Abstract
Background
Early management of patients with patent infarct-related artery (IRA) and optimal ST resolution in ST elevation myocardial infarction (STEMI) has never been assessed. We compared immediate vs delayed PCI in these patients.
Methods
Matched comparison of immediate vs delayed (24 h) PCI in STEMI patients presenting with patent IRA, thrombus-containing lesion and ST resolution ≥70%. Patients were matched for duration of symptoms, intervention type, angiographic data, diabetes. Patients in immediate PCI group received standard therapy in the cathlab. Patients in delayed PCI group received dual antiplatelet therapy, antithrombins, and GPIIb-IIIa inhibitors until PCI. Primary endpoint was procedural success. Secondary endpoints were enzyme release and in-hospital adverse events.
Results
Seventy-eight patients were included: 39 per group. Average age 62 years, 75% males. There was a significantly higher procedural success rate in the delayed PCI group (95% success, Vs. 77% in the immediate group, P = 0.008). Initial thrombus burden score did not differ between immediate and delayed PCI groups, but improved significantly in the delayed group between baseline angiography and time of PCI (P = 0.039). There was no difference in major adverse events or bleeding complications between groups. Peak CK levels were significantly higher in the immediate versus delayed PCI group (P = 0.02), although there was no difference between groups in peak CK-MB, peak troponin, or peak CK-MB ratio.
Conclusion
Our data suggest that in STEMI patients with patent IRA, optimal ST-segment resolution, and thrombus-containing lesion, deferred PCI when patients are given dual antiplatelet therapy, antithrombin agents, and GPIIb-IIIa inhibitors results in a higher procedural success rate, without an increased risk of MACE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of ST-segment elevation myocardial infarction (STEMI) has substantially evolved over the past ten years, due to the improvement in pharmacological and mechanical reperfusion strategies. Percutaneous coronary intervention (PCI) has emerged as the strategy of choice for re-establishing effective flow in occluded infarct-related arteries (IRA) in patients suffering from ST elevation myocardial infarction (STEMI). Several studies have shown the superiority of PCI in this indication over thrombolysis [1, 3, 5], but there has never been any assessment of the best strategy for early management of patients referred for primary or rescue PCI, and who present with patent IRA, thrombus-containing lesion and optimal ST-segment recovery at diagnostic angiography.
While immediate PCI is often the preferred strategy in this situation, distal embolisation is still observed in approximately 15% of patients after primary PCI, and is associated with incomplete ST-segment resolution, increased necrosis volume and poor outcome with an 8-fold increase in 5-year mortality [12]. To date, results achieved with thrombectomy or distal protection devices as an adjunct to immediate mechanical reperfusion in evolving STEMI have been disappointing. In addition, early administration of GP IIb/IIIa inhibitors appear to improve coronary patency in some studies, but are often administered relatively late, at the time of PCI [8, 16]. Recent data from the CLARITY and EXTRACT TIMI-25 studies showed that pre-treatment with clopidogrel and low molecular weight heparin (LMWH) reduced the incidence of major adverse cardiac events (MACE), improved patency of the IRA before PCI, and prevent early reocclusion [2, 21, 22]. In this context, delaying PCI by 24 h may seem to be a suitable alternative, although recurrent MI is observed in 5%–8% of patients within the first 30 days [18].
Therefore, we aimed to compare immediate (<12 h) versus delayed (24 h) PCI in STEMI patients referred for primary or rescue PCI, and presenting with TIMI 3 flow IRA, thrombus-containing lesion, and complete ST-segment resolution (≥70%) at diagnostic angiography.
Methods
Study population
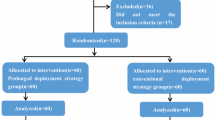
We examined the timing of PCI in STEMI patients admitted to our Department between January 2004 and January 2007. Patients were included in the comparison if referred for primary or rescue PCI for acute STEMI (<12 h after onset of symptoms), without cardiogenic shock. Further inclusion criteria were presence of TIMI 3 flow, thrombus-containing lesion grade 2, 3 or 4 at diagnostic angiography, as well as ST-segment resolution ≥70% [6, 11]. Patients who underwent PCI within 12 h were assigned to the “immediate” group, and matched with similar patients in whom PCI had been delayed by 24 h (“delayed PCI” group). The decision to delay PCI was at the operator’s discretion at the time of diagnostic angiography. The sheath was left in place for 24 h while awaiting delayed PCI.
Endpoints
The primary endpoint of the study was procedural success, defined as final diameter stenosis <30%; TIMI 3 flow; and no occurrence of slow flow, no-reflow or distal embolisation. The secondary objectives were enzyme release, and in-hospital major adverse cardiac events (MACE defined as death, recurrent ischaemia, target vessel revascularization or bleeding). Bleeding was classified according to the TIMI criteria [18].
Electrocardiographic analysis
Each patient had paired electrocardiograms (ECGs) at diagnosis and immediately before initial coronary angiogram. ST-segment recovery was defined as the percent reduction in the summed ST-segment elevation score between ECGs obtained at diagnosis and immediately before initial coronary angiogram according to Schroeder classification [11]. ST-segment elevation was calculated 20 ms after the J point. The sum of ST-segment elevation was calculated in leads I, aVL, and V1 through V6 for anterior myocardial infarctions, and leads II, III, aVF, V5, and V6 for non anterior myocardial infarctions. ST-segment recovery was considered as optimal when assessed as ≥70%.
Angiographic analysis
At diagnostic angiography, epicardial blood flow was assessed by TIMI flow grade, and corrected TIMI frame count [6, 11]. Slow flow was defined as a decrease in TIMI flow from 3 to 2 during the procedure, no-reflow was defined as TIMI flow decreasing from 3 to either 0 or 1 during the procedure. Thrombus burden grade was defined according to Gibson et al. [10]. Only patients with a thrombus-containing lesion grade 2, 3 or 4 at diagnostic angiography were included. In brief, in TIMI thrombus grade 0, no cineangiographic characteristics of thrombus are present; in TIMI thrombus grade 1, possible thrombus is present, with such angiography characteristics as reduced contrast density, haziness, irregular lesion contour, or a smooth convex “meniscus” at the site of total occlusion, suggestive, but not diagnostic of thrombus; in TIMI thrombus grade 2, there is definite thrombus, with greatest dimensions ≤1/2 the vessel diameter; in TIMI thrombus grade 3, there is definite thrombus but with greatest linear dimension >1/2 but <2 vessel diameters; in TIMI thrombus grade 4, there is definite thrombus with the largest dimension 2 vessel diameters; and in TIMI thrombus grade 5, there is total occlusion. Distal embolisation was defined as convex filling defect, partially or completely obstructing a coronary vessel distal to the culprit lesion [12].
In-hospital medication
Prior to initial diagnostic angiography, all patients were treated with aspirin (500 mg iv followed by 75 mg administered orally once daily), and clopidogrel (300 mg loading dose followed by 75 mg once daily). All patients received enoxaparin (30 mg iv bolus followed by 1 mg/kg subcutaneously every 12 h), except patients older than 75 years or with renal dysfunction (creatinine clearance <30 ml/min), who were given unfractionated heparin (UFH; 60 U/kg, maximum 4,000 U followed by infusion at a rate of 12 U/kg/h, maximum 1,000 U/h). Enoxaparin (or UFH) was maintained until hospital discharge.
In patients who underwent immediate PCI, abciximab was given only in those referred for primary PCI (i.e. those who did not undergo initial thrombolysis), and was maintained for 12–24 h (intravenous bolus of 0.25 mg/kg followed by a continuous infusion of 10 µg/min). In the immediate PCI group, patients referred for rescue PCI (i.e. those who underwent initial thrombolysis) were not given abciximab unless slow flow, no-reflow or distal embolization was observed.
Patients who were assigned to the delayed PCI group were maintained under enoxaparin (or UFH), aspirin and clopidogrel until the procedure. In this group, abciximab therapy was started immediately after initial diagnostic angiography in patients not previously submitted to thrombolysis, and within the first 2 h following initial diagnostic angiography in those who had initially received thrombolysis. Abciximab was maintained until delayed PCI (Fig. 1).
Enzyme release
During the hospital stay, total creatine kinase (CK), creatine kinase MB (CK-MB), and troponin levels were routinely checked every 12 h for the first 24 h, and once daily thereafter. The peak value from 4 to 6 serial measurements up to 48 h after PCI was reported. Patients with elevated CK-MB levels were stratified into categories according to peak CK-MB ratio of 0–1, 1–3, 3–5, 5–10 and >10× upper limit of normal value (ULN), where the peak CK-MB ratio was actual peak CK-MB value divided by the ULN for CK-MB.
Matching process and statistical analysis
Matching process was performed by a computerized program. The matching parameters in order of sequential selection were: (1) delay between onset of symptoms and initial angiogram; (2) primary PCI or rescue PCI; (3) lesion site; (4) vessel size reference diameter ± 0.3 mm; (5) diabetes.
Statistical analysis was performed using the Statview statistical package (Statview 5, SAS Institute, Cary, North Carolina). Quantitative variables are presented as mean ± SD, and categorical variables as number of cases (percentage). Comparison between groups was performed by the McNemar test for paired categorical data. The paired student t test was performed for continuous data. All tests were two sided, a P value < 0.05 was considered significant.
Results
Between January 2004 and January 2007, a total of 651 patients underwent PCI in the setting of acute myocardial infarction in our institution. Out of this population 429 patients (66%) had rescue PCI and 222 patients (34%) had primary PCI. Immediate PCI was performed in 589 patients (90.5%), while 62 patients (9.5%) underwent delayed PCI. In total, 78 patients were included in the study; 39 in the immediate PCI group, matched with 39 patients in the delayed PCI group. Average age was 62 years, and 75% were males. There were no significant differences between the two groups in terms of baseline demographic characteristics (Table 1). According to the protocol design, there was a significant difference in time to PCI between groups. In the delayed PCI group, angioplasty was performed on average 16 h later as compared with the immediate PCI group. The rate of thrombolysis was 41% in the immediate PCI group and 46% in the delayed PCI group (P = 0.65).
Angiographic and procedural results
The baseline angiographic and procedural data were similar in both groups, as shown in Table 2. In particular, there was no significant difference between the two groups in terms of corrected TIMI frame count or thrombus score. Thromboaspiration or distal protection devices were used in only 23% of patients in the immediate PCI group, compared with 15% of patients in the delayed PCI group (P = 0.39). Conversely, there was a significant difference in the rate of use of glycoprotein IIb/IIIa inhibitors, as all patients in the delayed PCI received abciximab, compared to only 49% in the immediate PCI group (P < 10−5).
Table 3 shows the post-procedure angiographic results. All patients underwent PCI and stent implantation, except four patients who no longer had significant coronary stenosis at the time of the delayed procedure. A significantly higher procedural success rate was achieved in the delayed PCI group, with a 95% success rate, compared to only 77% in the immediate PCI group (P = 0.008). This higher procedural success rate was related to a significant reduction in distal embolisation (−71%; P = 0.008) and no reflow or slow flow (−83%; P = 0.03) during PCI. Consequently, TIMI 3 flow was significantly more frequently observed at the end of the procedure in the delayed PCI group. Corrected TIMI frame count was significantly reduced by 55% in this group, as compared with the immediate PCI group.
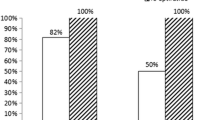
Thrombus burden score was similar between groups at baseline angiography. Conversely, there was a significant improvement in thrombus burden in patients in the delayed PCI group between the time of baseline angiography at presentation, and the time of the PCI procedure, 24 h later. In the delayed PCI group at baseline angiography, there were 17%, 39% and 44% of patients with thrombus scores 2, 3 and 4 respectively, compared to 62%, 20% and 18% for scores 2, 3 and 4 respectively at the time of PCI, P = 0.039 (Fig. 2).
In-hospital adverse events
Overall, there was no significant difference in MACE between patients submitted to immediate PCI and those in whom PCI was delayed (Table 4). No ischemic event occurred in the delayed PCI group between initial angiography and postponed coronary intervention. There was one death, and one target vessel revascularization in each group. Two patients in the immediate PCI group suffered recurrent ischemia, compared to only one patient in the delayed PCI group. In all three patients, recurrent ischemia occurred after PCI (whether immediate or delayed). Control angiography showed normal IRA patency and acute stent thrombosis in the two immediate PCI patients, and a large flow-limiting dissection in the delayed PCI patient. Conversely, one patient suffered a major bleed in the immediate PCI group, compared to two major bleeds among patients submitted to delayed PCI (2.6% Vs. 5.2%, P = 0.56). There was no emergency coronary artery bypass graft surgery in either group. Four patients in each group suffered more than one adverse event. Left ventricular ejection fraction was similar in both groups at discharge, at an average of 53%, and length of hospital stay was also similar, at an average of 6.5 days.
Peak CK levels were significantly higher in the immediate versus delayed PCI group (1,634 ± 810 Vs. 1,215 ± 745, immediate Vs. delayed, P = 0.02), but there was no difference between groups in peak CK-MB, peak troponin, or peak CK-MB ratio.
Discussion
Our data would suggest that in STEMI patients presenting with patent IRA, optimal ST-segment resolution, and thrombus-containing lesion, PCI can safely be deferred for approximately 24 h when patients are given dual antiplatelet therapy, antithrombin agents, and GPIIb-IIIa inhibitors. In this setting, deferred PCI results in a higher procedural success rate, fewer angiographic complications, without an increased risk of MACE or bleeding. Peak CK levels were significantly higher in the immediate vs delayed PCI group, although there was no difference between groups in peak CK-MB, peak troponin, or peak CK-MB ratio.
Although several studies have shown the superiority of PCI over thrombolysis in this indication, immediate PCI is associated with distal embolisation, and can lead to reduced myocardial reperfusion, more extensive myocardial damage, and a poor prognosis [12]. On the other hand, delaying PCI incurs the risk of recurrent ischemia, myocardial infarction, or death, likely due to abrupt reocclusion [18]. Both these scenarios unfavourably impact long-term outcome. Data from the CARESS in AMI study recently reported that high risk patients presenting with evolving STEMI who undergo thrombolytic therapy, should be transferred for PCI early after thrombolysis, regardless of the success of thrombolytic therapy [20]. However, the results of this study may not apply to the specific management of patients presenting with TIMI III flow IRA, optimal ST-segment resolution, and thrombus-containing lesion, particularly in patients who were not submitted to thrombolytic therapy. In addition, it should be underlined that approximately half of our study patients were not submitted to thrombolytic therapy.
To date, several mechanical and pharmacological approaches have been evaluated in the setting of acute STEMI, in order to optimise myocardial reperfusion and reduce procedure-related complications [4, 6, 11, 14, 15, 17, 26]. Myocardial protection devices are a logical approach in this setting. However, based on current knowledge, results with thrombectomy or distal protection devices are conflicting [28]. A recent randomized trial suggested that thrombectomy performed as routine therapy in primary PCI may have potentially deleterious effects, resulting in an increased final infarct size [13]. The Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction (TAPAS) Study found that thrombus aspiration resulted in improved myocardial reperfusion compared with conventional PCI, and may reduce the 1-year clinical mortality [27, 29].
In contrast, considerable progress has been made in recent years in the pharmacological approach to myocardial reperfusion, and prevention of reocclusion. Adjunctive abciximab therapy has been reported to improve the rate of IRA patency and to reduce the risk of reinfarction in STEMI patients, although reduced mortality with abciximab has only been observed in association with primary PCI [8, 16]. Moreover, in the FINESSE trial, pre-PCI treatment with abciximab did not significantly improve IRA patency and short-term outcome [20]. In our study, the longer duration of abciximab therapy could explain the significant improvement in thrombus score observed in patients in the delayed PCI group, who were maintained under aspirin, clopidogrel and LMWH while awaiting their PCI procedure. Indeed, four patients no longer had significant coronary stenosis at the time of the delayed procedure, the thrombus score having decreased from 4 to 2 in all four cases. The proportion of grade 2 thrombus burden increased from 17% to 62% between baseline angiography and PCI procedure in the delayed PCI group, undoubtedly contributing to the significantly higher success rate achieved among delayed PCI patients (77% Vs. 95%, immediate vs delayed PCI, P = 0.022). These findings reinforce the results of Di Pasquale et al. [9] who recently found in a comparison that delayed PCI was as effective, and possibly even superior to immediate PCI in STEMI patients.
Antithrombin agents are routinely used as part of a pharmacologic reperfusion regimen, because they are associated with a higher rate of IRA patency after initial fibrinolysis and a lower rate of recurrent myocardial infarction, even though they have not been reported to enhance initial clot lysis. After successful fibrinolysis, reocclusion can occur in 5%–8% of patients during index hospitalisation, almost tripling mortality [18]. The risk of reocclusion is related to the underlying degree of stenosis and residual thrombus. In this setting, low molecular weight heparin has emerged as an attractive alternative to UFH. In EXTRACT TIMI 25, enoxaparin significantly reduced the relative risk of reinfarction at 30 days, whereas UFH has not been shown to prevent reocclusion after successful fibrinolysis [2, 28].
Sabatine and co-workers [21, 22] recently showed that pre-treatment with clopidogrel in STEMI patients can significantly reduce the occurrence of MACE and improve patency in IRA before PCI. It is likely that this helps to maintain a stable hemodynamic state, leaving the drugs time to take effect, and giving the lesion time to “cool off” [7]. Clopidogrel appears to improve late coronary patency and clinical outcomes by preventing reocclusion of open arteries rather than by facilitating early reperfusion [7, 24]. This could explain why we did not observe any angiographic reocclusion between initial coronary angiogram and delayed PCI. In the setting of PCI, platelet activation is immediate [23] and therefore may be incompletely suppressed if clopidogrel is initiated only at the time of PCI [21].
The use of dual antiplatelet therapy, GP IIb/IIIa inhibitors plus antithrombin agents may have combined to create a safe environment for delaying PCI without recurrent ischaemic events. In this context, deferring PCI for 24 h in STEMI patients with TIMI 3 flow in the infarct related artery, and optimal ST resolution appears to be a suitable alternative approach.
Limitations
This study was conducted retrospectively, and treatment assignment was not randomised. In addition, the sample size is quite limited, and there is some heterogeneity in the population due to the amalgam of both rescue and primary PCI patients. Furthermore, the different treatment strategies obviously entailed different treatment regimens, and thus, there is also some heterogeneity between groups in terms of the medication administered.
Blush grade was not evaluated since this study was designed retrospectively. Although normal TIMI III flow grade does not necessarily mean that microvascular flow and myocardial perfusion have been normalized, complete ST-segment resolution has been reported to appropriately reflect optimal distal tissue perfusion [19, 25].
Lastly, there was a very low rate of use of thromb- ectomy or distal protection devices in this population, which could have influenced the outcome.
Conclusion
In summary, our findings would suggest that in STEMI patients referred for rescue or primary PCI and presenting with TIMI 3 flow IRA, thrombus-containing lesion and optimal ST resolution, delaying PCI by 24 h is safe and effective, and results in fewer angiographic complications. The delay makes it possible to reduce thrombus burden without increasing the risk of ischemic events. In view of the high level of long-term mortality associated with distal embolisation, delayed PCI could be a suitable alternative. Larger, randomized studies are warranted to evaluate the exact impact of this strategy in this population.
Abbreviations
- CABG:
-
Coronary artery bypass graft surgery
- IRA:
-
Infarct-related artery
- LMWH:
-
Low molecular weight heparin
- MACE:
-
Major adverse cardiac events
- MI:
-
Myocardial infarction
- MLD:
-
Minimal lumen diameter
- PCI:
-
Percutaneous coronary intervention
- STEMI:
-
ST elevation myocardial infarction
- UFH:
-
Unfractionated heparin
References
Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, Thayssen P, Abildgaard U, Pedersen F, Madsen JK, Grande P, Villadsen AB, Krusell LR, Haghfelt T, Lomholt P, Husted SE, Vigholt E, Kjaergard HK, Mortensen LS (2003) A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 349:733–742
Antman EM, Morrow DA, McCabe CH, Murphy SA, Ruda M, Sadowski Z, Budaj A, Lopez-Sendon JL, Guneri S, Jiang F, White HD, Fox KA, Braunwald E (2006) Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 354:1477–1488
Assessment of the safety and efficacy of a new treatment strategy with percutaneous coronary intervention (ASSENT-4 PCI) investigators (2006) Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 367:569–578
Beran G, Lang I, Schreiber W, Denk S, Stefenelli T, Syeda B, Maurer G, Glogar D, Siostrzonek P (2002) Intracoronary thrombectomy with the X-sizer catheter system improves epicardial flow and accelerates ST-segment resolution in patients with acute coronary syndrome: a prospective, randomized, controlled study. Circulation 105:2355–2360
Bonnefoy E, Lapostolle F, Leizorovicz A, Steg G, McFadden EP, Dubien PY, Cattan S, Boullenger E, Machecourt J, Lacroute JM, Cassagnes J, Dissait F, Touboul P (2002) Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: a randomised study. Lancet 360:825–829
Burzotta F, Trani C, Romagnoli E, Mazzari MA, Rebuzzi AG, De Vita M, Garramone B, Giannico F, Niccoli G, Biondi-Zoccai GG, Schiavoni G, Mongiardo R, Crea F (2005) Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol 46:371–376
Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS (2005) Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 366:1607–1621
De Luca G, Suryapranata H, Stone GW, Antoniucci D, Tcheng JE, Neumann FJ, Van de Werf F, Antman EM, Topol EJ (2005) Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA 293:1759–1765
Di Pasquale P, Cannizzaro S, Parrinello G, Giambanco F, Vitale G, Fasullo S, Scalzo S, Ganci F, La Manna N, Sarullo F, La Rocca G, Paterna S (2006) Is delayed facilitated percutaneous coronary intervention better than immediate in reperfused myocardial infarction? Six months follow up findings. J Thromb Thrombolysis 21:147–157
Gibson CM, de Lemos JA, Murphy SA, Marble SJ, McCabe CH, Cannon CP, Antman EM, Braunwald E (2001) Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation 103:2550–2554
Gick M, Jander N, Bestehorn HP, Kienzle RP, Ferenc M, Werner K, Comberg T, Peitz K, Zohlnhofer D, Bassignana V, Buettner HJ, Neumann FJ (2005) Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 112:1462–1469
Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, vant Hof AW, Hoorntje JC, Suryapranata H (2002) Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J 23:1112–1117
Kaltoft A, Bottcher M, Nielsen SS, Hansen HH, Terkelsen C, Maeng M, Kristensen J, Thuesen L, Krusell LR, Kristensen SD, Andersen HR, Lassen JF, Rasmussen K, Rehling M, Nielsen TT, Botker HE (2006) Routine thrombectomy in percutaneous coronary intervention for acute ST-segment-elevation myocardial infarction: a randomized, controlled trial. Circulation 114:40–47
Lefevre T, Garcia E, Reimers B, Lang I, di Mario C, Colombo A, Neumann FJ, Chavarri MV, Brunel P, Grube E, Thomas M, Glatt B, Ludwig J (2005) X-sizer for thrombectomy in acute myocardial infarction improves ST-segment resolution: results of the X-sizer in AMI for negligible embolization and optimal ST resolution (X AMINE ST) trial. J Am Coll Cardiol 46:246–252
Limbruno U, Micheli A, De Carlo M, Amoroso G, Rossini R, Palagi C, Di Bello V, Petronio AS, Fontanini G, Mariani M (2003) Mechanical prevention of distal embolization during primary angioplasty: safety, feasibility, and impact on myocardial reperfusion. Circulation 108:171–176
Montalescot G, Borentain M, Payot L, Collet JP, Thomas D (2004) Early vs late administration of glycoprotein IIb/IIIa inhibitors in primary percutaneous coronary intervention of acute ST-segment elevation myocardial infarction: a meta-analysis. JAMA 292:362–366
Napodano M, Pasquetto G, Sacca S, Cernetti C, Scarabeo V, Pascotto P, Reimers B (2003) Intracoronary thrombectomy improves myocardial reperfusion in patients undergoing direct angioplasty for acute myocardial infarction. J Am Coll Cardiol 42:1395–1402
Ohman EM, Califf RM, Topol EJ, Candela R, Abbottsmith C, Ellis S, Sigmon KN, Kereiakes D, George B, Stack R (1990) Consequences of reocclusion after successful reperfusion therapy in acute myocardial infarction: TAMI Study Group. Circulation 82:781–791
Poli A, Fetiveau R, Vandoni P, del Rosso G, D’Urbano M, Seveso G, Cafiero F, De Servi S (2002) Integrated analysis of myocardial blush and ST-segment elevation recovery after successful primary angioplasty: real-time grading of microvascular reperfusion and prediction of early and late recovery of left ventricular function. Circulation 106:313–318
Recio-Mayoral A, Kaski JC, McMurray JJ, Horowitz J, van Veldhuisen DJ, Remme WJ (2007) Clinical trials update from the European Society of Cardiology Congress in Vienna, 2007: PROSPECT, EVEREST, ARISE, ALOFT, FINESSE, Prague-8, CARESS in MI and ACUITY. Cardiovasc Drugs Ther 21:459–465
Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, Lewis BS, Murphy SA, McCabe CH, Braunwald E (2005) Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 294:1224–1232
Sabatine MS, Morrow DA, Montalescot G, Dellborg M, Leiva-Pons JL, Keltai M, Murphy SA, McCabe CH, Gibson CM, Cannon CP, Antman EM, Braunwald E (2005) Angiographic and clinical outcomes in patients receiving low-molecular-weight heparin versus unfractionated heparin in ST-elevation myocardial infarction treated with fibrinolytics in the CLARITY-TIMI 28 trial. Circulation 112:3846–3854
Scharf RE, Tomer A, Marzec UM, Teirstein PS, Ruggeri ZM, Harker LA (1992) Activation of platelets in blood perfusing angioplasty-damaged coronary arteries: flow cytometric detection. Arterioscler Thromb 12:1475–1487
Scirica BM, Sabatine MS, Morrow DA, Gibson CM, Murphy SA, Wiviott SD, Giugliano RP, McCabe CH, Cannon CP, Braunwald E (2006) The role of clopidogrel in early and sustained arterial patency after fibrinolysis for ST-segment elevation myocardial infarction: the ECG CLARITY-TIMI 28 Study. J Am Coll Cardiol 48:37–42
Sorajja P, Gersh BJ, Costantini C, McLaughlin MG, Zimetbaum P, Cox DA, Garcia E, Tcheng JE, Mehran R, Lansky AJ, Kandzari DE, Grines CL, Stone GW (2005) Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J 26:667–674
Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Dulas D, Rutherford BD, Antoniucci D, Krucoff MW, Gibbons RJ, Jones D, Lansky AJ, Mehran R (2005) Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA 293:1063–1072
Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F (2008) Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med 358:557–567
Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, Julian D, Lengyel M, Neumann FJ, Ruzyllo W, Thygesen C, Underwood SR, Vahanian A, Verheugt FW, Wijns W (2003) Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 24:28–66
Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F (2008) Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): a 1-year follow-up study. Lancet 371:1915–1920
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meneveau, N., Séronde, M.F., Descotes-Genon, V. et al. Immediate versus delayed angioplasty in infarct-related arteries with TIMI III flow and ST segment recovery: a matched comparison in acute myocardial infarction patients. Clin Res Cardiol 98, 257–264 (2009). https://doi.org/10.1007/s00392-009-0756-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-009-0756-z