PB-MNCs was similar between controls and patients with Buerger’s disease (Fig. 1a). The percentage of CD34+/KDR+ PB-MNCs was also similar in the two groups (Fig. 1a). These results indicate the preserved number of circulating EPCs and suggest the preserved mobilization capacity in patients with Buerger’s disease.
a The percentage of peripheral blood-derived mononuclear cells (PB-MNCs), expressing CD133+KDR+ or CD34+KDR+. In flow cytometric analysis, the percentages of circulating EPCs in patients with Buerger’s disease did not differ from those of age- and sex-matched controls. b Microscopic images of EPCs that were differentiated from PB-MNCs and adhered on fibronectin-coated plate after 4 days of a culture. The adherent EPCs were stained by the following three fluorescent colors: red Dil-acLDL incorporated into endothelial cell (EC), green Ulex-lectin binding to EC, blue DAPI staining nuclear. The adherent cell that was stained by all colors was defined as EPC. Scale bar 100 μm. c FACS analysis for the EPCs cultured from PB-MNCs in controls (C-EPC) and patients with Buerger’s disease (B-EPC). Gray color histograms indicate isotype-matched IgG controls. Both C-EPC and B-EPC similarly expressed CD31 and KDR, which were surface markers of endothelial cell, on the black color histograms. The same expression patterns were observed in three experiments. d Representative photos of Dil-acLDL-positive EPCs that were cultured from PB-MNCs in controls and patients with Buerger’s disease. The number of EPCs appeared smaller in a patient with Buerger’s disease than in a control. e The percentage of ex vivo generation of early outgrowth EPCs after 4 days of PB-MNCs culture. The early outgrowth EPCs generation was significantly reduced in Buerger’s disease (*p < 0.005 vs. controls). f–h For the adhesion, migration and invasion capacities of EPCs, no significant differences were observed between controls and patients with Buerger’s disease
After 4 days of PB-MNCs culture, all adherent cells on the culture plate were stained by three fluorescent dyes, Dil-acLDL, lectin and DAPI in all fields of view under fluorescence microscopy (Fig. 1b). In flow cytometric analysis, the adherent cells were positively expressed CD31 and KDR as endothelial lineage markers. The expression patterns were similar between controls and patients with Buerger’s disease (Fig. 1c). Taken together, we considered that the adherent cells were early outgrowth EPCs [12, 19, 23]. In patients with Buerger’s disease, the number of early outgrowth EPCs on each culture plate appeared smaller than that of the controls (Fig. 1d). In fact, the percentage of ex vivo EPC generation was significantly smaller (p < 0.005) in patients with Buerger’s disease (Fig. 1e). These results suggest that early outgrowth EPCs generation is reduced in Buerger’s disease. In the adhesion assay, the number of adherent cells on the fibronectin-coated well was similar between controls and patients with Buerger’s disease (Fig. 1f). Likewise, the migration (Fig. 1g) and invasion (Fig. 1h) capacities were similar between the two groups. In the ELISA assay, all the concentrations of VEGF, b-FGF, PDGF-BB, IGF-1, angiopoietin 1, angiopietin 2 and SDF-1α in the culture medium were similar between controls and patients with Buerger’s disease (Table 3).
Serum concentrations of hs-CRP and IL-6 were significantly increased in patients with Buerger’s disease. In a multiple linear regression analysis, the concentration of hs-CRP was significantly correlated with the concentration of IL-6 in serum (p < 0.05) and b-FGF in culture medium (p < 0.05). The concentration of IL-6 significantly was correlated with hyperlipidemia (p < 0.001), hs-CRP (p < 0.01) or adhesion capacity of early outgrowth EPCs (p < 0.05). By the use of multiple stepwise regression analysis, serum hs-CRP and IL-6 concentration was significantly related to IL-6 (p < 0.05) and hyperlipidemia (p < 0.001), respectively.
There were six patients in Fontaine class II and six in class IV in this study. The capacities of early outgrowth EPCs, patient characteristics and serum concentrations of neovascularization-related cytokines were not different between the two groups (data not shown). The migration and invasion capacities were significantly impaired in four current smokers of patients with Buerger’s disease (p < 0.05 vs. 4 current non-smokers in patients with Buerger’s disease, respectively), however; we were not able to draw any conclusion from the results because the number of smokers was too small.
Discussion
To assess the neovascularization-related capacities of early outgrowth EPCs in patients with Buerger’s disease, we examined the capacities in the multi-step process. The number of circulating EPCs in peripheral blood was similar between control subjects and patients with Buerger’s disease, however; the early outgrowth EPCs generation was reduced in Buerger’s disease.
Although it was previously reported in patients with coronary artery disease that the number of circulating EPCs was decreased [15, 16] and migration capacity was impaired [14] reports examining the number of circulating EPCs and ex vivo neovascularization capacities of EPCs in patients with Buerger’s disease are very few. In this study, we counted the number of PB-MNCs expressing the specific cell-surface markers of circulating EPCs, CD34+KDR+ or CD133+KDR+ [10], which were probably mobilized from the bone marrow into the circulation [8, 9]. The number of circulating EPCs was similar between controls and patients with Buerger’s disease, indicating the preserved mobilization capacity of EPCs in Buerger’s disease. Although the other functions, such as adhesion, migration, invasion and secretion capacities were similar to those of controls, only the generation of early outgrowth EPCs was reduced in patients with Buerger’s disease. Nishioka et al. [24] have previously reported that the number of circulating EPCs and migration capacity were not impaired in patients with Buerger’s disease, consisting with our results. It has been previously reported that EPCs number and functions were decreased in inflammatory diseases, such as rheumatoid arthritis [25, 26] and systemic lupus erythematosus [27, 28]. Although the reason is unclear at present, there may be several possibilities. First, hs-CRP was significantly higher than that of controls, but not so high when compared with these inflammatory diseases. Second, the early outgrowth EPCs in patients with Buerger’s disease might be less susceptible to inflammation than other inflammatory diseases.
The reason for the reduced generation of early outgrowth EPCs was not clarified in this study. Although it was reported that accumulation of risk factors for atherosclerosis impair functions of EPCs [11–14], the reduced generation of early outgrowth EPCs in Buerger’s disease is not likely due to differences in backgrounds because the number of the risk factors were similar between controls and patients with Buerger’s disease. Our results may be partially in compatible with those of Yamamoto et al. who have reported the decreased mRNA expressions of EPC-specific molecules in four patients with ischemic limbs, suggesting decreased angiogenic potentials in patients with peripheral artery occlusive disease [29]. Although we measured several kinds of angiogenic cytokines including VEGF, which play an important role in the differentiation of ECs [30–32], the concentrations of the cytokines secreted from EPCs into the culture medium were similar between controls and patients with Buerger’s disease. Some reported effects of hs-CRP and IL-6 on functions of EPCs [33, 34]. Accordingly, we examined the associations between EPCs functions and hs-CRP or IL-6. The serum concentration of hs-CRP and IL-6 was significantly elevated in patients with Buerger’s disease, however; the elevations did not correlate with the percentage of ex vivo generation of early outgrowth EPCs in a univariate analysis (data not shown).
Because smoking is major risk factor for Buerger’s disease [1], we examined the effects of smoking on the number of circulating EPCs and capacities of EPCs in controls and patients with Buerger’s disease. The migration and invasion capacities were significantly impaired only in current smokers with Buerger’s disease. We checked several neovascularization-related cytokines in the circulation and culture medium to clarify this mechanism, however; there were no relationships between the concentrations of these cytokines and functions of EPCs in patients with Burger’s disease (data not shown). Unfortunately, we did not test whether smoking cessation would recover the impaired migration and invasion capacities of the EPCs in patients with Buerger’s disease. Anyway, the number of smokers was too small to draw any conclusion from our results.
Limitations
This study had some limitations. First, the number of enrolled subjects was pretty small. Second, we assessed the several capacities of EPCs cultured from PB-MNCs not bone marrow-derived mononuclear cells (BM-MNCs). The results may be different when we used the EPCs cultured from BM-MNCs. Third, we assessed the several capacities of early, not late, outgrowth EPCs. Fourth, we did not determine colony forming units in early and late outgrowth EPCs. Fifth, we did not verify whether the reduced generation of early outgrowth EPCs in Buerger’s disease would lead to poor angiogenesis on ex vivo and/or in vivo experiment. In conclusion, the early outgrowth EPCs generation is reduced in patients with Buerger’s disease.
Abbreviations
- EPCs:
-
Endothelial progenitor cells
- PB-MNCs:
-
Peripheral blood-derived mononuclear cells
- TAO:
-
Thromboangiitis obliterans
- ECs:
-
Endothelial cells
- KDR:
-
Kinase domain receptor
- VEGF:
-
Vascular endothelial growth factor
- FACS:
-
Fluorescence-activated cell sorting
- HUVEC:
-
Human umbilical vein endothelial cell
- Dil-acLDL:
-
1,1′-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein
- DAPI:
-
4′,6-diamidino-2-phenylindole
- b-FGF:
-
Basic fibroblast growth factor
- PDGF-BB:
-
Platelet-derived growth factor BB
- IGF:
-
Insulin growth factor
- SDF-1α:
-
Stromal cell-derived factor-1α
- ELISA:
-
Enzyme-linked immunosorbent assay
- IL-1, -6, -8:
-
Interleukin-1, -6, -8
- TGF-1α:
-
Tumor growth factor-1α
- VCAM-1:
-
Vascular cell adhesion molecule-1
- MCP-1:
-
Monocyte chemotactic protein-1
- hs-CRP:
-
High-sensitivity C-reactive protein
- m-RNA:
-
Messenger ribonucleic acid
References
Olin JW (2000) Thromboangiitis obliterans (Buerger’s disease). N Engl J Med 343:864–869
von Winiwarter F (1879) Ueber eine eigenthumliche Form von Endarteriitis und Endophlebitis mit Gangran des Fusses. Arch Klin Chir 23:202–206
Buerger L (1908) Thrombo-angiitis obliterans: a study of the vascular lesions leading to presenile spontaneous gangrene. Am J Med Sci 136:567–580
Folkmann J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Risau W (2000) Mechanisms of angiogenesis. Nature 386:671–674
Cameliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–395
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A et al (1998) Evidence for circulating bone marrow-derived endothelial cells. Blood 92:362–367
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M et al (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95:343–353
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA et al (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348:593–600
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H et al (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89:E1–E7
Jung C, Fischer N, Fritzenwanger M, Thude H, Ferrari M, Fabris M et al (2009) Endothelial progenitor cells in adolescents: impact of overweight, age, smoking, sport and cytokines in younger age. Clin Res Cardiol 98:179–188
Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH et al (2004) Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 109:1615–1622
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A et al (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353:999–1007
Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U et al (2005) Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 111:2981–2987
Shionoya S (1998) Diagnostic criteria of Buerger’s disease. Int J Cardiol 66:S243–S245
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M et al (2000) Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 95:952–958
Hirschi KK, Ingram DA, Yoder MC (2008) Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28:1584–1595
Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR et al (2005) Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med 11:206–213
Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG et al (2001) Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci 938:36–45
Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M et al (2001) Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med 193:1005–1014
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM (2001) HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 108:391–397
Nishioka K, Higashi Y, Umemura T, Jituki D, Goto C, Nakamura S, et al. (2008) Vascular function and endothelial progenitor cells in thromboangiitis obliterans (Buerger’s disease). Circulation 118:S_635
Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D et al (2005) Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation 111:204–211
Herbrig K, Haensel S, Oelschlaegel U, Pistrosch F, Foerster S, Passauer J (2006) Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis 65:157–163
Moonen JR, de Leeuw K, van Seijen XJ, Kallenberg CG, van Luyn MJ, Bijl M et al (2007) Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Res Ther 9:R84
Ebner P, Picard F, Richter J, Darrelmann E, Schneider M, Strauer BE et al (2010) Accumulation of VEGFR-2+/CD133+ cells and decreased number and impaired functionality of CD34+/VEGFR-2+ cells in patients with SLE. Rheumatology (Oxford) 49:63–72
Yamamoto K, Kondo T, Suzuki S, Izawa H, Kobayashi M, Emi N et al (2004) Molecular evaluation of endothelial progenitor cells in patients with ischemic limbs: therapeutic effect by stem cell transplantation. Arterioscler Thromb Vasc Biol 24:e192–e196
Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS et al (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439–442
Fong GH, Klingensmith J, Wood CR, Rossant J, Breitman ML (1996) Regulation of flt-1 expression during mouse embryogenesis suggests a role in the establishment of vascular endothelium. Dev Dyn 207:1–10
Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML et al (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62–66
Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH et al (2004) C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation 109:2058–2067
Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W et al (2006) Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab 28:90–98
Acknowledgments
We thank to Kimiko Kimura in the cardiovascular research institute, Kurume University for her excellent technical supports in the flow cytometric analysis and ELISA. This study was supported by Research Grants from The Ministry of Education, Science and Culture, Research on Human Genome, Tissue Engineering Food Biotechnology, and the Ministry of Health, Labor and Welfare, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, Kaibara Morikazu Medical Science Promotion Foundation and Kimura Memorial Heart Foundation Research, Japan.
Conflict of interest statement
There is no conflict of interest or financial disclosure by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katsuki, Y., Sasaki, Ki., Toyama, Y. et al. Early outgrowth EPCs generation is reduced in patients with Buerger’s disease. Clin Res Cardiol 100, 21–27 (2011). https://doi.org/10.1007/s00392-010-0198-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-010-0198-7