Abstract
Background:
Human endothelial progenitor cells (EPCs) were first identified in the peripheral blood and later in the cord blood and bone marrow (BM) with different vascularization capacity and different surface marker profiles. However, their identity and functional roles in neovascularization have not been clearly demonstrated in vivo and in vitro.
Methods:
Characterization of BM-EPC like cells were performed by fluorescence-activated cell sorting, immunofluorescence staining, enzyme-linked immunosorbent assay, Matrigel tube formation assay, and western blot analysis.
Results:
BM-EPC like cells were identified by selective adhesion to fibronectin and collagen from BM mononuclear cells, which generate fast-growing colonies with spindle morphology, express surface markers of CD105, vWF, UEA-I lectin binding, secrete HGF, VEGF, TGF-beta1 but can be distinguished from circulating EPC and endothelial cells by no expression of surface markers such as CD31, CD309, CD45, and CD34. These BM-EPC like cells shared many cell surface markers of BM-mesenchymal stem cells (MSC) but also can be distinguished by their vasculogenic property and other unique surface markers. Furthermore, the vasculogenic capacity of BM-EPC like cells were enhanced by co-culture of BM-MSC or PDGF-BB priming. PDGF-BB stimulated cell migration, proliferation, and secretion of laminin β-1, which was proposed as one of the mechanisms involved in the better vascularization of BM-EPC like cells.
Conclusion
PDGF-BB priming may be applied to improve the potency and function of BM-EPC like cells as vasculogenic cell therapy for the ischemic vascular repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Endothelial progenitor cells (EPCs) have a critical role in neovascularization, angiogenesis, and tissue regeneration [1,2,3]. When tissue injury occurs, EPCs released from bone marrow (BM) may be mobilized into the injury site, where cytokines and growth factors such as stromal-derived growth factor-1α (SDF-1α), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF) are abundant [4,5,6]. At the damaged tissue, EPCs release abundant growth factors that promote tissue regeneration by augmenting differentiation, survival, migration, and proliferation of various cell types required for tissue regeneration [7, 8]. In addition, EPCs are the primary building block of new vessels and are able to differentiate into mature endothelial cells (ECs) with the aid of growth factors or cell–cell contact [4, 9]. The therapeutic potency of EPCs and their secretory molecules have been actively studied in vascular dysfunction-related diseases [10, 11].
In chronic diseases such as diabetes, hypertension, and aging, the function and homing ability of EPC are impaired [12,13,14]. This outcome is closely related to the high incidence of vascular diseases such as myocardial infarction, diabetic retinopathy, and critical limb ischemia [15,16,17]. Therefore, this pathological status limits the therapeutic potency of the clinical application of patients’ own EPC. Accordingly, applications of EPCs-derived from various origins, such as cord blood, peripheral blood, and induced pluripotent stem cell (iPSC) and ex vivo activation of patients’ own EPCs, have been attempted [18, 19]. Considering that EPCs and ECs are highly immunogenic and EPCs rapidly lose their cell proliferation capacity during ex vivo cell expansion [20], the application of cell priming technologies such as mechanical stretching and pretreatment of growth factors to enhance the cellular activity of patients’ own EPCs may be preferable for development of cell therapeutics.
EPCs are very heterogeneously defined in different tissue origins and different culture conditions. Among them, circulating EPC, mobilized by granulocyte-colony stimulating factor (G-CSF) treatment, has been classified as early EPC and late outgrowth EPC based on extracellular matrix (ECM) attachment and culture period [14, 21]. EPCs derived from the BM are not well defined, do not express many endothelial lineage specific markers, do not expand well, and change their phenotypes during ex vivo cell culture [22, 23]. Therefore, monitoring the cell phenotype of BM-EPCs in the cell expansion during repetitive subculture and functional characterization or cell priming mimicking the injury environment may be essential to advance the BM-EPC to the candidate cell therapeutics.
Platelet-derived growth factor (PDGF)-BB is a member of the PDGF growth factor family produced by platelets, pericytes, and some of activated endothelial cells [24]. PDGF-BB can bind to all PDGF receptors (PDGFR-α, PDGFR-β, and PDGFR-αβ), leading to PDGF receptor auto-phosphorylation, activation of the signaling pathway, and its internalization [24, 25]. The PDGF-BB-PDGFRβ signaling pathway effectively increases proliferation, migration, differentiation, and survival of various cell types [25,26,27]. Especially, PDGF-BB plays a vital role in recruiting pericytes in developing blood vessels [28]. However, PDGF-BB responsiveness is quite variable among EPCs and ECs from different origins and stages in differentiation, which means their high phenotypic plasticity [24, 29, 30]. Recently, it has been reported that EPCs overexpressing PDGFRβ promote vascular regeneration in carotid artery injury animal model [31]. Based on these studies, we hypothesized that BM-EPC priming with PDGF-BB at an early stage of the ex vivo cell expansion may retard its senescence and improve vascular regeneration.
In this study, we isolated human BM-EPC like cells as a colony forming cell and their phenotypic characteristics were observed at different passages. BM-EPC like cells themselves showed rather limited vasculogenic potential depending on microenvironment, which could be improved by priming with vasculogenic factors such as HGF, and PDGF-BB. Among them, PDGF-BB improved vessel forming ability and a possible mechanism underlying this PDGF-BB stimulation was explored in this study.
2 Materials and methods
2.1 Cell culture and characterization of BM-EPC like cells
Human BM mononuclear cells (MNCs) were purchased from STEMCELL Technologies Inc (Vancouver, Canada) and Lonza (Basel, Switzerland). For the culture of BM-EPC like cells, BM-MNCs (1 × 107 cells) were cultured in a 100 mm dish pre-coated with either fibronectin (Sigma, St. Louis, MO, USA) or type I collagen (Nitta Gelatin, Osaka, Japan) in endothelial growth media-2 (EGM-2, Lonza) with or without supplementation of 2% human platelet lysates (hPL, PL bioscience GmbH, German, Aachen). When cultured with hPL, FBS in the EGM-2 kit was excluded. The media was changed daily up to day 7, and every 3 days thereafter. The BM-EPC like cells were sub-cultured at 70 ~ 80% confluency. For mesenchymal stem cell (MSC) culture, BM-MNCs were cultured in a 100 mm dish in mesenchymal stem cell growth medium (MSCGM, Lonza). The media was changed every three days and the MSCs were sub-cultured at 70 ~ 80% confluency. More than 20 different donors of both men and women of different ages were cultured as described above, whose surface marker profiles and characteristics are the same. In this study, the data from BM-EPC like cells isolated from specific one donor was shown as a representative one but the same result was obtained in other donors under the similar experimental setting.
For immunofluorescence (IF) staining, BM-EPC like cells (1.0 × 104) were cultured on collagen type I coated coverslips, fixed with 3.7% formaldehyde in PBS, were permeabilized with 0.2% Triton x-100 buffer for 10 min, blocked in 20% normal goat serum. For Ulex europaeus agglutinin (UEA)-I lectin binding, fluorescein (FITC)-labeled UEA-I lectin (Sigma-Aldrich, St. Louis, MO, USA) was used. For IF staining, primary antibodies for Von Willebrand factor (vWF, Abcam, Cambridge, UK), PDGFRβ (Abcam), VEGFR2 (Abcam), Collagen type IV (Abcam), and Laminin (Santa Cruz, Santa Cruz, CA, USA) were used. Alexa fluor 488- or 568- conjugated secondary antibodies were used for the detection of primary antibodies. The coverslip was counterstained and mounted with mounting medium with DAPI (VECTOR, Burlingame, CA, USA). The images were obtained by fluorescent microscope (Leica, Wetzlar, Germany).
To confirm the expression level of cell surface markers, flow cytometry analysis was conducted. BM-EPC like cells were stained with FITC-conjugated UEA-I (VECTOR), phycoerythrin (PE) conjugated CD31, allophycocyanin (APC) conjugated CD309, APC conjugated CD105, FITC conjugated CD45 and PE conjugated CD34 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany) in 5% bovine serum albumin (BSA) in PBS. For vWF staining, BM-EPC like cells were fixed with methanol and permeabilized with 0.5% Tween20 in PBS. And the cells were incubated with Alexa Fluor 488-conjugated vWF antibody (Abcam). The cells were analyzed by Agilent flow cytometer and analyzed by NovoExpress software (Agilent, Santa Clara, CA, USA).
For conformation of TNF-α response of BM-EPC like cell, the cells (1.0 × 105) were cultured on collagen type I coated 6 well plates, incubated with 10 ng/ml TNF-α (PeproTech Inc, Rocky Hill, KY, USA) for 24 h, the cell lysate was used for western blot analysis of ICAM-1 (Abcam) and VCAM-1 (Santa Cruz).
2.2 Enzyme-linked immunosorbent assay (ELISA)
BM-EPC like cells (2.0 × 105 cells) were cultured in 100 mm culture dish and their culture supernatants were collected at day 4. Concentration of HGF, TGF-β1, VEGF, and PDGF-BB was measured by Quantikine ELISA (R&D systems, Minneapolis, MN, USA) according to manufacturer’s instructions. Absorbance was measured at 450 nm by microplate reader (Biotek, Winooski, VT, USA).
2.3 Tube formation assay
BM-EPC like cells from pooled multiple colonies were used in this study since BM-EPC like cells at just confluent state expressed most surface markers such as CD29, CD105, CD90, vWF at approximately 100% by fluorescence-activated cell sorting analysis. Matrigel (Corning Inc, Corning, NY, USA) was distributed to μ-slide (Ibidi, Grafelfing, Germary) at 10 μl per well. BM-EPC like cells (6.0 × 103) and BM-MSC (6.0 × 103) or a mixture of BM-EPC like cells (4.0 × 103) and MSCs (2.0 × 103) were suspended in α-MEM (GIBCO, NY, USA) supplemented with 0.2% FBS or 0.2% hPL and seeded on Matrigel and incubated in the presence of VEGF, HGF, and PDGF-BB (PeproTech) at a concentration of 0, 10, 20, and 40 ng/ml respectively. For fluorescence labeling of cells, BM-EPC like cells were stained with PKH-26 (Sigma) and BM-MSC were stained with PKH-67 (Sigma). The images were obtained by a microscope (Leica) and analyzed by NIH image J angiogenesis.
2.4 Cell viability assay and 5-Bromo-2’-deoxyuridine (BrdU) assay
For analysis of cell viability, BM-EPC like cells (1.0 × 104/well) were seeded onto collagen type I coated 96 well plates, starved with 0.2% FBS in endothelial basal media-2 (EBM-2, Lonza) for 18 h, and treated with PDGF-BB at 0, 10, 20 and 40 ng/ml for 24 h. WST assay was performed according to the manufacturer's instructions (Roche, Basel, Switzerland). Absorbance was measured at 450 nm in a multiplate reader (Biotek).
For BrdU cell labeling, BM-EPC like cells (1.0 × 104/well) were cultured on collagen type I coated coverslips, treated with 20 ng/ml PDGF-BB for 20 h in fresh media, incubated with 10 μM BrdU (Sigma-Aldrich) for the last 4 h, and then fixed in absolute methanol for 10 min. Then, the coverslip was treated with 2.0 N HCl for 1 h at 37 °C, neutralized with 0.1 M borate buffer, incubated with the primary antibody to detect BrdU (Roche), incubated with FITC conjugated secondary antibody (Invitrogen, Waltham, MA, USA), counterstained with PI (Sigma-Aldrich) for total cell number, and images were obtained by fluorescent microscope (Leica).
2.5 Western blot
BM-EPC like cell lysate was harvested using lysis buffer (Cell Signaling, Danvers, MA, USA) with 2 mM PMSF (Sigma-Aldrich). Protein concentration was measured using BCA assay (Thermo Scientific, Waltham, MA, USA). The lysates and conditioned medium were separated by SDS-PAGE and transferred to nitrocellulose membrane. The membrane was blocked with 5% skim milk. Primary antibodies to detect VCAM-1 (Santa Cruz), ICAM-1 (Abcam), collagen type IV (Abcam), laminin-β1 (Santa Cruz) and α-tubulin (Sigma-Aldrich) were used and detected with horseradish peroxidase conjugated-secondary antibody (BD, San Jose, CA, USA). The membrane was developed using EZ-Western Lumi Pico (Dogen, Seoul, Korea). The signals were detected using a chemilluminator (Vilber, Marne-la-vallée, France) and then quantified with NIH Image J software.
2.6 Wound healing assay (scratch assay)
Confluent BM-EPC like cell monolayer on collagen type I coated 6 well plates were starved in 0.2% FBS in EBM-2 for 18 h, scraped with 200 μl pipette tip, washed with 1 × PBS and incubated with 0.2% FBS in α-MEM containing 0, 10, and 20 ng/ml PDGF-BB. The wound healing process was observed using a microscope (Leica).
2.7 Statistical analysis
All data are presented as mean ± standard deviation. Statistical analysis of all the data was carried out using GraphPad Prism software. P values < 0.05 were considered statistically significant differences (*p < 0.05, **p < 0.01, ***p < 0.001).
3 Results
3.1 Identification of human BM-EPC like cells from MNC of BM aspirates and its characterization
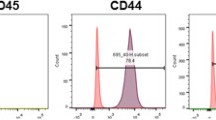
The colony forming cells of human BM-EPC like cells were selectively isolated from MNCs of BM aspirates by adhesion to fibronection or type I collagen in the EGM-2, whose morphology is in a spindle shape rather than a cobblestone one (Fig. 1A). In order to confirm the phenotype of BM-EPC like cells, immunofluorescence staining, ELISA, flow cytometry, and Matrigel tube formation assay were performed. UEA-1 lectin binding and vWF expression, general markers for endothelial lineage cells, were confirmed in human BM-EPC like cell culture by double fluorescence staining (Fig. 1B). Then, major angiogenic factors such as HGF, TGF-β1, VEGF, and PDGF-BB were measured in the BM-EPC like cell-conditioned medium at different passages by ELISA (Fig. 1C-F). Human BM-EPC like cells secreted HGF, TGF-β1, and VEGF, whose levels decreased along the cell passage but did not secrete PDGF-BB at all passages 2–4. The surface marker expression was also measured by flow cytometry (Fig. 1G). Human BM-EPC like cells at passages 1–4 exhibited low expression of CD31 (< 2%), CD309 (< 3%), CD45 (< 2%), and CD34 (< 1%), which is considered as surface markers for circulating EPC but exhibited high expression of CD105 (> 95%). The BM-EPC like cells also exhibited approximately 99% of UEA-I binding and vWF expression by flow cytometry analysis at passage 1–4, similar to that shown in the immunofluorescence staining. Based on the Matrigel tube formation assay, BM-EPC like cell showed poor vasculogenic potential. BM-MSCs aggregated easily and it made them could not maintain vascular network than BM-EPC like cell did (Fig. 1H). However, BM-EPC like cells co-culture with BM-MSC in a 2:1 ratio further improve their vasculogenic activity and also stimulated the recruitment of BM-MSC to the vascular network (Fig. 1 H,I).
Identification of human BM-EPC like cell from MNC of BM aspirates. A Representative colony of human BM-EPC like cells at passage 0 with different culture days (scale bar = 200 μm). B Double immunofluorescence staining of FITC-UEA-I binding (green) and vWF (red) counterstained with DAPI (blue) of human BM-EPC like cells (scale bar = 200 μm). C–F Concentration of growth factors in conditioned medium of BM-EPC like cells at passage 2–4. The conditioned medium was collected at day 2 after media change and normalized with the protein amount of cell lysates. (n = 3). G Cell surface marker expression of BM-EPC like cells at passages 1–4 analyzed by flow cytometry. H Matrigel tube formation assay on µ-slide of BM-EPC like cells, BM-MSC, and the mixture of BM-EPC like cells and BM-MSC. Tube formation was allowed for 16 h, and representative images are shown (scale bar = 200 μm). I PKH labeling of Matrigel tube formation assay on µ-slide of the mixture of BM-EPC like cells and BM-MSC. Representative images are shown (scale bar = 500 μm)
Collectively, BM-EPC like cells, which were selectively isolated from the MNCs of BM aspirates by fibronectin or collagen-1 adhesion, reveal its colony forming capacity and can be expanded by ex vivo culture with approximately 1.5 days of population doubling time up to passage 3 (Supplementary Fig. 1). Based on their phenotypic analysis at different passages, BM-EPC like cells do not reveal all the characteristics of mature endothelial cells at the early passage but express only some of them, which is rather similar to the early EPC identified in the blood previously [32].
3.2 TNF-α sensitivity of BM-EPC like cells in culture
TNF-α is known to upregulate pro-inflammatory adhesion molecules such as ICAM-1 and VCAM-1 on the ECs [33]. It was examined whether human BM-EPC like cells could induce pro-inflammatory response upon TNF- α treatment or not by western blot analysis. The BM-EPC like cells did not express ICAM-1 but significantly increased ICAM-1 expression upon TNF-α stimulation (Fig. 2A, C). Also, BM-EPC like cells markedly upregulated VCAM-1 expression upon TNF-α stimulation (Fig. 2B, D). Taken together, human BM-EPC like cells can initiate pro-inflammatory response upon TNF-α stimulation, which is also one of the important characteristics of endothelial-lineage cells.
Assay for TNF-α responsiveness of BM-EPC like cells. (A, B) Western blot analysis of ICAM-1 and VCAM-1 expression at 24 h after TNF-α treatment. α-tubulin was used as an internal loading standard. (C, D) Quantitative densitometry analysis of ICAM-1 and VCAM-1 expression at 24 h after TNF-α treatment. The α-tubulin was used as an internal loading standard and normalized for densitometry analysis. Values are mean ± SD of two independent experiments (**p < 0.01, compared with control)
3.3 PDGF-BB enhanced the vascular network forming capacity of human BM-EPC like cells in comparison to that of VEGF or HGF
EPCs or EPC like cells secrete a variety of angiogenic growth factors, which are important for pursuing their injury recruitment and vascular repairing capacity. As shown in Fig. 1, BM-EPC like cells secreted HGF, VEGF and TGF-β1 but did not secrete PDGF-BB. In order to investigate whether those factors could affect the vessel forming capacity of BM-EPC like cell which revealed rather limited vessel forming capacity, VEGF, HGF or PDGF-BB at different doses was provided to the tube formation assay of BM-EPC like cells at passage 3 (Fig. 3A). At 4 h after PDGF-BB treatment, elaborate interconnected tubular network was formed in a dose dependent manner, which was much better than VEGF or HGF treatment. As a quantitative analysis of vascular architecture, the number of meshes, junctions, segments between two junctions, and total length were measured, all of which are positively correlated with the structural maturity of the fine web-like network (Fig. 3B-E). VEGF did not increase the vessel forming capacity of BM-EPC like cells, but HGF did slightly increase tube forming ability of BM-EPC like cell only at high concentration. PDGF-BB, which was not secreted in BM-EPC like cells but abundant in the injury environment (Fig. 1F), markedly stimulated the vessel forming capacity of BM-EPC like cells at passage 3 in a dose dependent manner, whose tube formation was continuously monitored for 18 h (Supplementary Fig. 2). These results indicate that PDGF-BB effectively promotes the vessel forming capacity of human BM-EPC like cells, which may be applied to the cell priming for the better vessel formation after transplantation.
Comparison of endothelial tube forming capacity of BM-EPC like cells in vitro in the presence of VEGF, HGF, and PDGF-BB. The vessel forming capacity of BM-EPC like cell at passage 3 was estimated in the presence of VEGF, HGF, and PDGF-BB at concentrations of 0, 10, 20, and 40 ng/ml for 4 h. A Representative images of tube formation of BM-EPC like cell on Matrigel-coated μ-slide at 4 h after the treatment of each growth factor was shown (scale bar = 500 μm). B–E Quantitative analysis of tube architecture such as meshes (B), junctions (C), segments (D), and total lengths (E) using the NIH Image J angiogenesis program. Values are mean ± SD of three independent experiments (*p < 0.05, **p < 0.01, compared with 0 ng/ml growth factor treated group)
3.4 PDGF-BB stimulated BM-EPC like cell migration, viability, proliferation, laminin-β1 expression, and PDGFRβ endocytosis
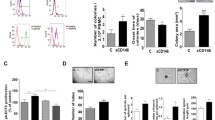
In an attempt to investigate the mechanism of action of PDGF-BB on the stimulation of vessel forming capacity of BM-EPC like cells, its effect on cell migration of BM-EPC like cells, viability, proliferation, and expression of VEGFR2, PDGFRβ, type IV collagen, and laminin-β1 were examined (Fig. 4). For the analysis of PDGF-BB effect on cell migration, the monolayer culture of BM-EPC like cells were scratched and the cell migration toward the wound edge was monitored at 0, 4, 8 h (Fig. 4A). Quantitative analysis of wound coverage showed that PDGF-BB significantly increased the migration of BM-EPC like cells compared to the control group (Fig. 4B). For analysis of viability, WST-1 assay was conducted at different passages (Fig. 4C). Compared with the control group, PDGF-BB increased BM-EPC like cells’s viability in a dose-dependent manner but did not at passage 4. BrdU cell labeling for s-phase entry was also increased by PDGF-BB treatment (Fig. 4D).
Effect of PDGF-BB on the cell migration, viability, expression of ECM and PDGFRβ of BM-EPC like cells. The mechanism of action of PDGF-BB on stimulating vessel formation of BM-EPC like cell was explored. Cell migration assay after the scratch wound of confluent BM-EPC like cell monolayer. A Representative images of the recovery from the scratch wound of BM-EPC like cells in the presence of PDGF-BB (0, 10, 20 ng/ml) at different time points (0, 4, 8 h) (scale bar = 500 μm). B Quantitative analysis of wound coverage. Wound coverage (%) = (wound area at 0 h–wound area at 4 or 8 h)/wound area at 0 h) × 100. Values are mean ± SD of eight independent experiments. C PDGF-BB effect on cell viability of BM-EPC like cell at different passages by WST assay at 24 h after PDGF-BB stimulation (0, 10, 20, 40 ng/ml). Values are mean ± SD of five independent experiments. D BrdU incorporation assay to estimate proliferating cells. Values are mean ± SD of eight independent experiments. E–I Immunofluorescence staining of type IV collagen (E) and laminin-β1 (F) and western blot analysis of type IV collagen and laminin-β1 (G) in the conditioned media of BM-EPC like cells culture at 24 h after PDGF-BB stimulation (0, 10, 20 ng/ml). Representative images are shown (scale bar = 100 μm). H–I Densitometry quantification was normalized by proteins of total cell lysates. Values are mean ± SD of two independent experiments. J Immunofluorescence staining of VEGFR2 and PDGFRβ in BM-EPC like cells at 24 h after PDGF-BB stimulation (0, 10, 20 ng/ml). Representative images of VEGFR2 (green) and PDGFRβ (red) were shown (scale bar = 100 μm). *p < 0.05, **p < 0.01, ***p < 0.001, compared with 0 ng/ml PDGF-BB treated group
For vascular tube formation of BM-EPC like cells seeded on Matrigel, the cell migration and expression of extracellular matrix proteins are essential. Expression of type IV collagen and laminin-β1 after PDGF-BB treatment was examined by immunofluorescence staining and western blot analysis (Fig. 4E-I). PDGF-BB did not increase the expression of type IV collagen significantly but increased the expression of laminin-β1, especially at 20 ng/ml PDGF-BB, which strongly supports the cell migration of BM-EPC like cells on laminin-enriched substratum, possibly through laminin receptor, α6β4 integrin.
Based on immunofluorescence staining, most of the PDGFRβ in BM-EPC like cells was localized at the cell surface diffusely, which was clearly relocated to the endocytic compartment upon PDGF-BB treatment (Fig. 4J). Thus, PDGF-BB-induced PDGFRβ endocytosis may be involved in the PDGF-BB mediated endothelial tube formation of BM-EPC like cells. Taken together, PDGF-BB-PDGFRβ signaling and its endocytosis enhanced directional cell migration, viability, proliferation, and laminin-β1 expression of BM-EPC like cells.
4 Discussion
EPCs play an important role in vascular endothelial repair by incorporating into the blood vessel or releasing angiogenic growth factors that accelerate wound healing [1, 6, 8]. Therefore, EPCs are a promising candidate for a variety of ischemic vascular diseases such as ischemic limb diseases, diabetic wounds, stroke, myocardial infarction, and so on. However, the patients who need such cell therapy using EPCs have a deficient number of these cells, whose functions are insufficient for treatment [13]. Considering that EPC therapy is suitable for auto-graft rather than allograft because of its immunogenicity, a proper preconditioning or priming strategy aiming to improve the vasculogenic function and harvest sufficient numbers of EPCs will be necessary for their therapeutic applications.
Although numerous research on EPCs derived from a variety of tissue sources have been reported, their cellular characterizations are quite diverse and their functional role in vasculogenesis is not well defined. In this study, the BM-EPC like cells were selectively isolated from the bone marrow aspirates by ECM adhesion, whose surface marker expression profiles are quite different from those of circulating EPC but retain vasculogenic capacity distinctly distinguished from BM-MSC derived from BM-MNCs. Furthermore, BM-EPC like cell can be expanded with population doubling time of approximately 1.5 days at early passage, secrete abundantly angiogenic factors such as VEGF, HGF, and TGF-β1 and generate elaborate vascular network upon combination with BM-MSC, which is expected to play a functional role as a pericyte in this context. Therefore, BM-EPC like cells along with their combination with BM-MSC may be further explored as a candidate cell therapeutics for the treatment of a variety of ischemic and chronic vascular diseases.
One of common features of endothelial cells or their precursor cells is to sensitively respond to a variety of inflammatory stimuli by expressing ICAM and VCAM. In this study, BM-EPC like cells revealed TNF-alpha sensitivity by increasing expression of ICAM and VCAM in response to TNF-alpha, which also supports that BM-EPC like cells may be potentially considered as EPCs. However, it was not confirmed in this study whether TNF-alpha can stimulate or inhibit vessel formation abilities of BM-EPC like cells even though TNF-alpha mediated inhibition or leakage of vascular network is highly expected. This possible scenario, which may happen in the inflammatory tissue environment, may be significantly explored for the advancement of potential cell therapeutics in the future study.
BM-EPC like cells seem to be rather immature and do not express genuine endothelial phenotype overtly at an early stage. BM-EPC like cells themselves did generate rather limited vascular networks in Matrigel tube assay but their co-culture with BM-MSCs or supplementation of PDGF-BB, known as one of the angiogenic growth factors of BM-MSC [34], further increased the vascular tubule forming capacity of BM-EPC like cells. In contrast, supplementation of VEGF and HGF did not further improve the vasculogenic capacity, suggesting whose autocrine effect has been met in the culture of the BM-EPC like cells. However, priming of BM-EPC like cells with PDGF-BB, deficient in the conditioned medium of BM-EPC like cells, may efficiently increase PGDF-BB mediated vascularizing capability, possibly through PDGFR and its endocytic related directional cell migration [24, 25]. The lack of VEGF responsiveness may be explained by no expression of low affinity VEGF receptor, VEGFR2 in BM-EPC like cells. With this regard, BM-EPC-like cells may not utilize VEGF as their major growth factors but PDGF-BB, which may be supplied by migrating pericytes [24, 28] or MSCs, may be very critical in the cell proliferation and migration of BM-EPC like cells for the vascular network formation. Actually, PDGF-BB is a well-known growth factor that promotes wound healing [30]. Especially in the growing vasculature, PDGF-BB is mainly secreted by activated and migrating ECs and recruits pericytes for vessel maturation [28]. Besides, a previous study demonstrated that PDGF-BB promoted proliferation, migration, and secretion of angiogenic growth factors from EPCs isolated from rat BM [31]. However, VEGF and HGF, secreted abundantly by BM-EPC like cells, may be expected to play a paracrine role in angiogenesis and vascularization after transplantation in vivo instead of their autocrine effect.
How can PDGF-BB priming affect the positively vascularizing capacity of BM-EPC like cells? As shown in Fig. 4, PDGF-BB stimulated BM-EPC like cells’ migration, viability, and proliferation, which are very important for the tubular association of separately inoculated cells. Furthermore, PDGF-BB selectively enhanced laminin-β1 expression but not type IV collagen, which may also expedite the migration of BM-EPC like cells by outbalancing laminin-enriched substratum and then may also be engaged in the reformation of tubular basement membrane along with type IV collagen in the preexisting tubules. As one of mechanisms of PDGF-BB-stimulated cell migration, PDGF-BB-induced endocytosis of PDGF receptor is proposed, which leads to the receptor downregulation as well as a novel signaling pathway for cell migration through the complex formation of DOCK4 and Dynamin and Rac-1 activation at the leading edge [35]. Therefore, the PDGF-BB signaling cascade may mainly enhance the migration of BM-EPC like cells and accordingly lead to the higher capacity of tubular network formation of BM-EPC like cells with PDGF-BB priming.
Conclusively, it was identified in this study that a colony forming EPC like cells from BM aspirates with limited expressions of endothelial lineage markers could be stimulated to generate fine capillary networks by PDGF-BB priming. Therefore, BM-EPC like cells can be activated in vitro to be better engaged in vascular repair. This can be further developed as a donor EPCs to treat a variety of vascular diseases.
Especially, BM-EPC like cells in the bone marrow are expected to be more premature than circulating ones and may retain the trafficking capacity from the bone marrow to circulation under the peripheral ischemic condition.
References
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–6.
Keighron C, Lyons CJ, Creane M, O’Brien T, Liew A. Recent advances in endothelial progenitor cells toward their use in clinical translation. Front Med. 2018;5:354.
Haider KH, Salim A, Al-Reshidi MA. Endothelial progenitor cells for cellular angiogenesis and repair: lessons learned from experimental animal models. Regen Med. 2017;12:969–82.
Patry C, Stamm D, Betzen C, Tönshoff B, Yard BA, Beck GC, et al. CXCR-4 expression by circulating endothelial progenitor cells and SDF-1 serum levels are elevated in septic patients. J Inflamm (Lond). 2018;15:10.
Li L, Liu H, Xu C, Deng M, Song M, Yu X, et al. VEGF promotes endothelial progenitor cell differentiation and vascular repair through connexin 43. Stem Cell Res Ther. 2017;8:237.
Maeng YS, Choi HJ, Kwon JY, Park YW, Choi KS, Min JK, et al. Endothelial progenitor cell homing: prominent role of the IGF2-IGF2R-PLCβ2 axis. Blood. 2009;113:233–43.
Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, et al. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PloS One 2019;4:e5643.
Wang T, Fang X, Yin ZS. Endothelial progenitor cell-conditioned medium promotes angiogenesis and is neuroprotective after spinal cord injury. Neural Regen Res. 2018;13:887.
Kaushik K, Das A. Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy. 2019;21:1137–50.
Maki T, Morancho A, Martinez-San Segundo P, Hayakawa K, Takase H, Liang AC, et al. Endothelial progenitor cell secretome and oligovascular repair in a mouse model of prolonged cerebral hypoperfusion. Stroke. 2018;49:1003–10.
Zhang R, Yang J, Yuan J, Song B, Wang Y, Xu Y. The therapeutic value of bone marrow-derived endothelial progenitor cell transplantation after intracerebral hemorrhage in rats. Front Neurol. 2017;8:174.
Wils J, Favre J, Bellien J. Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacol Ther. 2017;170:98–115.
Berezin AE. The endothelial progenitor cell dysfunction in hypertension: the diagnostic and predictive values. Vessel Plus. 2018;2:22.
Williamson K, Stringer SE, Alexander MY. Endothelial progenitor cells enter the aging arena. Front Physiol. 2012;3:30.
Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep. 2008;60:119.
Li CS, Neu MB, Shaw LC, Grant MB. Endothelial progenitor dysfunction in the pathogenesis of diabetic retinopathy: treatment concept to correct diabetes-associated deficits. EPMA Journal. 2010;1:88–100.
Teraa M, Sprengers RW, Westerweel PE, Gremmels H, Goumans MJT, Teerlink T, et al. Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. PLoS One. 2013;8:e55592.
Domingues A, Bujko K, Kucia M, Ratajczak J, Ratajczak MZ. Efficient ex vivo expansion of highly purified human umbilical cord blood-derived very small CD34+ lin-CD45-stem cells into functional endothelial cells in vitro in chemically identified, feeder layer-free medium supplemented with nicotinamide. Blood. 2019;134:4882.
Shen WC, Chou YH, Huang HP, Sheen JF, Hung SC, Chen HF. Induced pluripotent stem cell-derived endothelial progenitor cells attenuate ischemic acute kidney injury and cardiac dysfunction. Stem Cell Res Ther. 2018;9:344.
Lu H, Mei H, Wang F, Zhao Q, Wang S, Liu L, et al. Decreased phosphorylation of PDGFR-β impairs the angiogenic potential of expanded endothelial progenitor cells via the inhibition of PI3K/Akt signaling. Int J Mol Med. 2017;39:1492–504.
Bhatwadekar AD, Shaw LC, Grant MB. Promise of endothelial progenitor cell for treatment of diabetic retinopathy. Expert Rev Endocrinol Metab. 2010;5:29–37.
Amini AR, Laurencin CT, Nukavarapu SP. Differential analysis of peripheral blood-and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J Orthop Res. 2012;30:1507–15.
Fang J, Guo Y, Tan S, Li Z, Xie H, Chen P, et al. Autologous endothelial progenitor cells transplantation for acute ischemic stroke: a 4-year follow-up study. Stem Cells Transl Med. 2019;8:14–21.
Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312.
Evrova O, Buschmann J. In vitro and in vivo effects of PDGF-BB delivery strategies on tendon healing: a review. Eur Cell Mater. 2017;34:15–39.
Xiang DN, Feng YF, Wang J, Zhang X, Shen JJ, Zou R, et al. Platelet-derived growth factor-BB promotes proliferation and migration of retinal microvascular pericytes by up-regulating the expression of C-X-C chemokine receptor types 4. Exp Ther Med. 2019;18:4022–30.
Liang T, Zhu L, Gao W, Gong M, Ren J, Yao H, et al. Coculture of endothelial progenitor cells and mesenchymal stem cells enhanced their proliferation and angiogenesis through PDGF and Notch signaling. FEBS Open Bio. 2017;7:1722–36.
Kemp SS, Aguera KN, Cha B, Davis GE. Defining endothelial cell-derived factors that promote pericyte recruitment and capillary network assembly. Arterioscler Thromb Vasc Biol. 2020;40:2632–48.
Fang J, Huang X, Han X, Zheng Z, Hu C, Chen T, et al. Endothelial progenitor cells promote viability and nerve regenerative ability of mesenchymal stem cells through PDGF-BB/PDGFR-β signaling. Aging. 2020;12:106.
Rolny C, Nilsson I, Magnusson P, Armulik A, Jakobsson L, Wentzel P, et al. Platelet-derived growth factor receptor-β promotes early endothelial cell differentiation. Blood. 2006;108:1877–86.
Wang H, Yin YG, Huang H, Zhao XH, Yu J, Wang Q, et al. Transplantation of EPCs overexpressing PDGFR-β promotes vascular repair in the early phase after vascular injury. BMC Cardiovasc Disord. 2016;16:179.
Spigoni V, Picconi A, Cito M, Ridolfi V, Bonomini S, Casali C, et al. Pioglitazone improves in vitro viability and function of endothelial progenitor cells from individuals with impaired glucose tolerance. PLoS One. 2012;7:e48283.
Piao J, Park JS, Hwang DY, Hong HS, Son Y. Substance P blocks β-aminopropionitrile-induced aortic injury through modulation of M2 monocyte-skewed monocytopoiesis. Transl Res. 2021;228:76–93.
Zhang M, Ahn W, Kim S, Hong HS, Quan C, Son Y. Endothelial precursor cells stimulate pericyte-like coverage of bone marrow-derived mesenchymal stem cells through platelet-derived growth factor-BB induction, which is enhanced by substance P. Microcirculation. 2017;24:e12394.
Kawada K, Upadhyay G, Ferandon S, Janarthanan S, Hall M, Vilardaga JP, et al. Cell migration is regulated by platelet-derived growth factor receptor endocytosis. Mol Cell Biol. 2009;29:4508–18.
Acknowledgements
This work was supported by Korean Health Technology R&D Project grant, Grant/Award Number: (HI18C1492), the Technology Innovation Program (1415179737,20018551) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea), and the Korean Fund for Regenerative Medicine(KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare), (23C0110L1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflicts.
Ethical statement
This article does not contain any studies involving human participants or experimental animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, D.Y., Park, G., Hong, H.S. et al. Platelet-Derived Growth Factor-BB Priming Enhances Vasculogenic Capacity of Bone Marrow-Derived Endothelial Precursor Like Cells. Tissue Eng Regen Med 20, 695–704 (2023). https://doi.org/10.1007/s13770-023-00546-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00546-9