Abstract
Background
Drug-eluting balloon (DEB) catheters coated with paclitaxel in a water-soluble matrix have shown beneficial effects in the treatment and prevention of restenosis in the porcine coronary overstretch model and in clinical trials. Adherence of paclitaxel, same dose, on another recently introduced coated percutaneous coronary intervention (PCI) catheter (DIOR®) is mediated by a roughened balloon surface. Only scarce experimental and clinical data has been published on the new coating method. The aim of the present study was to compare the safety and efficacy of the two coatings in the porcine model.
Methods and results
Twenty-eight stainless steel stents were implanted in the left anterior descending and circumflex coronary arteries of 14 domestic pigs using either matrix-coated (n = 8), roughened DEB (n = 9), or uncoated PCI catheters, which served as control (n = 11). After 28 days, quantitative angiography and histomorphometry of the stented arteries were performed. Matrix-coated DEB led to a highly significant (P < 0.01) reduction of all parameters indicating neointimal proliferation compared to both, uncoated control and the roughened DEB; late lumen loss in-segment was 0.4 ± 0.2, 1.9 ± 0.5, and 1.4 ± 0.5 mm, respectively. In contrast, the roughened DEB failed to produce statistically significant effects on angiographic measures of stenosis or morphometric parameters such as maximal neointimal thickness and luminal area, except for neointimal area (5.7 ± 1.5 mm2 in the control group, 4.1 ± 1.7 mm2 roughened DEB, P < 0.05 vs. control, and 2.5 ± 0.8 mm2 matrix-coated DEB, P < 0.01 vs. control).
Conclusion
Inhibition of neointimal proliferation in the porcine coronary overstretch model by paclitaxel depends critically on the coating method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The finding that sustained release of paclitaxel is not a precondition for long-lasting inhibition of restenosis using drug-eluting balloons (DEBs) led to the development of a novel method for local drug delivery to the vessel wall [11]. Coating of conventional percutaneous coronary intervention (PCI) balloon catheters with paclitaxel using a new matrix-coating technique (PACCOCATH®) allows immediate and complete drug release upon inflation. Cells retain paclitaxel in vivo for 6 days even when plasma levels fall far below the detection limit [7]. In vitro, even brief contact with antiproliferative agents results in prolonged inhibition of vascular smooth muscle cell proliferation [9–11, 17].
Paclitaxel-coated DEB catheters based on the matrix technology have shown excellent effects in the treatment and prevention of restenosis in the porcine coronary overstretch model [11, 17], in coronary in-stent restenosis in patients [1, 12, 14], and in human peripheral arteries [18]. Although a product making use of this coating method is approved in several countries under the trade mark of SeQuent® Please a CE marked commercial product is not yet available. The favorable results obtained so far suggest that the DEB has the potential to become established as the currently most advanced alternative to drug-eluting stents (DES) for local drug delivery.
The CE-certified DIOR® balloon catheter has recently been introduced for paclitaxel delivery in PCI. Although the paclitaxel dose is similar to that in the matrix-coated balloon, coating is done in a different way. Adherence of the drug to the balloon surface is mediated by roughening of the membrane. Few experimental and clinical data regarding safety and efficacy have been published. The aim of the present study was to compare the safety and efficacy of PACCOCATH® and DIOR® DEB in inhibiting neointimal proliferation in the porcine coronary overstretch model.
Methods
Restenosis study
Bare metal stents (Bavaria Medizin Technologie, Oberpfaffenhofen, Germany) were implanted with an oversize ratio of ≈1.2. According to the randomization list, the 14 domestic pigs (body weight 24.8 ± 1.1 kg) received a total of 28 stents (diameter 3.5 mm, length 18 mm) into the left anterior descending (LAD) and circumflex (CX) arteries. The stents were premounted (crimping device produced by Machine Solutions Inc., Flagstaff, Arizona, USA) on either uncoated (control, n = 11 vessels) or paclitaxel-coated PCI balloon catheters (matrix-coating, catheters Bavaria Medizin Technologie, coating MB, Charité, Berlin, Germany: n = 8 vessels; roughened-surface coating, DIOR®, Eurocor GmbH, Bonn, Germany: n = 9 vessels). According to the manufacturers (Eurocor, DIOR® product brochure, Rev. No. 0807 B7), the paclitaxel dose on the balloon surfaces was 3 μg/mm2 in each case. Implantation pressures (control: 12.0 ± 0.0 atm, matrix-coating: 11.6 ± 1.3 atm, roughened-surface coating: 12.0 ± 0.0 atm) and inflation times (60 ± 0 s) were equal in all treatment groups. After inflation, stent deposition, and balloon withdrawal through the guiding catheter, the paclitaxel content of the balloons was determined by HPLC [11].
Animals were pre-sedated by intramuscular injection of ketamine and xylazine. A venous access was provided and anesthesia initiated by intravenous injection of propofol followed by orotracheal intubation, maintained with 1.0–2.0 vol% isoflurane, 70 vol% N2O, and 30 vol% oxygen. All pigs received 5,000 IU of heparin, 250 mg aspirin, and intracoronary nitroglycerin. The coronary arteries were imaged using a standard angiographic technique via the left carotid artery. Target segments were selected in the LAD and CX coronary arteries. After stent implantation, prophylactic intramuscular streptomycin and penicillin were given; pigs were allowed to recover and were monitored for 4 weeks. Oral aspirin 100 mg and ticlopidine 250 mg per day were administered starting 3 days before the procedure and continuing until sacrifice. After 4 weeks, the animals were sacrificed by means of an intravenously injected overdose of pentobarbital after follow-up angiography. Hearts were rapidly excised, the coronary system flushed with 0.9% saline, and the arteries were fixed by perfusion with 4% buffered formalin under physiological pressure and overnight immersion. The target segments were then dissected and samples for histology obtained. All experiments were conducted in accordance with the guidelines for animal experiments set forth by the local animal protection committee.
Quantitative coronary angiography (QCA)
Coronary imaging was done using a Philips PolyArc fluoroscope connected to a digitizer and an Apple Macintosh Power PC. The CAAS II System (Pie Medical, the Netherlands) was used for quantitative coronary angiography (QCA) by two experienced cardiologists blinded to the treatment groups. Discrepancies were resolved by mutual consensus. The following parameters were evaluated: reference diameter, stent diameter at baseline angiography, and minimal lumen diameter (MLD) at follow-up angiography. Late lumen loss was calculated as the difference between stent diameter immediately after implantation and MLD at follow-up angiography.
Histology
Stented coronary arteries were dissected from the formalin-fixed hearts and immersed in methyl-methacrylate (Merck, Darmstadt, Germany). Three representative cross sections (proximal, mid, distal) per stent were separated from the blocks with a coping saw, polished, and glued on acrylic plastic slides. Final specimens were stained by HE technique. After digitizing, histomorphometric measurements were performed with the NIH image program (PC version ‘Scion Image’, Scion Corporation, MD, USA). The parameters determined were: maximal neointimal thickness, vessel area, luminal area, and neointimal area. An injury score was assigned as previously described by Schwartz et al. [15], an inflammation score as described by Kornowski et al. [5].
Statistical analysis
Histomorphometric variables of the three cross-sectional planes were averaged to obtain a mean value per stent. Continuous variables of quantitative coronary angiography were compared by one-way, repeated-measures ANOVA and Student’s t test after normal distribution was confirmed by a statistical program (SPSS 15.0 for Windows, SPSS Inc.). Data are presented as mean ± SD.
Results
There were no acute thrombotic complications and no significant adverse events in terms of ECG parameters during or after the coronary interventions. After inflation, stent deposition, and withdrawal of the balloon through the guiding catheter, the paclitaxel content of the balloons was measured. The postprocedural content was 50.4 ± 11.5% of the initial dose (specified by Eurocor, DIOR® product brochure) on the roughened PCI balloon catheters (P = 0.001 vs. control), and 4.5 ± 0.7% on matrix-coated balloons (P = 0.001 vs. control, P = 0.001 vs. roughened DEB).
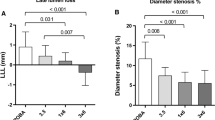
Quantitative angiography and histomorphometry of the stented arteries ascertained the statistical equality of baseline parameters between the control and treatment groups (Table 1). Quantitative coronary angiography revealed no edge effects. Treatment with the matrix-coated balloon resulted in a marked and statistically highly significant reduction of late lumen loss and highly significant increase in MLD compared to control (P < 0.001) and the roughened DEB (P < 0.001) (Figs. 1, 2; Table 1). Uncoated balloon PCI led to a late lumen loss of 1.85 ± 0.52 mm (n = 11) within the stented segments. Angioplasty with the roughened DEB failed to show a significant reduction in late lumen loss compared to control (1.41 ± 0.48 mm, n = 9). In contrast, late lumen loss in vessel segments treated with the matrix-coated DEB was reduced to 0.43 ± 0.20 mm (n = 8, P < 0.001 vs. control, P < 0.001 vs. roughened DEB) 28 days after intervention (Fig. 2; Table 1).
After 4 weeks, histological evaluation showed that all the stents were sufficiently expanded (Fig. 3). Complete endothelialization was present in all samples (Fig. 3). Inflammation scores were similar in the different treatment groups (Fig. 4; Table 1). Corresponding to the angiographic findings, histomorphometry showed a statistically significant increase in lumen diameter and luminal area in the vessels treated with the matrix-coated DEB compared to control and the roughened DEB (Fig. 4; Table 1). In contrast, treatment with the roughened DEB resulted only in a slight decrease in neointimal hyperplasia, in comparison to the control group. Four weeks after intervention, uncoated balloon angioplasty led to a maximal neointimal thickness of 1.24 ± 0.59 mm (n = 11). Vessels treated with the matrix-coated DEB displayed a highly significant reduction of maximal neointimal thickness to 0.42 ± 0.14 mm (n = 8, P = 0.001 vs. control, P < 0.01 vs. roughened DEB; Fig. 4; Table 1). However, neointimal thickness was not significantly reduced by the roughened DEB (0.86 ± 0.37 mm, n = 9). Neointimal area was highly significantly (P = 0.001) reduced by treatment with the matrix-coated DEB (2.48 ± 0.78 mm2, n = 8 vs. 5.72 ± 1.51 mm2, n = 11 (control)). In contrast, the roughened DEB reduced the neointimal area to only 4.05 ± 1.65 mm2 (n = 11), which reached the defined level of statistical significance (P = 0.03 vs. control; Fig. 4; Table 1).
Discussion
Paclitaxel has been coated on stents in different ways, preferably mixed with polymers but also directly on the metal surface, on the surface of a pretreated metal or in tiny cavities created in the abluminal surface of the stent struts. Some of these paclitaxel-eluting stents have shown impressive anti-restenotic effects after PCIs [3, 4, 13, 16]. Efficacy of other stents coated with the same drug and the same or an even higher dose was not satisfactory and the development was discontinued [6, 16]. In these cases, the lack of efficiency of the DES was attributed to too fast drug release [16].
Paclitaxel admixed to a suitable matrix on balloon catheters has shown similar or superior effects in the treatment and prevention of restenosis in preclinical [11, 17] and clinical trials [1, 12, 14, 18]. Thus, the DEB is by all means the currently most advanced potential alternative to DES to prevent restenosis. However, pure paclitaxel coating on plain PCI balloons alone seems to be insufficient to achieve adequate inhibition of neointimal proliferation. The effect in restenosis inhibition obtained with the matrix-coated DEB was established in extensive animal studies investigating numerous coating variations [9–11, 17]. As the matrix of a DES is at least as important as the antiproliferative drug itself, the same holds true for DEB coating. The challenge posed by DEBs is to ensure adhesion of the drug on the balloon while transferring it to the target vessel segment. Upon inflation, which should be as short as possible, an effective dose has to be released to ensure transfer of a large enough amount of the drug into the vessel wall. In our initial animal studies, several coatings failed to cause sufficient neointimal inhibition [11]. Finally, a specific matrix coating (paclitaxel to which a small amount of the hydrophilic X-ray contrast medium iopromide, the iodinated component in Ultravist®, was added) resulted in substantial neointimal inhibition [11, 17] in animals. These effects have since been confirmed in clinical trials [1, 12, 14, 18]. Matrix coating results in a porous surface with a large contact surface between the lipophilic drug molecules and the vessel wall. It provides uniform and complete release of the drug after the first balloon expansion. These attributes result in a high bioavailability of paclitaxel at the target site, a precondition for rapid drug absorption by the vessel wall [11].
One limitation of our present study is the lack of paclitaxel tissue concentration measurement. However, data were already published for the matrix-coated DEB [11] and recently also for the DIOR® balloon [8]. Posa et al. [8] demonstrated that DIOR® angioplasty leads to measurable, although very low tissue concentrations in porcine coronary arteries. In this study no angiographic follow-up was performed to show efficacy in restenosis inhibition. In the present study only the paclitaxel-coated DEB based on the matrix technology led to a major reduction of neointimal proliferation in the porcine coronary overstretch model.
The DIOR® balloon has a ‘nanoporous’ balloon surface containing paclitaxel microcrystals following dimethyl sulfate treatment (probably dimethyl sulfoxide meant) [2]. In contrast, the PACCOCATH® matrix coating uses small amounts of iopromide (Ultravist®) as matrix to enhance the release and dissolution of the drug, possibly also the adherence to the vessel wall. Iopromide is used anyway as X-ray contrast medium in angioplasty [9, 10, 17]. Only 30–45% of the drug coating is released from roughened balloons during the recommended balloon inflation time of 45–60 s [2]. These findings are consistent with the results of the present study, showing a postprocedural paclitaxel content of 50.4% on balloons with comparatively high variance (SD 11.5%). In contrast, the iopromide matrix was found to release the full amount of the drug (4.5 ± 0.7% of paclitaxel dose on ballons after the procedure), which may contribute to the superiority in restenosis inhibition.
Therefore, the favorable preclinical and clinical findings with the paclitaxel–iopromide-coated balloon cannot necessarily be generalized to other balloon coatings. Preclinical [8] and clinical [2] trials with paclitaxel on the roughened balloon membrane did not yet demonstrate efficacy in restenosis inhibition. The clinical data are limited to a registry of 20 patients with bifurcation lesions [2]. The fact that MACE rates were not elevated after 4 months in this trial indicates that the coating of this balloon is well tolerated but without angiographic follow-up the benefit of the coating is difficult to assess.
The data presented demonstrate that the challenge with DEBs is to achieve fast release upon inflation and persistent efficacy in restenosis prevention and safety without long-lasting double antiplatelet therapy.
References
Clever YP, Rosenkranz S, Böhm M, Scheller B (2008) Hotline update of clinical trials and registries presented at the ACC and SCAI-ACCi2 meeting 2008 in Chicago. Clin Res Cardiol 97:409–417
Fanggiday JC, Stella PR, Guyomi SH, Doevendans PA (2008) Safety and efficacy of drug-eluting balloons in percutaneous treatment of bifurcation lesions: the DEBIUT (drug-eluting balloon in bifurcation Utrecht) registry. Catheter Cardiovasc Interv 71:629–635
Fattori R, Tommaso P (2003) Drug-eluting stents in vascular intervention. Lancet 361:247–249
Heldman AW, Cheng L, Jenkins GM, Heller PF, Kim DW, Ware M Jr, Nater C, Hruban RH, Rezai B, Abella BS, Bunge KE, Kinsella JL, Sollott SJ, Lakatta EG, Brinker JA, Hunter WL, Froehlich JP (2001) Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 103:2289–2295
Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB (1998) In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol 31:224–230
Krucoff MW, Kereiakes DJ, Petersen JL, Mehran R, Hasselblad V, Lansky AJ, Fitzgerald PJ, Garg J, Turco MA, Simonton CA 3rd, Verheye S, Dubois CL, Gammon R, Batchelor WB, O’Shaughnessy CD, Hermiller JB Jr, Schofer J, Buchbinder M, Wijns W, COSTAR II Investigators Group (2008) A novel bioresorbable polymer paclitaxel-eluting stent for the treatment of single and multivessel coronary disease: primary results of the COSTAR (cobalt chromium stent with antiproliferative for restenosis) II study. J Am Coll Cardiol 51:1543–1552
Mori T, Kinoshita Y, Watanabe A, Yamaguchi T, Hosokawa K, Honjo H (2006) Retention of paclitaxel in cancer cells for 1 week in vivo and in vitro. Cancer Chemother Pharmacol 58:665–672
Posa A, Hemetsberger R, Petnehazy O, Petrasi Z, Testor M, Glogar D, Gyöngyösi M (2008) Attainment of local drug delivery with paclitaxel-eluting balloon in porcine coronary arteries. Coron Artery Dis 19:243–247
Scheller B, Speck U, Romeike B, Schmitt A, Sovak M, Böhm M, Stoll HP (2003) Contrast media as a carrier for local drug delivery: successful inhibition of neointimal proliferation in the porcine coronary stent model. Eur Heart J 24:1462–1467
Scheller B, Speck U, Schmitt A, Böhm M, Nickenig G (2003) Addition of paclitaxel to cotrast media prevents restenosis after coronary stent implantation. J Am Coll Cardiol 42:1415–1420
Scheller B, Speck U, Abramjuk C, Bernhardt U, Böhm M, Nickenig G (2004) Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 110:810–814
Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Böhm M, Speck U (2006) Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med 355:2113–2124
Scheller B, Kühler M, Cremers B, Mahnkopf D, Böhm M, Boxberger M (2007) Short and long-term effects of a novel paclitaxel coated stent in the porcine coronary model. Clin Res Cardiol 97:118–123
Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Böhm M, Speck U (2008) Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. Clin Res Cardiol 97:773–781
Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, Holmes DR (1992) Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 19:267–274
Silber S, Borggrefe M, Böhm M, Hoffmeister HM, Dietz R, Ertl G, Heusch G (2007) Positionspapier der DGK zur Wirksamkeit und Sicherheit von Medikamente freisetzenden Koronarstents (DES). Der Kardiologe 1:84–111
Speck U, Scheller B, Abramjuk C, Breitwieser C, Dobberstein J, Böhm M, Hamm B (2006) Neointima inhibition: comparison of effectiveness of non-stent-based local drug delivery and a drug-eluting stent in porcine coronary arteries. Radiology 240:411–418
Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, Claussen CD, Oldenburg A, Scheller B, Speck U (2008) Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 358:689–699
Conflict of interest statement
Prof. Scheller report being coinventor of a patent application for various methods of inhibiting restenosis (including the technique used in this trial), which was submitted by Charité University Hospital in Berlin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cremers, B., Biedermann, M., Mahnkopf, D. et al. Comparison of two different paclitaxel-coated balloon catheters in the porcine coronary restenosis model. Clin Res Cardiol 98, 325–330 (2009). https://doi.org/10.1007/s00392-009-0008-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-009-0008-2